Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Halogen bonding BODIPY-appended pillar[5]arene for the optical sensing of dicarboxylates and a chemical warfare agent simulant†
Andrew J.
Taylor‡
 ,
Jamie T.
Wilmore‡
,
Jamie T.
Wilmore‡
 and
Paul D.
Beer
and
Paul D.
Beer
 *
*
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford OX1 3TA, UK. E-mail: paul.beer@chem.ox.ac.uk
First published on 2nd September 2024
Abstract
A pillar[5]arene host, functionalised with halogen bonding (XB) recognition sites and BODIPY fluorophores, demonstrates strong binding and optical sensing of environmentally relevant dicarboxylates and a chemical warfare agent simulant, in organic and competitive aqueous-organic media – enabled by the unprecedented combination of fluorophore-conjugated XB interactions with the hydrophobic pillar[5]arene host cavity.
As significant intermediates in many biosynthetic pathways,1 dicarboxylic acids (DCAs) and dicarboxylates (DCBs) (Fig. 1a) are implicated in a variety of chronic illnesses, including kidney and liver disease.2 In addition, DCBs are commonly used in the manufacturing of plastics and as linkers in the ever-increasing production of MOFs,3,4 and hence are emerging as environmental pollutants of concern.3 As a result, the recognition and sensing of DCAs and DCBs is of intense interest for applications in health monitoring, industrial material production and environmental protection.5–7
Since its first use in World War I, sulfur mustard (Fig. 1b) has been deployed in several conflicts as a chemical warfare agent (CWA), causing blistering effects soon after exposure, in addition to potentially fatal respiratory system effects and its carcinogenic and mutagenic potential.8 Clearly, sensing sulfur mustard and other CWAs is of great importance, and an optical, supramolecular, integrated assembly approach to doing so has a number of advantages, including low sensor loadings and good reversibility.9–11
First developed in 2008,12 pillar[5]arenes (P5As) are a class of highly symmetric macrocyclic host compounds,13 which have emerged as useful supramolecular scaffolds for molecular recognition,14 on account of their unique host–guest properties.15 P5As have an electron-rich, hydrophobic, cavity,16 which has been demonstrated to include a variety of electron-deficient molecular species in organic solvents17,18 and hydrophobic molecules in aqueous media.19–21
In recent years, halogen bonding (XB) has been utilised as a powerful non-covalent supramolecular host–guest interaction, especially, but not exclusively, for anion recognition purposes in competitive aqueous solvent media.22–28 Despite exhibiting significantly greater potency and hydrophobic character than conventionally employed hydrogen bonding (HB) interactions,29 decoration of the hydrophobic pillararene cavity with XB donors, for the recognition of target negatively charged and neutral environmental contaminants in aqueous media, has yet to be achieved.
Herein, we report a bis-XB-functionalised P5A, appended with very bright BODIPY fluorophores,30,31 (Fig. 2), which demonstrates sensing of DCBs and a sulfur mustard simulant in organic and aqueous media.32 The XB receptor shows enhanced binding of the analytes over its HB analogue, particularly in aqueous media, in which binding is hydrophobically enhanced for both receptors.
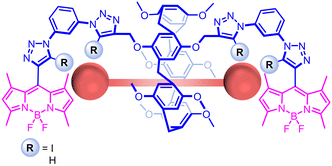 | ||
| Fig. 2 Structures of pillar[5]arene-based sensors, showing the proposed binding mode of DCBs and CEES. | ||
The receptor design sought to append one bis-iodotriazole XB donor motif to each rim of a P5A, wherein the stringent linearity of XB, coupled with its improved performance in aqueous solvents, was expected to give enhanced selectivity and binding affinity for DCBs over previously reported urea-appended P5As.28,33 The XB P5A recognition site was paired with BODIPY fluorophores to enable an optical response to guest binding, allowing in situ detection of the target guest.34
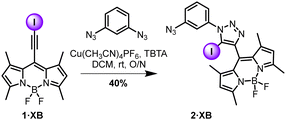
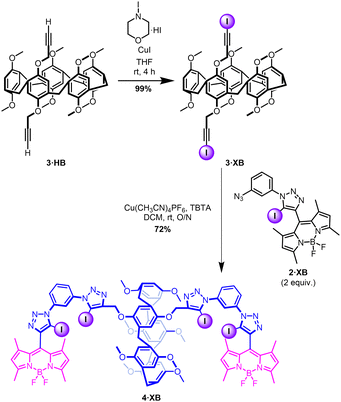
The BODIPY iodo-alkyne, 1·XB, was prepared in accordance with literature procedures.35–37 Mono-functionalised intermediate 2·XB was prepared via a CuAAC reaction (Scheme 1).37 Exploiting the dichloroethane solvent-templated synthesis of P5As,38 the bis alkyne-functionalised P5A 3·HB was obtained in moderate yield (12%) by statistical condensation of 1,4-bis(prop-2-yn-1-yloxy)benzene and 1,4-dimethoxybenzene with paraformaldehyde under conventional P5A synthesis conditions.39 Iodination of 3·HB by N-iodomorpholine hydroiodide afforded the P5A bis-iodoalkyne 3·XB in quantitative yield (Scheme 2). Subsequently, the host 4·XB was afforded in 72% yield by a further CuAAC reaction between 3·XB and two equivalents of 2·XB (Scheme 2). The hydrogen bonding receptor analogue 4·HB was prepared in a similar manner (Schemes S1 and S2, ESI†). Both receptors were fully characterised by 1H and 13C NMR, optical spectroscopy, and high-resolution mass spectrometry (Fig. S4–S9, ESI†).
The ability of the receptors to respond to DCBs in organic media (CHCl3) was first investigated by fluorescence host–guest binding studies with DCBs with a range of alkyl chain lengths, in which the intensity of the BODIPY emission was measured upon successive additions of a DCB tetrabutylammonium (TBA) salt, prepared by neutralisation of the corresponding DCA with TBA hydroxide.33
Pleasingly, XB host 4·XB demonstrated a marked ‘switch-on’ fluorescence response upon the addition of a range of aliphatic straight chain DCBs with the number of carbon atoms in the backbone, n, ranging from n = 8–16 (Fig. 3). Such a response likely arises from an inhibition in the ability of the BODIPY chromophore to rotate about its meso position upon anion binding.37 Qualitative 1H NMR studies in CDCl3 showed a marked upfield shift in the P5A phenyl proton resonance, Hi, coupled with an upfield shift in the DCB methylene proton resonances upon addition of a DCB guest solution to the host, confirming the threading of the DCB through the P5A cavity (Fig. 4).40
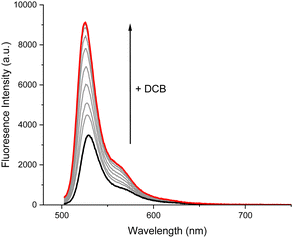 | ||
| Fig. 3 Fluorescence response of receptor 4·XB upon addition of −O2C(CH2)10CO2−. ([4·XB] = 1 μM, [DCB]max = 142 μM, CHCl3, 298 K). | ||
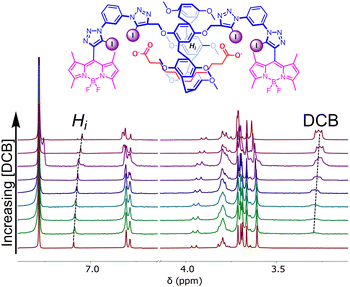 | ||
| Fig. 4 Stacked 1H NMR spectra of 4·XB upon addition of increasing concentrations of −O2C(CH2)10CO2−. (1 mM, CDCl3, 298 K). | ||
Calculation of the host–guest association constants K,41via global fitting of the fluorescence emission isotherms at a range of wavelengths, demonstrated binding affinity is highly dependent on the length of the carbon backbone, with the strongest binding for n = 12, likely due to size complementarity of the DCB guest with the separation of the XB host binding sites (Table 1). Interestingly, the first association of −OAc, fitted with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest binding stoichiometry, was 30 times lower than for any of the DCBs, highlighting the importance of the DCB-P5A threading interaction for the recognition event. In stark contrast, no fluorescence response was measured for 4·HB upon addition of DCB salts, despite a 1H NMR experiment demonstrating threading of the TBA salt of dodecanedioic acid (Fig. S13 and S22, ESI†). This suggests that the interaction between the carboxylate functional group and the HB binding site of 4·HB is insufficient to elicit a fluorescence response from the BODIPY reporter group, highlighting the previously observed superior signal transduction characteristics of XB in the context of fluorescent anion sensing.42
2 host–guest binding stoichiometry, was 30 times lower than for any of the DCBs, highlighting the importance of the DCB-P5A threading interaction for the recognition event. In stark contrast, no fluorescence response was measured for 4·HB upon addition of DCB salts, despite a 1H NMR experiment demonstrating threading of the TBA salt of dodecanedioic acid (Fig. S13 and S22, ESI†). This suggests that the interaction between the carboxylate functional group and the HB binding site of 4·HB is insufficient to elicit a fluorescence response from the BODIPY reporter group, highlighting the previously observed superior signal transduction characteristics of XB in the context of fluorescent anion sensing.42
| n (−O2C(CH2)n−2CO2−) | K (M−1) |
|---|---|
a Determined in CHCl3 at 298 K by global fitting of fluorescence isotherms to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model and errors <5% unless otherwise noted.
b Error <7%.
c Fitted to 1 1 binding model and errors <5% unless otherwise noted.
b Error <7%.
c Fitted to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest binding model, K11 reported.
d Determined by global fitting over an extended concentration range. Error <12%. 2 host–guest binding model, K11 reported.
d Determined by global fitting over an extended concentration range. Error <12%.
|
|
| 8 | 6800 |
| 10 | 8400 |
| 12 | 9900 |
| 14b | 8600 |
| 16 | 6700 |
| TBAOAcc | 240 |
| CEESd | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Attention then turned to exploiting the hydrophobicity of the P5A cavity and XB donors for the enhanced recognition of DCBs in 50% ACN: H2O (buffered to pH = 8.0 with 50 mM HEPES). Fluorescence titration experiments were conducted with the TBA salt of dodecanedioic acid, as it had demonstrated the strongest binding in CHCl3. In contrast to the experiments in CHCl3, both 4·XB and 4·HB exhibited ‘switch-off’ fluorescence responses upon the addition of the DCB (Fig. 5 and Fig. S14 and S15, ESI†) which may be a consequence of reduced meso rotation of the BODIPY in this more viscous solvent mixture,43 leading to anion binding-induced quenching acting as the dominant quenching method, consistent with previous studies.44 Impressively however, the dianionic carboxylate was bound with high affinity by both 4·XB and 4·HB, with K = 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 and 28
000 M−1 and 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1, respectively (Table 2). This represents a 10-fold enhancement in binding for the XB receptor upon changing to the aqueous organic mixture, notably illustrating the potency of the hydrophobic effect employed by this receptor.45 It is also important to note the 4-fold stronger anion affinity demonstrated by the XB receptor over its HB counterpart, showing its potency in aqueous-containing media.45
500 M−1, respectively (Table 2). This represents a 10-fold enhancement in binding for the XB receptor upon changing to the aqueous organic mixture, notably illustrating the potency of the hydrophobic effect employed by this receptor.45 It is also important to note the 4-fold stronger anion affinity demonstrated by the XB receptor over its HB counterpart, showing its potency in aqueous-containing media.45
| Guest | K (M−1) | |
|---|---|---|
| 4·XB | 4·HB | |
a Determined at 298 K by global fitting of fluorescence isotherms to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model.
b 50% ACN: 50% H2O pH = 8.0 (50 mM HEPES buffer) and errors <15%.
c 50% ACN: 50% H2O and errors <7%.
d Average of 3 repeats. 1 binding model.
b 50% ACN: 50% H2O pH = 8.0 (50 mM HEPES buffer) and errors <15%.
c 50% ACN: 50% H2O and errors <7%.
d Average of 3 repeats.
|
||
| −O2C(CH2)10CO2− | 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000b 000b |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500b 500b |
| CH3CH2SCH2CH2Cl (CEES) | 528![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000c,d 000c,d |
145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000c 000c |
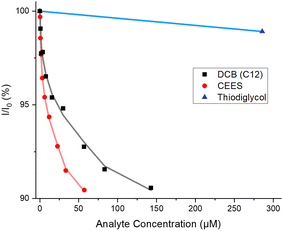
In relation to sensing, sulfur mustard and its simulants have similar molecular properties to DCBs. In particular, it contains a hydrophobic central core appended with halo-chlorine electron-rich end groups which could themselves be bound by XB or HB interactions. Indeed, such interactions have formed the basis of catalysts for nucleophilic substitution reactions.46 Therefore, fluorescence titrations of 4·XB and 4·HB with 2-chloroethyl ethyl sulfide (CEES), a simulant of sulfur mustard, in 50% ACN: H2O, were undertaken (Fig. S16 and S17, ESI†).47 For both receptors, a modest switch-off response (ca. −10%) was observed upon addition of CEES (Fig. 5) and binding constants of 528![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 and 145
000 M−1 and 145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 were observed for 4·XB and 4·HB respectively (Table 2). The increase in affinity for CEES over the TBA salt of dodecanedioic acid was attributed to the increased hydrophobicity of the former. The limit of detection of CEES remained very low for both receptors (0.13 μM and 0.40 μM for 4·XB and 4·HB respectively), highlighting the sensitivity of the fluorescence technique (see Section S6, ESI†). Titrations of CEES with 4·XB were also attempted in CHCl3 and a small but significant decrease in emission intensity (ca. −9%) was observed (Fig. S18, ESI†). An association constant of 32
000 M−1 were observed for 4·XB and 4·HB respectively (Table 2). The increase in affinity for CEES over the TBA salt of dodecanedioic acid was attributed to the increased hydrophobicity of the former. The limit of detection of CEES remained very low for both receptors (0.13 μM and 0.40 μM for 4·XB and 4·HB respectively), highlighting the sensitivity of the fluorescence technique (see Section S6, ESI†). Titrations of CEES with 4·XB were also attempted in CHCl3 and a small but significant decrease in emission intensity (ca. −9%) was observed (Fig. S18, ESI†). An association constant of 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 was determined (Table 1), which is an order of magnitude weaker than in the aqueous-containing media, emphasising the importance of the hydrophobic effect in this recognition event.
000 M−1 was determined (Table 1), which is an order of magnitude weaker than in the aqueous-containing media, emphasising the importance of the hydrophobic effect in this recognition event.
It is noteworthy that neither tributyl phosphate, a nerve agent simulant,48,49 nor the non-toxic hydrolysis product of sulfur mustard, thiodiglycol, elicited a significant fluorescent response upon addition to solutions of either the XB or HB receptor, highlighting the likely selectivity of 4·XB and 4·HB to sulfur mustard itself (Fig. 5 and Fig. S19–S21, ESI†).
In summary, we report the synthesis of a novel XB, BODIPY-appended, P5A host molecule 4·XB, as an optical molecular sensor for a range of anthropogenically generated molecules of environmental interest. Upon addition of DCBs to organic solutions of 4·XB, marked turn-ON emission of the BODIPY reporter groups was observed, while no such response was observed for HB analogue 4·HB, highlighting the superiority of XB-based systems as binding event signal transducers. 1H NMR binding studies confirmed the DCBs thread through the P5A cavity. On account of the hydrophobic nature of the P5A cavity, a marked increase in host–guest binding affinity is observed in highly competitive 50% ACN: H2O aqueous-organic solvent mixtures. Such increased affinity demonstrates the combination of guest threading through a hydrophobic P5A cavity and the use of hydrophobic XB binding groups as a uniquely potent strategy for the sensing of pollutants in aqueous media. Most impressively, the system demonstrates very high binding affinities for a charge-neutral CWA mustard simulant, CEES, demonstrating the power of combining the supramolecular toolkit for sensing both charged and neutral environmental pollutants.
J. T. W. thanks the EPSRC for funding (Centre for Doctoral Training in Inorganic Chemistry for Future Manufacturing (OxICFM), EP/S023828/1, and a Doctoral Prize, EP/W524311/1). A. J. T. thanks the EPSRC for a studentship (EP/T517811/1). Dr Robert Hein (University of Münster) is thanked for helpful discussions.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- D. M. Greenberg, Chemical pathways of metabolism, Academic Press, 2014 Search PubMed.
- B. Hoppe, Nat. Rev. Nephrol., 2012, 8, 467–475 CrossRef CAS PubMed.
- P. A. Przybylińska and M. Wyszkowski, Ecol. Chem. Eng., 2016, 23, 347–356 Search PubMed.
- S. Dang, Q.-L. Zhu and Q. Xu, Nat. Rev. Mater., 2017, 3, 17075 CrossRef.
- S. M. Butler and K. A. Jolliffe, Org. Biomol. Chem., 2020, 18, 8236–8254 RSC.
- D. Curiel, M. Más-Montoya and G. Sánchez, Coord. Chem. Rev., 2015, 284, 19–66 CrossRef CAS.
- W. Chen, C. Guo, Q. He, X. Chi, V. M. Lynch, Z. Zhang, J. Su, H. Tian and J. L. Sessler, J. Am. Chem. Soc., 2019, 141, 14798–14806 CrossRef CAS PubMed.
- Institute of Medicine (US) Committee on the Survey of the Health Effects of Mustard Gas and Lewisite, in Veterans at Risk: The Health Effects of Mustard Gas and Lewisite, ed. C. M. Pechura and D. P. Rall, National Academies Press, US, Washington (DC), 1993 Search PubMed.
- M. R. Sambrook and S. Notman, Chem. Soc. Rev., 2013, 42, 9251 RSC.
- H. M. Tay and P. Beer, Org. Biomol. Chem., 2021, 19, 4652–4677 RSC.
- J. T. Wilmore and P. D. Beer, Adv. Mater., 2024, 36, 2309098 CrossRef CAS PubMed.
- T. Ogoshi, S. Kanai, S. Fujinami, T.-A. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed.
- T. Ogoshi, T.-a Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed.
- X.-Y. Lou, S. Zhang, Y. Wang and Y.-W. Yang, Chem. Soc. Rev., 2023, 52, 6644–6663 RSC.
- A. Mapp, J. T. Wilmore, P. D. Beer and J. M. Goicoechea, Angew. Chem., Int. Ed., 2023, 62, e202309211 CrossRef CAS PubMed.
- T. Ogoshi, J. Inclusion Phenom. Macrocyclic Chem., 2012, 72, 247–262 CrossRef CAS.
- T. Ogoshi, D. Yamafuji, T. Aoki, K. Kitajima, T. A. Yamagishi, Y. Hayashi and S. Kawauchi, Chem. – Eur. J., 2012, 18, 7493–7500 CrossRef CAS PubMed.
- K. Khamphaijun, P. Namnouad, A. Docker, A. Ruengsuk, J. Tantirungrotechai, R. Díaz-Torres, D. J. Harding and T. Bunchuay, Chem. Commun., 2022, 58, 7253–7256 RSC.
- T. Ogoshi, M. Hashizume, T.-A. Yamagishi and Y. Nakamoto, Chem. Commun., 2010, 46, 3708 RSC.
- M. Bojtár, A. Paudics, D. Hessz, M. Kubinyi and I. Bitter, RSC Adv., 2016, 6, 86269–86275 RSC.
- Y. Li, X. Lou, C. Wang, Y. Wang, Y. Jia, Q. Lin and Y. Yang, Chin. Chem. Lett., 2023, 34, 107877 CrossRef CAS.
- A. J. Taylor, A. Docker and P. D. Beer, Chem. – Asian J., 2023, 18, e202201170 CrossRef CAS PubMed.
- A. K. A. Jaini, L. B. Hughes, M. M. Kitimet, K. J. Ulep, M. C. Leopold and C. A. Parish, ACS Sens., 2019, 4, 389–397 CrossRef CAS PubMed.
- J. Pancholi and P. D. Beer, Coord. Chem. Rev., 2020, 416, 213281 CrossRef CAS.
- P. A. Gale and C. Caltagirone, Coord. Chem. Rev., 2018, 354, 2–27 CrossRef CAS.
- Y. Gao, L. Zhang, Z. Wang and L. Lu, ChemPlusChem, 2023, 88 Search PubMed.
- T. Bunchuay, K. Boonpalit, A. Docker, A. Ruengsuk, J. Tantirungrotechai, M. Sukwattanasinitt, P. Surawatanawong and P. D. Beer, Chem. Commun., 2021, 57, 11976–11979 RSC.
- J. Y. Lim and P. D. Beer, Chem. Commun., 2015, 51, 3686–3688 RSC.
- A. Borissov, I. Marques, J. Y. C. Lim, V. Félix, M. D. Smith and P. D. Beer, J. Am. Chem. Soc., 2019, 141, 4119–4129 CrossRef CAS PubMed.
- Y. Cheong Tse, R. Hein, E. J. Mitchell, Z. Zhang and P. D. Beer, Chem. – Eur. J., 2021, 27, 14550–14559 CrossRef CAS PubMed.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed.
- B. Picard, I. Chataigner, J. Maddaluno and J. Legros, Org. Biomol. Chem., 2019, 17, 6528–6537 RSC.
- Q. Duan, W. Xia, X. Hu, M. Ni, J. Jiang, C. Lin, Y. Pan and L. Wang, Chem. Commun., 2012, 48, 8532 RSC.
- N. L. Strutt, R. S. Forgan, J. M. Spruell, Y. Y. Botros and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 5668–5671 CrossRef CAS PubMed.
- M. Albrecht, A. Lippach, M. P. Exner, J. Jerbi, M. Springborg, N. Budisa and G. Wenz, Org. Biomol. Chem., 2015, 13, 6728–6736 RSC.
- K. Hiratsuka, F. T. Salim, K. Takahashi, T. Nakamura and Y. Sagara, Bull. Chem. Soc. Jpn., 2022, 95, 1474–1480 CrossRef CAS.
- A. J. Taylor, R. Hein, S. C. Patrick, J. J. Davis and P. D. Beer, Angew. Chem., Int. Ed., 2024, 63, e202315959 CrossRef CAS PubMed.
- T. Ogoshi, N. Ueshima, T. Akutsu, D. Yamafuji, T. Furuta, F. Sakakibara and T.-A. Yamagishi, Chem. Commun., 2014, 50, 5774–5777 RSC.
- J. Bi, X. Zeng, D. Tian and H. Li, Org. Lett., 2016, 18, 1092–1095 CrossRef CAS PubMed.
- T. Ogoshi, R. Iizuka, D. Kotera and T.-A. Yamagishi, Org. Lett., 2015, 17, 350–353 CrossRef CAS PubMed.
- D. Brynn Hibbert and P. Thordarson, Chem. Commun., 2016, 52, 12792–12805 RSC.
- F. Zapata, A. Caballero, N. G. White, T. D. W. Claridge, P. J. Costa, V. T. Félix and P. D. Beer, J. Am. Chem. Soc., 2012, 134, 11533–11541 CrossRef CAS PubMed.
- Y. V. Zatsikha, N. O. Didukh, R. K. Swedin, V. P. Yakubovskyi, T. S. Blesener, A. T. Healy, D. E. Herbert, D. A. Blank, V. N. Nemykin and Y. P. Kovtun, Org. Lett., 2019, 21, 5713–5718 CrossRef CAS PubMed.
- A. J. Taylor and P. D. Beer, Chem. Commun., 2024, 60, 7983–7986 RSC.
- M. J. Langton, S. W. Robinson, I. Marques, V. Félix and P. D. Beer, Nat. Chem., 2014, 6, 1039–1043 CrossRef CAS PubMed.
- S. M. Walter, F. Kniep, E. Herdtweck and S. M. Huber, Angew. Chem., Int. Ed., 2011, 50, 7187–7191 CrossRef CAS PubMed.
- B. M. Smith, Chem. Soc. Rev., 2008, 37, 470–478 RSC.
- K. Kim, O. G. Tsay, D. A. Atwood and D. G. Churchill, Chem. Rev., 2011, 111, 5345–5403 CrossRef CAS PubMed.
- M. R. Sambrook, J. C. Vincent, J. A. Ede, I. A. Gass and P. J. Cragg, RSC Adv., 2017, 7, 38069–38076 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc03748k |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2024 |