Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nickel-based perovskite-catalysed direct phenol-to-aniline liquid-phase transformations†
Anna Adél
Ádám‡
a,
Sándor Balázs
Nagy‡
 a,
Ákos
Kukovecz
a,
Ákos
Kukovecz
 b,
Zoltán
Kónya
b,
Zoltán
Kónya
 bc,
Pál
Sipos
bc,
Pál
Sipos
 a and
Gábor
Varga
a and
Gábor
Varga
 *b
*b
aDepartment of Molecular and Analytical Chemistry and Materials and Solution Structure Research Group, University of Szeged, Dóm tér 7, Szeged, H-6720, Hungary
bDepartment of Applied and Environmental Chemistry and Interdisciplinary Excellence Centre, Institute of Chemistry, University of Szeged, Rerrich Béla tér 1, Szeged, H-6720, Hungary. E-mail: gabor.varga5@chem.u-szeged.hu
cHUN-REN-SZTE Reaction Kinetics and Surface Chemistry Research Group, Rerrich Béla tér 1, H-6720 Szeged, Hungary
First published on 29th August 2024
Abstract
Liquid phase direct amination of phenols to primary anilines with hydrazine was achieved using commercial NiLa-perovskite catalysts as bifunctional Lewis acid/redox-active catalysts without adding any external hydride sources. The amination strategy took place efficiently in the absence of any amount of reducing gasses (H2/NH3) and noble metals under mild conditions.
Primary anilines are of unique importance in both academia and industry as parent molecules of primary amines and consequently of many agrochemicals and pharmaceuticals.1,2 In the last decade, considerable efforts have been made to replace the conventional but still widely used preparation methods, mainly at the industrial level, based on the two-step arene nitration–nitrobenzene reduction process such as the Buchwald–Hartwig, Chan–Lam and Ullmann cross-coupling reactions.3 The reason for this is the considerable limitations of these methods, as they use petroleum-based raw materials, have low atom and energy efficiency and produce hazardous wastes.
Among the newly developed sustainable strategies for the selective production of primary anilines or cyclohexylamines,4–9 the precious metal-based, in particular Pd/C-catalysed, direct liquid-phase reductive amination of phenols has proven to be one of the most promising, rapidly industrializable tools.5,7,8 However, this method heavily relies on the setting of high temperatures (160–200 °C), the use of non-green solvents (toluene or 1,4-dioxane) and the use of large excess amounts of ammonia and/or hydrogen gasses, conditions that are far from the requirements of green chemistry.5,7,8 The latter disadvantage has already been overcome by the introduction of hydrazine as an amine and hydrogen source.7 Furthermore, there is no catalytic route to replace noble metals in this strategy to produce anilines.7 In particular, when using NH3/H2 gas mixtures, Ni(0)-containing catalysts proved to be active and selective promoters when using Lewis acidic supports, but exclusively for cyclohexylamines.9 In contrast, there has been no attempt to introduce a catalytic route for the phenol-to-aniline transformation using catalysts involved in the Ni(I/III) catalytic cycle. In addition, there has been no study on the use of Ni catalysts in phenol conversion using hydrazine as a hydrogen source.
Considering the key role of the Ni(I/III) cycle in nitrogen reduction and/or hydrazine oxidation reactions as well as in C–N coupling reactions,10–12 we hypothesized that these specimens might be suitable for both dehydrogenative activation of hydrazine in the initialization step of a direct phenol-to-aniline transformation and the further amination step. Furthermore, it also seemed obvious that the use of a co-catalyst with Lewis acidic character is essential for this process.5,6,9 Since both the stabilization of the Ni(III) oxidation state in a solid phase13 and the formation of a properly intimate interaction between catalyst and co-catalyst2 are difficult to realize, we strongly believed that the use of a mixed oxide fulfilling both functions could be the solution. To test our hypothesis, LaNiO3 perovskite as one of the most stable Ni(III) compounds and its Ruddlesden–Popper-phase layered counterparts have been synthesized, characterized and used as catalysts.13 Herein, we present a low-temperature Ni(III)-containing perovskite-catalysed reductive phenol-to-aniline route using hydrazine as hydrogen and nitrogen source in green solvents.
Our study was initiated by screening various potentially active catalysts (Table 1) from the combination of different nickel specimens with different oxidation states and Lewis acidic supports in the above-mentioned phenol-to-aniline transformations. The actual composition of the as-prepared structures were determined by XRD (Fig. S1A and B, ESI†) and ICP-MS (Table S1, ESI†). The test reactions were carried out under reaction conditions which were optimized for Pd/C-catalysed phenol-to-aniline reaction except for setting lower temperature (reflux; T ≈ 101 °C).7 As expected, the Ni(0)-containing catalysts did not perform as well at low temperatures (aniline yield: 0–12 mol%; entries 2–5) – regardless of the quality of the Lewis acidic support – as they did at higher temperature in the presence of an NH3/H2 gas mixture (aniline yield: 99 mol%).8 It can also be seen that the composites with Ni(II)-containing moieties were completely inactive (entries 6–9). In sharp contrast, all Ni(III)-containing catalyst candidates showed moderate to good aniline yields (17–43 mol%, entries 10–17) with at least reasonable aniline selectivity (81–100 mol%). This indicates, in good agreement with the literature11 that the Ni(I/III) catalytic cycle surpasses the performance of the Ni(0/II) cycle in the dehydrogenative activation of hydrazine. It has also become evident that the efficiency of the catalytic system with active Ni centres of each oxidation state was higher in the presence of a Lewis acidic support than in its absence (Table 1). Among these structures, LaNiO3 exhibited the best catalytic performance, clearly outperforming the others, especially in phenol conversion. This underpins our hypothesis that a sufficiently close interaction between the catalytic centres can lead to increased efficiency of the catalysts. We also found that the layered counterparts of LaNiO3 performed worse than the pure perovskite. However, this trend was not monotonically decreasing and did not correspond to the decrease in the number of Ni(III) centres due to the appearance of Ni(II) centres determined by TPR measurements (Fig. S2A, ESI†). (See below for an explanation of this phenomenon). Finally, La2O3 appeared to be more suitable as a host structure for cooperation with Ni(III)-containing active specimens than other Lewis acidic supports (entries 10–17 in Table 1). This is likely explained by the ability of La-containing oxides to stabilize high-valent oxidation states.14 Remarkably, the observed differences cannot be related to the differences in the measured specific surface area of the catalyst (Table S1, ESI†), which shows a random distribution compared to the catalytic performance.
| Entry | Catalyst | Phenol conversion (mol%) | Aniline selectivity (mol%) | Aniline yield (mol%) |
|---|---|---|---|---|
| 1 | — | <1 | — | — |
| 2 | Ni-NP | 2 | — | — |
| 3 | Ni/Al2O3 | 14 | 86 | 12 |
| 4 | Ni/La2O3 | 9 | 90 | 8 |
| 5 | Ni/MgO | 6 | 80 | 5 |
| 6 | NiO | — | — | — |
| 7 | NiO/Al2O3 | 4 | — | — |
| 8 | NiO/La2O3 | 2 | — | — |
| 9 | NiO/MgO | — | — | — |
| 10 | Ni2O3 | 19 | 81 | 24 |
| 11 | Ni2O3/Al2O3 | 26 | 92 | 31 |
| 12 | Ni2O3/La2O3 | 30 | 96 | 29 |
| 13 | Ni2O3/MgO | 20 | 86 | 17 |
| 14 | LaNiO 3 | 43 | 100 | 43 |
| 15 | La 2 NiO 4 | 36 | 98 | 35 |
| 16 | La 3 Ni 2 O 7 | 26 | 95 | 25 |
| 17 | La 4 Ni 3 O 10 | 31 | 98 | 30 |
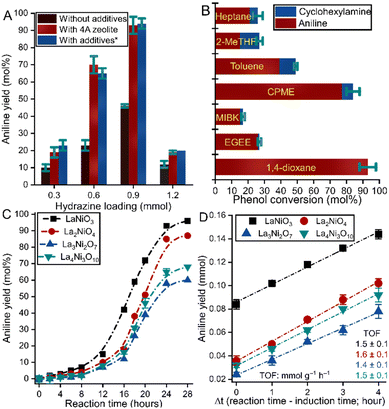
Although all this must be considered as proof of our concept, the reported catalytic markers fall far short of those that can be realized by precious metal-based systems.7,8 To determine whether the perovskite was competitive enough with the benchmark catalyst (Pd/C), the optimal reaction conditions for it had to be investigated. Initially, continuously increasing the absolute concentration of reactants lead to a significant increase in aniline yields (43 mol% → 65 mol%; Fig. S3A, ESI†). With a further increase in reactant concentrations, the aniline yields began to decrease (65 mol% → 55 mol%), as the observed product selectivity decreased significantly (100 mol% → 83%), with hydrazobenzene appearing as a by-product. The observed excess in conversions is plausibly related to the avoided diffusion limitations characteristic of non-porous catalysts. However, the observed reduction in selectivity could be related to the differences between the reactants and intermediates in terms of the strength of their binding to the active surface. Hydrazobenzene, which is a key intermedier,7 probably has a lower affinity to bind to oxyphilic active sites than phenol. Therefore, the hydrazobenzene is forced by the excess phenol to leave the surface before the last step of the catalytic cycle to obtain aniline.
The effects of the actual molar ratio of the reactants on the efficiency of the catalytic system were then investigated. As can be seen, the catalytic markers depended to a significant extent on the actual molar ratio of hydrazine to phenol (Fig. 1A). A four times higher amount of hydrazine than phenol must be adjusted to maximize the aniline yield (65 mol% → 93 mol%) while maintaining the unique selectivity towards aniline, which agrees well with the results for the Pd/C-catalysed system.7 This is likely due to the slow decomposition of hydrazine to generate H2, which can be accelerated by increasing the hydrazine content.
When altering the catalyst loading (Fig. S3B, ESI†), the observed change in the aniline yield can be described by a maximum curve, which shows a maximum yield at a catalyst loading of 10 mol% (aniline yield of 93 mol%). When a higher catalyst loading is used, lower product yields can be determined due probably to the fast aggregation of the perovskite particles.
Besides, it is presented that the use of almost all additives during the phenol conversion can be neglected, with the exception of the molecular sieves, without which less than half of the aniline yield was obtained (93 mol% → 45 mol%; Fig. 1A). This indicates that the formed water molecules poison the active sites, similar to the Pd-containing systems. The reduction in the number of additives is a remarkable advance over the Pd/C-catalysed systems, which require large amounts of LiOH as a hydride source.7 The replacement of 1,4-dioxane with other commonly used solvents showed to be impossible (Fig. 1B). On the contrary, when the green alternatives to 1,4-dioxane were tested, the transformation took place with slightly reduced efficiency in cyclopentyl methyl ether (CPME; aniline yield: 93 mol% → 76 mol%, aniline selectivity: 100 mol% → 92 mol%). Since we used the reflux temperature in all cases – which is a notable advance over the optimum of 170 °C for Pd/C-promoted systems – this also predicted that the use of a lower temperature than 100 °C is likely unfavourable for this transformation. Indeed, the productivity of this strategy decreased sharply at temperatures below (Fig. S3C, ESI†) 100 °C when using e.g. CPME. Noticeably, the introduction of higher temperatures than the reflux ones led to a moderate increase in phenol conversion (60 mol% → 68 mol% in 1,4-dioxane), but in combination with a measurable decrease in aniline selectivity (100 mol% → 63 mol%) enabling the formation of cyclohexylamine by-product.
To determine the optimum reaction time for this transformation, the kinetic profile of the phenol-to-aniline reaction was determined over perovskite catalysts (Fig. 1C). The aniline yield went to saturation after 24 hours in all cases, which was chosen as the optimum reaction time. To properly interpret the kinetic profile, toolbox of mesokinetic analysis supposed to be used but the bifunctional feature of the catalysts makes difficult to define the active centres without any doubt.15,16 It is noteworthy that the Pd/C-catalysed system can enable almost complete conversion of phenol selectively in a shorter time, albeit under harsh reaction conditions (reductive gas mixture in excess at 170 °C).8 More interestingly, an induction period was observed in the kinetic curves for all reactions promoted by perovskites (Fig. 1C). This period is probably due to the dynamic changes of the surface of the catalyst under reaction condtions.17 By determining the TOF values, which are almost the same for each perovskite (1.4–1.6 mmol (aniline) g−1 (catalyst) h−1; Fig. 1D), it can be claimed that the same specimens play an active role in different perovskites which are probably the Ni(III) centres.
With the optimized reaction conditions in our hands, test reactions over potentially active Ni-based catalysts (Table S2, ESI†) were repeated. On the one hand, all catalyst candidates proved to be completely inactive except for the Ni(III)-containing ones, which gave good conversions with almost exclusive aniline selectivity. On the other hand, the previously mentioned differences in the catalytic performance of the perovskites persisted. Fortunately, NH3-TPD measurements (Fig. S2B, ESI†) pointed out that the differences in Lewis acidity of the as-prepared structures followed the same trend as the observed catalytic performances (Fig. 1C). This demonstrates the influential role of the lanthanum oxide matrix as a Lewis acidic co-catalyst in this process.
The control experiments revealed the heterogeneity of the liquid phase transformation in the presence of the perovskite catalyst (Fig. 2A). When removing the solid perovskite from the slurry by simple centrifugation after half of the maximum aniline yield has been reached, no further phenol conversion can be observed. Furthermore, both the recyclability and the stability of the perovskite structure can be demonstrated. Due to the difficulties in separating the solid component of the reaction slurry, the reaction system without molecular sieve was investigated for reuse (Fig. 2B and Table S3, ESI†). After a phenol-to-aniline reaction carried out under the optimized reaction conditions, the catalyst was separated from the reaction mixture, washed, heat-treated, and reused under the optimized reaction conditions. The phenol conversion decreased slightly after the first run and then no significant loss of conversion was observed up to five runs. Thereafter, a significant decrease in conversion occurred in the 6th and 7th runs. This deactivation is probably related to the phase transformation of the perovskite confirmed by XRD (Fig. S1C, ESI†). However, this negative effect can be readily overcome by regenerating the spent catalyst at high temperature (T = 600 °C) in an inert atmosphere, which almost completely restores the original catalytic performance. Furthermore, no significant change in exclusive aniline selectivity was observed during recycling. Therefore, the perovskite catalyst can be regarded as a reusable heterogeneous catalyst, particularly as no leaching of the active components was observed by ICP-MS.
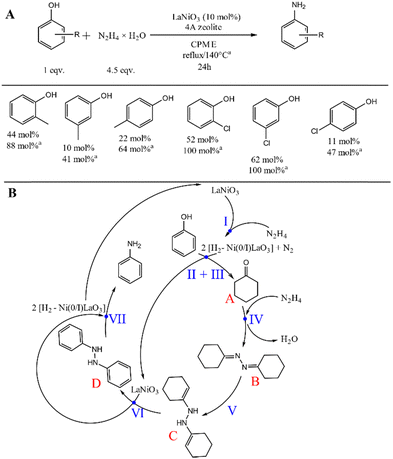
The versatility of this strategy was also demonstrated by the inclusion of phenol derivatives in the study (Fig. 3A). Both cresols and chloro-substituted phenols could be converted into their corresponding aniline counterparts in CPME. Remarkably, the unique selectivity of the system remained unchanged regardless of the reactant used. However, to obtain as good aniline yields as from phenol, a significantly higher reaction temperature (T = 140 °C) must be used. It must be noted at this point that no logical directing effect was observed during these studies, the reason for which is not yet clear.
Based on the literature data7 and our control experiments (Scheme S1, ESI†), which reinforced the ability of perovskite to catalyse both isomerisation (1), transfer hydrogenation (2), and C–N bond formation (3), a plausible reaction mechanism was proposed (Fig. 3B). In the first step, hydrazine molecules (I) decompose at the catalyst surface to form active Ni(I) or Ni(0)–hydrogen specimens that can reduce phenol to cyclohexenol (II) which then undergoes an isomerisation, likely catalysed by the Lewis acidic centres (III), to afford cyclohexanone (A) (control experiment (4)). Cyclohexanone can condense with another hydrazine molecule (IV) to give 1,2-dicyclohexylhydrazine (B), which undergoes hydrazone–enehydrazine tautomerization (V) to produce the intermediate (C). Subsequently, a rapid dehydrogenative–aromatization (VI) of intermediate (C) occurs which results in intermediate (D) and active nickel–hydrogen specimens. Finally, reductive N–N cleavage (VII) of this latter intermediate provides the final product (aniline).
In conclusion, a catalytic strategy was developed based on the use of Ni(III)-containing bifunctional perovskite-type catalysts operating in the Ni(I/III) catalytic cycle for the direct reductive phenol-to-aniline transformation with hydrazine. This strategy proved to be an alternative to the well-known noble metal and Ni(0)-catalysed strategies, which work well under harsh reaction conditions in non-green solvents requiring considerable amount of additives. In contrast, Ni-perovskite systems allow efficient (83 mol% phenol conversion), selective (92 mol% aniline selectivity) transformation under milder reaction conditions (T = 100–110 °C) in CPME. Furthermore, in contrast to its Pd/C counterpart, the perovskite catalyst appears to be recyclable and stable during phenol conversion. This work was supported by the University of Szeged Open Access Fund (Grant number: 7229).
Data availability
The data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Schranck and A. Tlili, ACS Catal., 2018, 8, 405–418 CrossRef CAS.
- M. Kocsis, K. Baán, S. B. Ötvös, Á. Kukovecz, Z. Kónya, P. Sipos, I. Pálinkó and G. Varga, Catal. Sci. Technol., 2023, 13, 3069–3083 RSC.
- J. F. Hartwig, S. Shekhar, Q. Shen and F. Barrios-landeros, Synthesis of Anilines in The chemistry of anilines, ed. Z. Rappoport, John Wiley & Sons, Inc., 2007, pp. 455–536 Search PubMed.
- Y. Koizumi, X. Jin, T. Yatabe, R. Miyazaki, J. Y. Hasegawa, K. Nozaki, N. Mizuno and K. Yamaguchi, Angew. Chem., Int. Ed., 2019, 58, 10893–10897 CrossRef CAS.
- X. Liu, W. Chen, J. Zou, L. Ye and Y. Yuan, ACS Sustainable Chem. Eng., 2022, 10, 6988–6998 CrossRef CAS.
- K. Chen, Q. K. Kang, Y. Li, W. Q. Wu, H. Zhu and H. Shi, J. Am. Chem. Soc., 2022, 144, 1144–1151 CrossRef CAS PubMed.
- Z. Qiu, L. Lv, J. Li, C. C. Li and C. J. Li, Chem. Sci., 2019, 10, 4775–4781 RSC.
- T. Cuypers, P. Tomkins and D. E. De Vos, Catal. Sci. Technol., 2018, 8, 2519–2523 RSC.
- T. Cuypers, T. Morias, S. Windels, C. Marquez, C. Van Goethem, I. Vankelecom and D. E. De Vos, Green Chem., 2020, 22, 1884–1893 RSC.
- N. X. Gu, P. H. Oyala and J. C. Peters, Angew. Chem., Int. Ed., 2021, 60, 4009–4013 CrossRef CAS PubMed.
- X. Gu, X. Li, S. Wu, J. Shi, G. Jiang, G. Jiang and S. Tian, RSC Adv., 2016, 6, 8070–8078 RSC.
- V. Arun, K. Mahanty and S. De Sarkar, ChemCatChem, 2019, 11, 2243–2259 CrossRef CAS.
- J. Yu, J. Sunarso, Y. Zhu, X. Xu, R. Ran, W. Zhou and Z. Shao, Chem. – Eur. J., 2016, 22, 2719–2727 CrossRef CAS PubMed.
- Q. Wu, J. Xiong, Y. Zhang, X. Mei, Y. Wei, Z. Zhao, J. Liu and J. Li, ACS Catal., 2019, 9, 3700–3715 CrossRef CAS.
- W. Chen, G. Qian, Y. Wan, D. Chen, X. Zhou, W. Yuan and X. Duan, Acc. Chem. Res., 2022, 55, 3230–3241 CrossRef CAS PubMed.
- W. Chen, W. Fu, X. Duan, B. Chen, G. Qian, R. Si, X. Zhou, W. Yuan and D. Chen, Engineering, 2022, 14, 124–133 CrossRef CAS.
- R. D. Bradley, B. D. McManus, J. G. Yam, V. Carta and A. Bahamonde, Angew. Chem., Int. Ed., 2023, 62, e202310753 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental part and supporting data. See DOI: https://doi.org/10.1039/d4cc03638g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |