Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The lithium effect in ketenyl anion chemistry†
Prakash
Duari
 ,
Sunita
Mondal
,
Mike
Jörges
and
Viktoria H.
Gessner
,
Sunita
Mondal
,
Mike
Jörges
and
Viktoria H.
Gessner
 *
*
Faculty of Chemistry and Biochemistry, Ruhr-University Bochum, 44801 Bochum, Germany. E-mail: viktoria.gessner@rub.de
First published on 2nd August 2024
Abstract
Ketenyl lithium compounds of type [RC(Li)CO] (with R = Ph2P(E), E = O, S, Se) were found to exhibit lower thermal stabilities than their potassium analogues due to the stronger coordination of the oxygen of the ketene moiety to the harder metal cation, resulting in a more pronounced ynolate character. Using additional ligands allows manipulation of the O–Li interaction, thereby influencing the stability and reactivity of the ketenyl anions.
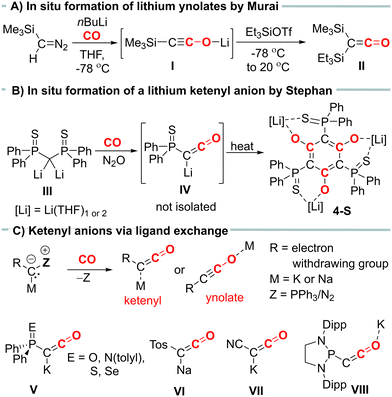
Ketenes are important intermediates in synthetic organic chemistry, offering access to a variety of carbonyl containing compounds.1 In general, ketenes are highly reactive species due to their polarized cumulated double bond.2 Therefore, they are often generated in situ and directly converted to the desired products. Over the years, several synthetic methods to ketenes have been developed, including the Wolff rearrangement of diazoketones,3 photolytic or pyrolytic reactions of suitable precursors,4 or the carbonylation of carbenes or metal carbenes.5 However, many of these methods have drawbacks, such as requiring harsh conditions or unstable starting materials, resulting in low yields and poor selectivity, or having limitations regarding substrate scope.
Ketenyl anions, [RC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]−, offer an alternative entry into ketene chemistry. In the past, these anions were synthesized by carbonylation of diazomethanides (e.g.I, Fig. 1A) and used without isolation.6,7 Recently, we developed a novel synthetic route to ketenyl anions through a mild PPh3/CO exchange at the ylidic carbon center in α-metalated ylides.8,9 This method enabled the first isolation and structure elucidation of a ketenyl anion, revealing an intermediate bonding situation in the anion between the ketenyl and ynolate form (Fig. 1C). Using this synthetic pathway, we isolated the complete series of phosphinoyl-substituted ketenyl anions V,10 the tosyl-ketenyl anion (VI),11 and the cyano-substituted ketenyl anion (VII).12 Furthermore, the Liu group reported the isolation of ketenyl anion IIX using the N2/CO exchange in diazomethanides.13
O]−, offer an alternative entry into ketene chemistry. In the past, these anions were synthesized by carbonylation of diazomethanides (e.g.I, Fig. 1A) and used without isolation.6,7 Recently, we developed a novel synthetic route to ketenyl anions through a mild PPh3/CO exchange at the ylidic carbon center in α-metalated ylides.8,9 This method enabled the first isolation and structure elucidation of a ketenyl anion, revealing an intermediate bonding situation in the anion between the ketenyl and ynolate form (Fig. 1C). Using this synthetic pathway, we isolated the complete series of phosphinoyl-substituted ketenyl anions V,10 the tosyl-ketenyl anion (VI),11 and the cyano-substituted ketenyl anion (VII).12 Furthermore, the Liu group reported the isolation of ketenyl anion IIX using the N2/CO exchange in diazomethanides.13
Remarkably, all isolated ketenyl anions reported to date have had either sodium or potassium as counter cation. No lithium salt has been isolated yet.14 In 2021, Stephan and coworkers reported the formation of Li[Ph2P(S)CCO] (IV) from the reaction of dilithiomethandiide III with CO and N2O (Fig. 1B),15 but in contrast to its potassium analogue V, it was unstable and cyclotrimerized to compound 4-S. Thus, we became interested in understanding the effect of lithium on the stability of ketenyl anions. We recently demonstrated that changes in the electronegativity of the substituents at carbon affect the relative preference of the ynolate versus the ketenyl form.10 Therefore, we hypothesized that a similar shift can be induced by the metal cation depending on the strength and site (C versus O) of the cation–anion interaction.
We began our investigations with the preparation of lithium yldiides 2 with different phosphinoyl moieties P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E (E = O, S, Se), the potassium analogues of which we have recently reported.10 Lithium diisopropylamide (LDA) was found to be a suitable base to selectively generate the α-metalated ylides 2 (Scheme 1).15 Due to their reactivity, yldiides 2 were not isolated but used as in situ prepared reagents. The formation of 2 was confirmed in each case by 31P{1H} NMR spectroscopy, which showed a significant high-field shift of the signal compared to the parent ylides 1 (see ESI†).16 For 2b and 2c, few crystals suitable for X-ray diffraction (XRD) analysis were obtained by the slow diffusion of hexane into toluene/THF solutions, further confirming the successful metalation.
E (E = O, S, Se), the potassium analogues of which we have recently reported.10 Lithium diisopropylamide (LDA) was found to be a suitable base to selectively generate the α-metalated ylides 2 (Scheme 1).15 Due to their reactivity, yldiides 2 were not isolated but used as in situ prepared reagents. The formation of 2 was confirmed in each case by 31P{1H} NMR spectroscopy, which showed a significant high-field shift of the signal compared to the parent ylides 1 (see ESI†).16 For 2b and 2c, few crystals suitable for X-ray diffraction (XRD) analysis were obtained by the slow diffusion of hexane into toluene/THF solutions, further confirming the successful metalation.
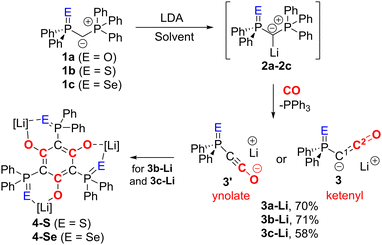 | ||
| Scheme 1 Synthesis of yldiide 2, ketenyl anion 3 and their decomposition products 4. Solvent = toluene (for E = O), toluene/THF mixture (E = S, Se). | ||
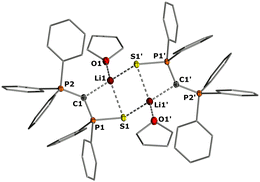
In the crystal, the metalated ylides 2b and 2c form centrosymmetric dimers, with the lithium cations being coordinated by the S and Se atom, respectively, the ylidic carbon atom, and an additional THF molecule (see Fig. 2 for 2b, the ESI† for 2c). In 2b, the P–C distances (1.671(2) and 1.651(2) Å) are significantly shorter than in the ylide precursor (1.729(1) and 1.696(1) Å) due to increased electrostatic interactions within the P–C–P linkage upon metalation. A similar trend has been observed for the corresponding potassium compound with 18-crown-6.17
Once the selective formation of the metalated ylides 2 was confirmed, we examined their reactivity towards CO and selected 2c as first test system. Its reaction with CO in toluene/THF solution led to the selective formation of PPh3, indicated by a signal at −5.26 ppm in the 31P{1H} NMR spectrum, and another species characterized by a signal at 5.57 ppm, which we assigned to the targeted lithium ketenyl 3c-Li. 3c-Li could be isolated as a colourless solid in 58% yield. The lithium ketenyl is further characterized by a doublet at 5.9 ppm with 1JPC coupling constant of 184.9 Hz for the C1 carbon atom and another doublet at 139.8 ppm with 2JPC coupling constant of 45.0 Hz for the C2 carbon atom in 13C{1H} NMR spectrum (Table 1). The larger JPC coupling constants of the C1 and C2 carbon atom compared to those in the potassium analogue 3c-K (164.0 and 39.0 Hz) indicate a larger P–C–C angle and the preference for the ynolate form in 3c-Li relative to 3c-K.18
| 3a-Li | 3a-K | 3b-Li | 3b-K | 3c-Li | 3c-K | |
|---|---|---|---|---|---|---|
| δ(P) | 12.3 | 13.2 | 21.6 | 22.5 | 5.6 | 6.2 |
| δ(C1) | 2.3 | 3.1 | 6.7 | 2.4 | 5.9 | 2.5 |
| 1 J PC | 218.3 | 210.0 | 193.8 | 175.0 | 184.9 | 164.0 |
| δ(C2) | 141.9 | 140.8 | 138.3 | 142.7 | 139.8 | 143.9 |
| 2 J PC | 48.8 | 47.5 | 46.0 | 40.7 | 45.0 | 39.0 |
Interestingly, lithium ketenyl 3c-Li was found to be unstable in solution. At room temperature, a THF solution converted to a new compound within a few days. This compound was identified as the hexa-substituted benzene derivative 4-Se, formed by trimerization of the ketenyl anion (see ESI†). This trimerization is consistent with the observation made by the Stephan group (Fig. 1B) but contrasts the stability of the potassium analogue of IV, which showed no analogous trimerization reaction at room temperature.15
Given the lower stability of the lithium salt 3c-Li relative to its potassium salt 3c-K, we next evaluated whether this was a general trend. To this end, the lithium ketenyls 3a-Li and 3b-Li were isolated as colourless solids in high yields of over 70% after stirring of the metalated ylide in toluene/THF solution under 1 atm. of CO overnight (Scheme 1). Like the selenide, 3a-Li and 3b-Li show two characteristic doublets in the 13C{1H} NMR spectrum for the C1 and C2 carbon atom, with 1JPC and 2JPC coupling constants larger than those observed for the corresponding potassium compounds (Table 1). Thus, the oxide and the sulfide confirm the trend observed for the selenide, further suggesting that the lithium compounds exhibit a more pronounced ynolate character. Nonetheless, 3b-Li was found to be surprisingly stable in THF solution at room temperature, but it began to trimerize to 4-S when heated to 70 °C. Full conversion was observed after 7 days (Fig. 3). The phosphine oxide 3a-Li demonstrated even greater stability. After heating a THF solution of 3a-Li at 70 °C for 2 weeks, no noticeable changes were observed in the 31P{1H} NMR spectrum. These observations suggest that increased ynolate character generally decreases the stability of the lithium compounds, but this trend varies with different substituents. According to the JPC coupling constants, the ynolate character increases from the phosphine selenide to the oxide, yet the stability increases in the same order. We assumed that this contradictory trend is due to lithium binding to both the ketenyl and the phosphinoyl moiety. The strength of the E–Li interaction increases with the hardness of the donor (O > S > Se), leading to a greater electron density shift towards the Group 16 element. Thus, the JPC coupling constant increases due to a stronger C–P(E) bond, rather than an increased ynolate character.
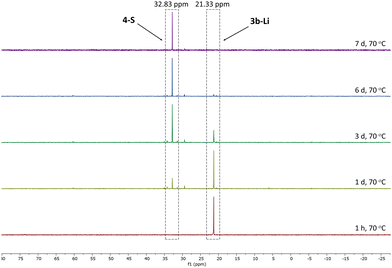 | ||
| Fig. 3 Monitoring of the conversion of 3b-Li into 4-S by 31P{1H} NMR spectroscopy in THF-d8 at 70 °C. | ||
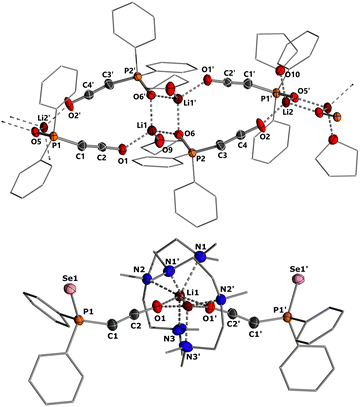
To gain structural insights into the ketenyl lithium compounds, we attempted to obtain single crystals for XRD analysis. Suitable crystals of 3a-Li were grown by slow diffusion of hexane into a saturated toluene/THF solution (Fig. 4, top). In the solid state, 3a-Li forms a polymeric structure with a Li2O2 four-membered ring as central structural motif.19 The lithium ions are each coordinated by the phosphinoyl moiety of two molecules of 3a, the ketenyl oxygen of two neighboring molecules and an additional THF molecule. No contact to the ketenyl C1 carbon atom is observed. The C1–C2 bond in the ketene moiety is slightly shorter (∼1.230 Å) and C–O bond (∼1.214 Å) is slightly longer than in the potassium salt 3a-K with [2,2,2]-cryptand (1.240 Å for C1–C2 and 1.212 Å for C2–O), indicating a stronger contribution of the ynolate form 3′ in the lithium salt as suggested by the JPC coupling constants.10 Accordingly, the P–C–C angle (∼160.5°) in 3a-Li is also larger than in the potassium salt (148.9°), almost approaching linearity.
Since we could not obtain suitable crystals for the THF complexes of 3b-Li and 3c-Li, we isolated the lithium salts 3 with PMDETA (N,N,N′,N′′N′′-pentamethyldiethylenetriamine)20 as additional ligand to gain further information about the coordination chemistry of the lithium ketenyls.21 We hypothesized that the coordination of PMDETA would weaken the O–Li interaction and thereby increase stability. Stirring of the α-metalated ylides 2 with 1 eq. of PMDETA in toluene/THF solvent under an atmosphere of CO led to the precipitation of the corresponding complexes 3-Li(PMDETA), which were isolated as colourless solids in moderate to good yields (see the ESI†). In contrast to the THF complexes, all PMDETA complexes turned out to be stable and did not trimerize to the corresponding benzene derivatives 4, demonstrating the advantageous influence of the ligand on stability. Single crystals of 3c-Li(PMDETA) were obtained by slow diffusion of hexane into a toluene/THF solution at −30 °C. In the solid-state, 3c-Li(PMDETA) forms a centrosymmetric dimer with the lithium cations coordinated by PMDETA and the oxygen of the ketenyl moiety (Fig. 4, bottom). The C–C and C–O bond lengths in the ketene moiety (1.243(8) and 1.222(7) Å) slightly change towards the values of the free anion (1.244(5) and 1.207(4) Å),10 indicating that the additional ligand weakens the O–Li interaction, thereby increasing the ketenyl character in C–C–O moiety and enhancing its stability.
Given that the structural changes are minimal, we aimed to further confirm that the strength of the lithium–oxygen interaction affects the bonding situation by examining the IR vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. Generally, the IR stretch of ketenyl anions falls between those of ketenes and alkynyl ethers,22 with the vibration of ketenes (e.g., 2080 cm−1 for (SiR3)(PhCH2CH2)C
O group. Generally, the IR stretch of ketenyl anions falls between those of ketenes and alkynyl ethers,22 with the vibration of ketenes (e.g., 2080 cm−1 for (SiR3)(PhCH2CH2)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) appearing distinctly red-shifted compared to those of alkynyl ethers (e.g., 2280 cm−1 for PhCH2CH2–C
O) appearing distinctly red-shifted compared to those of alkynyl ethers (e.g., 2280 cm−1 for PhCH2CH2–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–OSiMe3).23,24 Thus, the C
C–OSiMe3).23,24 Thus, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch is highly diagnostic for determining the ketenyl and ynolate character. IR measurements of anions 3-Li, with and without PMDETA, were performed in the solid state as well as in THF solution to exclude solid-state effects (Table 2). In solid state, the lithium ketenyls without PMDETA exhibit stretching frequencies higher than 2125 cm−1. In contrast, their potassium analogues show frequencies lower than 2100 cm−1, thus corroborating with a more pronounced ynolate character of the lithium ketenyls. In addition, the value for the phosphine oxide 3a-Li is lower than those of the sulfide (3b-Li) and selenide (3c-Li), corroborating with its higher stability. The stretching frequencies of 3-Li with PMDETA are lower than those without it, corroborating with a shift towards increased ketenyl character and the enhanced stability of the ketenyl anions in presence of the additional ligand. In THF solution, the frequencies were found to be independent of the presence or absence of the PMDETA ligand, likely due to decreased cation–anion interactions in the coordinating THF solvent.
O stretch is highly diagnostic for determining the ketenyl and ynolate character. IR measurements of anions 3-Li, with and without PMDETA, were performed in the solid state as well as in THF solution to exclude solid-state effects (Table 2). In solid state, the lithium ketenyls without PMDETA exhibit stretching frequencies higher than 2125 cm−1. In contrast, their potassium analogues show frequencies lower than 2100 cm−1, thus corroborating with a more pronounced ynolate character of the lithium ketenyls. In addition, the value for the phosphine oxide 3a-Li is lower than those of the sulfide (3b-Li) and selenide (3c-Li), corroborating with its higher stability. The stretching frequencies of 3-Li with PMDETA are lower than those without it, corroborating with a shift towards increased ketenyl character and the enhanced stability of the ketenyl anions in presence of the additional ligand. In THF solution, the frequencies were found to be independent of the presence or absence of the PMDETA ligand, likely due to decreased cation–anion interactions in the coordinating THF solvent.
| Solid state | THF solution | |||
|---|---|---|---|---|
| Ligand-free | +PMDETA | Ligand-free | +PMDETA | |
| 3a-Li | 2129.83 | 2109.72 | 2093.91 | 2093.92 |
| 3b-Li | 2142.03 | 2103.26 | 2126.31 | 2125.52 |
| 3c-Li | 2138.44 | 2098.23 | 2124.80 | 2124.08 |
In conclusion, we have isolated lithium salts of phosphinoyl-substituted ketenyl anions through carbonylation of lithium yldiides through elimination of PPh3. These lithium ketenyl anions are less thermally stable towards trimerization than their potassium analogues. NMR and IR spectroscopy as well as crystallographic studies showed that this lower stability is accompanied by lithium's preferential coordination by the oxygen atom of the ketene moiety. This coordination favours the ynolate character, indicated by larger coupling constants, blue-shifted C–C–O vibrations, and shorter C–C bonds. The bonding situation can be further influenced by using an additional ligand like PMDETA, weakening the Li–O interaction. These findings highlight the flexibility of the bonding situation in ketenyl anions, which can be regulated through ion-pairing. These effects decisively impact the stability of the anions and can be utilized for reactivity control essential for future applications.
This work was supported by RESOLV, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – EXC-2033 – project number 390677874 and by the European Research Council (Starting Grant: YlideLigands 677749). P. D. acknowledges the German Academic Exchange Service for a PhD scholarship.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest.Notes and references
- H. Staudinger, Chem. Ber., 1905, 38, 1735–1739 CrossRef.
- (a) T. T. Tidwell, Eur. J. Org. Chem., 2006, 563–576 CrossRef CAS; (b) A. D. Allen and T. T. Tidwell, Eur. J. Org. Chem., 2012, 1081–1096 CrossRef CAS; (c) A. D. Allen and T. T. Tidwell, ARKIVOC, 2016, 415–490 Search PubMed.
- (a) Y. Chiang, A. J. Kresgeand and V. V. Popik, J. Am. Chem. Soc., 1999, 121, 5930 CrossRef CAS; (b) B. D. Wagner, B. R. Arnold, G. S. Brown and J. Lusztyk, J. Am. Chem. Soc., 1998, 120, 1827 CrossRef CAS.
- A. D. Allen and T. T. Tidwell, Chem. Rev., 2013, 113, 7287–7342 CrossRef CAS PubMed.
- Z. Zhang, Y. Zhang and J. Wang, ACS Catal., 2001, 1, 1621 CrossRef.
- (a) J. C. Brahms and W. P. Dailey, J. Am. Chem. Soc., 1990, 112, 4046–4047 CrossRef CAS; (b) P. W. Antoni, J. Reitz and M. M. Hansmann, J. Am. Chem. Soc., 2021, 143, 12878–12885 CrossRef CAS PubMed; (c) W. Feuerstein, P. Varava, F. Fadaei-Tirani, R. Scopelliti and K. Severin, Chem. Commun., 2021, 57, 11509–11512 RSC.
- H. Kai, K. Iwamoto, N. Chatani and S. Murai, J. Am. Chem. Soc., 1996, 118, 7634–7635 CrossRef CAS.
- M. Jörges, F. Krischer and V. H. Gessner, Science, 2022, 378, 1331–1336 CrossRef PubMed.
- M. M. Hansmann and G. Bertrand, J. Am. Chem. Soc., 2016, 138(49), 15885–15888 CrossRef CAS PubMed.
- M. Jörges, S. Mondal, M. Kumar, P. Duari, F. Krischer, J. Löffler and V. H. Gessner, Organometallics, 2024, 43, 585–593 CrossRef PubMed.
- F. Krischer, M. Jörges, T.-F. Leung, H. Darmandeh and V. H. Gessner, Angew. Chem., Int. Ed., 2023, 62, e202309629 CrossRef CAS PubMed.
- (a) F. Krischer, V. S. S. N. Swamy, K.-S. Feichtner, R. J. Ward and V. H. Gessner, Angew. Chem., Int. Ed., 2024, 63, e202403766 CrossRef CAS PubMed; (b) T. Wang, Z. Guo, L. E. English, D. W. Stephan, A. R. Jupp and M. Xu, Angew. Chem., Int. Ed., 2024, 63, e202402728 CrossRef CAS PubMed.
- R. Wei, X.-F. Wang, D. A. Ruiz and L. L. Liu, Angew. Chem., Int. Ed., 2023, 62, e202219211 CrossRef CAS PubMed.
- F. Krischer and V. H. Gessner, JACS Au, 2024, 4, 1709–1722 CrossRef CAS PubMed.
- M. Xu, T. Wang, Z.-W. Qu, S. Grimme and D. W. Stephan, Angew. Chem., Int. Ed., 2021, 60, 25281–25285 CrossRef PubMed.
- (a) M. Jörges, A. Kroll, L. Kelling, R. Gauld, B. Mallick, S. Huber and V. H. Gessner, ChemistryOpen, 2021, 10, 1089–1094 CrossRef PubMed; (b) T. Scherpf, R. Wirth, S. Molitor, K.-S. Feichtner and V. H. Gessner, Angew. Chem., Int. Ed., 2015, 54, 8542–8546 CrossRef CAS PubMed; (c) H. Darmandeh, T. Scherpf, K.-S. Feichtner, C. Schwarz and V. H. Gessner, Z. Allg. Anorg. Chem., 2020, 646, 835–841 CrossRef CAS PubMed; (d) T. Baumgartner, B. Schinkels, D. Gudat, M. Nieger and E. Niecke, J. Am. Chem. Soc., 1997, 119, 12410–12411 CrossRef CAS; (e) S. Goumri-Magnet, H. Gornitzka, A. Baceiredo and G. Bertrand, Angew. Chem., Int. Ed., 1999, 38, 678–680 CrossRef CAS; (f) A. Garduno-Alva, R. Lenk, Y. Escudié, M. Lozano González, L. Bousquet, N. Saffon-Merceron, C. A. Toledano, X. Bagan, V. Branchadell, E. Maerten and A. Baceiredo, Eur. J. Inorg. Chem., 2017, 3494 CrossRef CAS.
- M. Jörges, A. J. Gremillion, D. Knyszek, S. P. Kelley, J. R. Walensky and V. H. Gessner, Chem. Commun., 2024, 60, 3190 RSC.
- The J coupling constant depends on the bond angle and s-character of the involved orbitals. With increasing bond angle, the s-character increases. (a) J. Geertsen, J. Oddershede and W. T. Raynes, Magn. Reson. Chem., 1993, 31, 722 CrossRef CAS; (b) E. Y. Tupikina, G. S. Denison, A. S. Antonov and P. M. Tolstoy, Phys. Chem. Chem. Phys., 2020, 22, 1994 RSC And references therein.
- (a) B. Cetinkaya, I. Gumrukcu, M. F. Lappert, J. L. Atwood and R. Shakir, J. Am. Chem. Soc., 1980, 102(6), 2086–2088 CrossRef CAS; (b) F. M. Kerton, C. M. Kozak, K. Lüttgen, C. E. Willans, R. J. Webster and A. C. Whitwood, Inorg. Chim. Acta, 2006, 359, 2819–2825 CrossRef CAS.
- (a) T. Tatic, H. Ott and D. Stalke, Eur. J. Inorg. Chem., 2008, 3765–3768 CrossRef CAS; (b) D. E. Anderson, A. Tortajada and E. Hevia, Angew. Chem., Int. Ed., 2023, 62, e202218498 CrossRef CAS PubMed; (c) D. R. Armstrong, C. M. M. Harris, A. R. Kennedy, J. J. Liggat, R. McLellan, R. E. Mulvey, M. D. T. Urquhart and S. D. Robertson, Chem. – Eur. J., 2015, 21, 14410–14420 CrossRef CAS PubMed.
- M. Granitzka, A.-C. Pöppler, E. K. Schwarze, D. Stern, T. Schulz, M. John, R. Herbst-Irmer, S. K. Pandey and D. Stalke, J. Am. Chem. Soc., 2012, 134(2), 1344–1351 CrossRef CAS PubMed.
- M. A. McAllister and T. T. Tidwell, Can. J. Chem., 1994, 72, 882–887 CrossRef CAS.
- C. J. Kowalski, G. S. Lal and M. S. Haque, J. Am. Chem. Soc., 1986, 108, 7127–7128 CrossRef CAS.
- M. Ito, E. Shirakawa and H. Takaya, Synlett, 2002, 1329–1331 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2362606–2362609. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc03167a |
| This journal is © The Royal Society of Chemistry 2024 |