Streamlining Si–O bond formation through cobalt-catalyzed dehydrocoupling†
Ewelina
Szafoni
,
Dariusz
Lewandowski
,
Marcin
Gruszczyński
,
Konstancja
Broniarz
,
Hanna
Stachowiak-Dłużyńska
,
Krzysztof
Kuciński
 * and
Grzegorz
Hreczycho
* and
Grzegorz
Hreczycho
 *
*
Faculty of Chemistry, Adam Mickiewicz University, Poznan, Uniwersytetu Poznanskiego St. 8, 61-614, Poznan, Poland. E-mail: kucinski.k@amu.edu.pl; g.h@amu.edu.pl
First published on 5th September 2024
Abstract
Herein we report a strategy for the synthesis of organosilicons, including siloxanes, silyl ethers, and aminosilanes, via Co-catalyzed dehydrogenative coupling between hydrosilanes and nucleophiles. This discovery represents an expansion of the synthetic toolkit for organosilicon synthesis, forging Si–O and Si–N bonds in the presence of cobalt complexes with salen-type ligands.
The Si–O motif frequently appears in molecules of interest to both chemists and industry.1,2 Undoubtedly, the primary use of silyl ethers is their role in protecting OH groups.3 However, it is also worth mentioning their application in coupling reactions.4 In contrast, siloxanes – compounds identified by the presence of at least one Si–O–Si motif, have been the subject of numerous discussions not only in the context of fundamental research but also in practical applications.2 Silicones are omnipresent in our daily lives,5 just as they were when humanity first set foot on the moon. Silazanes, in brief, are classified as compounds containing Si–N bonds and are much less well understood due to their sensitivity to moisture.6–10
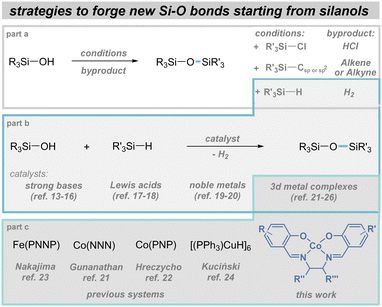
The main method for forming Si–O (and Si–N bonds as well) involves the use of halosilanes (Fig. 1, part a).1,6 Despite the significant advantages of the widespread availability of chlorosilanes and the simplicity of their transformation, this approach also has several drawbacks. Key issues include the formation of reactive, corrosive by-products and significant challenges in achieving the desired chemoselectivity. As a result, scientists have begun seeking alternatives to chlorosilanes.11 Among the most promising options are hydrosilanes1 and carbosilanes such as alkenyl-,12 and alkynylsilanes13,14 (Fig. 1, part a). Considering the atom economy of the process and the market availability of substrates, the utilization of hydrosilanes has garnered the most interest (Fig. 1, part b). This interest has spurred the development of various catalytic methods involving strong bases,15,16 Lewis acids,17,18 4d/5d metal complexes,19,20 and more recently, 3d metal species. Among the latter, the use of cobalt21,22 and iron pincer complexes,23 as well as the use of copper cluster,24 is well known (Fig. 1, part 3). In the case of Co and Fe, the use of these complexes also required the prior synthesis of their ligands, whereas the Cu compound, although commercially available, is characterized by very high chemical sensitivity. Taking this into account, we concluded that the use of readily available salen-type ligands and their cobalt complexes should provide easy access to a range of organosilicons, such as siloxanes, silyl ethers, and aminosilanes.
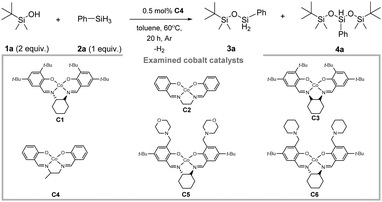
Initial optimization of the transformation was performed using tert-butyldimethylsilanol (1a) as the model substrate (Table 1).
| Entry | Variation from above | Conversion of 2a [%]b | Selectivity [%]d [3a]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [4a] [4a] |
|---|---|---|---|
| a General conditions: 1a (2 equiv., 4 mmol), 2a (1 equiv., 2 mmol), C4 (0.5 mol%), under an argon atmosphere, 60 °C, 20 h. b Conversion of 2a determined by GC. c Isolated yield. d Selectivity of [mono]:[double] dehydrocoupling products determined by GC. | |||
| 1 | None | 99 (98)c | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 2 | No catalyst | 0 | — |
| 3 | 1 equiv. of 1a with C6 | 99 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 4 | CoCl2 instead of C4 | 5 | — |
| 5 | C1 instead of C4 | 82 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 88 88 |
| 6 | C2 instead of C4 | 80 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91 91 |
| 7 | C3 instead of C4 | 84 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
| 8 | C5 instead of C4 | 96 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 9 | C6 instead of C4 | 98 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 10 | 0.25 mol% of C4 | 90 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 11 | In 40 °C | 43 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 12 | Under air | 34 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 13 | In tetrahydrofuran | 98 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 14 | In chlorobenzene | 87 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86 86 |
| 15 | Neat | 89 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
When 2 equiv. of 1a were mixed with 1 equiv. of phenylsilane (2a) in the presence of 0.5 mol% of C4 in toluene at 60 °C, 98% of the O-silylated product 4a was obtained (Table 1, entry 1). The use of equimolar ratio of both mentioned substrates in the presence of catalyst C6 resulted in quantitative formation of mono-O-silylated product 3a (Table 1, entry 3). The reaction in the absence of any cobalt catalyst does not proceed (Table 1, entry 2). Substituting C4 with alternative cobalt sources led to a decrease in conversion (Table 1, entries 4–9). Replacing toluene with other solvents led to a slightly decreased yield of 4a (Table 1, entries 13 and 14). Interestingly, when the reaction was performed under solvent-free conditions, a satisfactory 89% conversion of the substrates to 4a was achieved. This result suggests the potential for solvent-free dehydrocoupling (Table 1, entry 15). Finally, product 4a could also be obtained under an air atmosphere, albeit in lower yield, likely indicating the potential for synthesizing unsymmetrical siloxanes in the presence of oxygen (without traces of symmetrical siloxane formation; Table 1, entry 12).
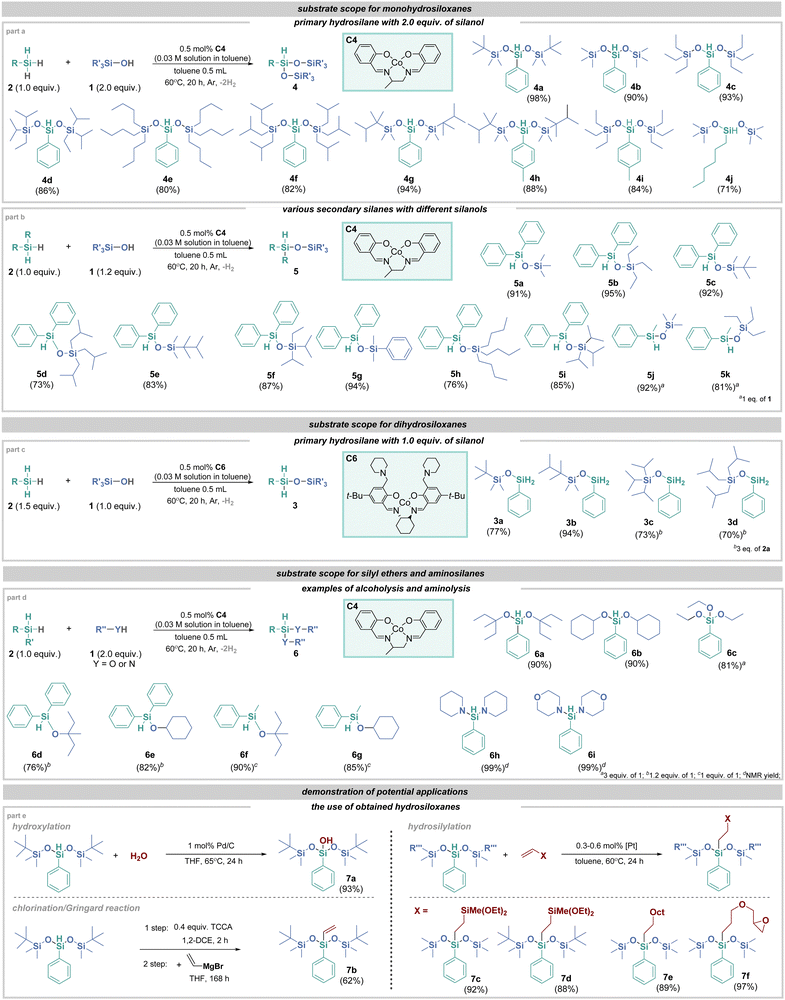
With the optimal conditions in hand, we examined the scope of the transformation (Fig. 2). All primary hydrosilanes exhibited high reactivity, and several trisiloxanes were obtained via double dehydrogenative coupling (Fig. 2, part a). Similarly, secondary hydrosilanes were compatible with the reaction, leading to the isolation of several unsymmetrical disiloxanes in very good yields (Fig. 2, part b). In both cases, mono-hydrosiloxanes were formed, retaining one hydrogen atom attached to silicon. This feature allows for their further use in subsequent reactions, such as hydrosilylation. Unfortunately, tertiary hydrosilanes remain outside the scope of the transformation, likely due to the steric hindrance. After establishing the generality of mono-hydrosiloxane synthesis, we aimed to apply this catalytic system to the production of dihydrosiloxanes. To achieve this, we focused on using catalyst C6 with primary hydrosilanes, which could potentially yield the corresponding siloxanes with two hydrogen atoms attached to silicon. In the case of catalyst C6 (likely due to steric effects), only dihydrosiloxanes were observed when silane was in excess relative to silanol. For the other catalysts studied, traces of monohydrosiloxanes were also observed (<5%). In line with previous results, a dehydrogenative coupling reaction was conducted using an excess of 2a and various silanols (Fig. 2, part c). As a result, we obtained four dihydrosiloxanes with yields of up to 94%. Given that cobalt complexes have already been successfully used in reactions between alcohols and hydrosilanes, we also undertook the task of testing their activity in the synthesis of silyl ethers. As shown in Fig. 2, part d, the desired products were obtained with high yields, regardless of the alcohol's steric hindrance. A natural extension of our research was the application of amines. In recent years, many new catalytic solutions have been developed,10 including two examples involving cobalt compounds.25,26 In this study, it was demonstrated that both piperidine and the much less nucleophilic morpholine can be successfully used in the aminolysis process. In the next phase, our goal was to demonstrate the functionalization potential of the hydrosiloxanes we synthesized (Fig. 2, part e). We began by hydroxylating the previously obtained product 4a using 1 mol% of Pd/C,27 which resulted in the formation of silanol 7a with a 93% yield. Additionally, we performed sequential chlorination followed by a Grignard reaction to produce vinylsilane 7b. We then focused on using products 4a and 4b in a hydrosilylation process with platinum as the catalyst. Pt-based catalysts are extensively used and well-studied in this area.28 The reaction with various alkenes led to the formation of products 7c–7f, with yields reaching up to 97%. These reactions collectively underscore the significant application potential of the hydrosiloxanes synthesized in our study.
Moreover, we successfully demonstrated the scalability of the original siloxane synthesis by performing the reaction on a larger scale (Fig. 3).
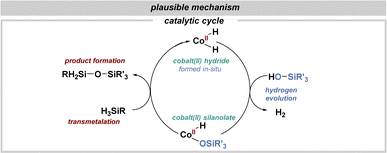
To gain mechanistic insights into the Co-catalyzed reaction, we conducted preliminary experiments. Silanolysis in the presence of the radical scavenger TEMPO showed similar efficiency, indicating that radical pathways are unlikely (details in ESI†). Testing with the metal scavenger Quadra-Pure TU revealed no product formation, suggesting homogeneous catalysis (details in ESI†). NMR experiments did not clarify potential intermediates. However, based on these observations and existing literature,29–31 we propose a plausible mechanism (Fig. 4).
In conclusion, we developed a simple dehydrogenative coupling method for forming unsymmetrical siloxanes, silyl ethers, and aminosilanes using cobalt catalysts with salen-type ligands. These ligands also show great potential for asymmetric siloxane synthesis, which will be explored in future research, along with the reaction's mechanism.
This work was supported by a National Science Centre Grant UMO-2018/30/E/ST5/00045 (G.H.). H. S.-D. acknowledges the Foundation for Polish Science (FNP START 2024 Scholarship). D. L. acknowledges an Adam Mickiewicz University Foundation Scholarship in the 2023/2024 academic year.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Kuciński, H. Stachowiak-Dłużyńska and G. Hreczycho, Coord. Chem. Rev., 2022, 459, 214456 CrossRef.
- T. Köhler, A. Gutacker and E. Mejía, Org. Chem. Front., 2020, 7, 4108–4120 RSC.
- T. W. Greene and P. G. M. Wuts, Protective Groups in Organic Synthesis, John Wiley & Sons, 4th edn, 2007 Search PubMed.
- P. Nareddy, F. Jordan and M. Szostak, Org. Lett., 2018, 20, 341–344 CrossRef.
- A. C. Marmo and M. A. Grunlan, ACS Macro Lett., 2023, 12, 172–182 CrossRef.
- M. B. Reuter, K. Hageman and R. Waterman, Chem. – Eur. J., 2021, 27, 3251–3261 CrossRef PubMed.
- V. Verma, A. Koperniku, P. M. Edwards and L. L. Schafer, Chem. Commun., 2022, 58, 9174–9189 RSC.
- K. Kuciński, H. Stachowiak, D. Lewandowski, M. Gruszczyński, P. Lampasiak and G. Hreczycho, J. Organomet. Chem., 2022, 961, 122127 CrossRef.
- B. E. Leland, J. Mondal and R. J. Trovitch, Chem. Commun., 2023, 59, 3665–3684 RSC.
- M. B. Reuter, D. M. Seth, D. R. Javier-Jiménez, E. J. Finfer, E. A. Beretta and R. Waterman, Chem. Commun., 2023, 59, 1258–1273 RSC.
- B.-B. Yang, P. Guo, X. He and K.-Y. Ye, Org. Chem. Front., 2024, 11, 4125–4130 RSC.
- Y. R. Yeon, Y. J. Park, J. S. Lee, J. W. Park, S. G. Kang and C. H. Jun, Angew. Chem., Int. Ed., 2008, 47, 109–112 CrossRef.
- K. Kuciński, H. Stachowiak and G. Hreczycho, Eur. J. Org. Chem., 2020, 2020, 4042–4049 CrossRef.
- K. Kuciński and G. Hreczycho, Chem. Commun., 2022, 58, 11386–11389 RSC.
- K. Kuciński, H. Stachowiak and G. Hreczycho, Inorg. Chem. Front., 2020, 7, 4190–4196 RSC.
- E. L. Coz, S. Kahlal, J.-Y. Saillard, T. Roisnel, V. Dorcet, J.-F. Carpentier and Y. Sarazin, Chem. – Eur. J., 2019, 25, 13509–13513 CrossRef.
- S. Shinke, T. Tsuchimoto and Y. Kawakami, Silicon Chem., 2007, 3, 243–249 CrossRef.
- H. Gao, A. Battley and E. M. Leitao, Chem. Commun., 2022, 58, 7451–7465 RSC.
- Z. M. Michalska, Transition Met. Chem., 1980, 5, 125–129 CrossRef.
- Y. Satoh, M. Igarashi, K. Sato and S. Shimada, ACS Catal., 2017, 7, 1836–1840 CrossRef.
- S. Pattanaik and C. Gunanathan, ACS Catal., 2019, 9, 5552–5561 CrossRef.
- E. Szafoni, K. Kuciński and G. Hreczycho, J. Catal., 2023, 423, 1–9 CrossRef.
- T. Takeshita, K. Sato and Y. Nakajima, Dalton Trans., 2018, 47, 17004–17010 RSC.
- M. Markwitz, K. Łyczek, Q. Bu and K. Kuciński, Inorg. Chem. Front., 2024, 11, 4855–4866 RSC.
- A. Sharma, R. H. Bean, T. E. Long and R. J. Trovitch, ACS Sustainable Chem. Eng., 2023, 11, 11172–11180 CrossRef CAS.
- E. Szafoni, K. Kuciński and G. Hreczycho, ChemCatChem, 2024, 16, e202400143 CAS.
- D. Brząkalski, M. Walczak, J. Duszczak, B. Dudziec and B. Marciniec, Eur. J. Inorg. Chem., 2018, 2018, 4905–4910 CrossRef.
- H. Stachowiak-Dłużyńska, M. Gruszczyński, M. Kubicki and G. Hreczycho, J. Catal., 2024, 433, 115494 CrossRef.
- K. Matsubara, T. Mitsuyama, S. Shin, M. Hori, R. Ishikawa and Y. Koga, Organometallics, 2021, 40, 1379–1387 CrossRef.
- P. Schiltz, N. Casaretto, A. Auffrant and C. Gosmini, Chem. – Eur. J., 2022, 28, e202200437 CrossRef.
- A. T. Latha and P. C. A. Swamy, Chem. – Eur. J., 2024, 30, e202401841 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization Data and Spectra. See DOI: https://doi.org/10.1039/d4cc04144e |
| This journal is © The Royal Society of Chemistry 2024 |