Urchin-like covalent organic frameworks templated Au@Ag composites for SERS detection of emerging contaminants†
Xiaoya
Yuan
a,
Weihua
Wang
b,
Mantang
Chen
c,
Lijin
Huang
 a,
Qin
Shuai
a and
Lei
Ouyang
a,
Qin
Shuai
a and
Lei
Ouyang
 *a
*a
aState Key Laboratory of Biogeology and Environmental Geology, Faculty of Materials Science and Chemistry, China University of Geosciences, Wuhan 430074, China. E-mail: ouyanglei@cug.edu.cn
bHubei Key Laboratory of Resources and Eco-Environment Geology (Hubei Geological Bureau), Wuhan 430034, China
cZhengzhou Tobacco Research Institute of CNTC, Zhengzhou 450001, China
First published on 9th July 2024
Abstract
Au@Ag core–shell composites were successfully fabricated on urchin-like covalent organic frameworks (COFs), providing a platform with numerous hot spots for the detection of two categories of emerging contaminants: sulfonamide antibiotics and nanoplastics, using surface-enhanced Raman spectroscopy (SERS). Au seeds (∼10 nm) were generated on the COFs, leveraging the reducing properties of the vinyl and imino groups within the framework. This ensured the growth of dense and uniformly distributed Ag nanoparticles. The COFs exceptionally large surface area (2324 m2 g−1) and high adsorption capacity, significantly contributed to the enrichment and detection of trace pollutants. As a result, using a portable Raman spectrometer, limits of detection of 0.008 μmol L−1 for sulfamethoxazole and 0.029 mg L−1 for polystyrene nanoplastics were achieved.
Emerging contaminants (ECs) exhibit pronounced biological toxicity, enduring environmental persistence, and significant bioaccumulation potential.1 Antibiotics and micro(nano)plastics are two prime examples of ECs that are prevalent in environment.2,3 Specifically, sulfamethoxazole (SMX), an antibiotic, has been detected in aquatic systems at concentrations reaching nanomolar per liter (nmol L−1) levels.4,5 Additionally, the extensive production and utilization of plastics lead to the generation of micron- or even nanometer-sized plastic fragments.5 They have been detected in rivers,6 lakes,7 oceans,8 sediments,9 and other aquatic habitats. In comparison to microplastics, nanoplastics pose even greater ecological and biological threats due to their longer persistence and higher penetrability within living organisms.10
Currently, the detection methods for ECs are constrained. Antibiotics are commonly quantified using liquid chromatography coupled with mass spectrometry.11,12 While these technologies offer excellent sensitivity and accuracy, but they are often time-consuming and costly.13 The analysis of nanoplastics can be achieved by thermal decomposition gas chromatography-mass spectrometry (TED-GC-MS), but the sample preparation process is intricate.14 Infrared spectrum (IR) and Raman spectrum can also be utilized for the detection of nanoplastics, yet their sensitivity requires enhancement.5 Among these techniques, surface-enhanced raman spectroscopy (SERS) stands out as a promising analytical tool for trace detection of ECs, owing to its fingerprint spectroscopy capabilities, convenient detection procedures, and simplified pretreatment steps.15 Zhou et al. achieved trace SERS detection of sulfadiazine using Au nanoparticles (AuNPs) with a limit of detection (LOD) of 1 μg L−1.13 Qin et al. detected 20 nm nanoplastics down to 0.1 mg L−1.16 Recently, researchers have tried to synthesis various nano-beacon materials to replace traditional colloidal gold, including composite nanomaterials,17 hollow nanomaterials,18 and magnetic particles,19 and so on. The sensitivity of SERS is heavily influenced by two crucial factors: the density and arrangement of the precious metal nanoparticles, which determine the hot spots for enhancing the Raman signal; and the affinity between the substrate's surface and the target analytes, as only those targets within close proximity to the substrate (<10 nm) can experience signal enhancement.20 However, surface-active nanoparticles tend to aggregate when they come into close proximity, and the chemical affinity of bare noble metal particles towards ECs is relatively weak, thereby restricting the applicability of SERS in ECs detection.21 In this regard, researchers seek to solve this problem with new nanomaterials such as metal–organic frameworks (MOFs) and covalent organic frameworks (COFs).22,23 COFs are class of porous organic structures that offer controllable pore structures, large specific surface areas and excellent adsorption properties.24 These characteristics make COFs an ideal choice as three-dimensional supports for noble nanoparticles in SERS.
In the current study, an urchin-like COF (UCOF) with a particle size of approximately 500 nm was successfully constructed using 1,3,5-tris(4-aminophenyl) benzene (TAPB) and 2,5-divinylterephthalaldehyde (DVA) as the building blocks (Fig. 1a and b). The presence of lattice fringes and polycrystalline diffraction rings in the high-resolution images obtained from transmission electron microscopy (TEM) analysis provided compelling evidence of the excellent crystallinity of the UCOF (Fig. S1 in ESI†). Good crystallinity was further validated by the X-ray diffraction (XRD) pattern, which exhibited distinct peaks at 2.87°, 4.82°, 5.65°, 7.45°, 9.80° and 25.26°. The pattern is in good agreement with the simulated results (Fig. S2a, ESI†).25 Furthermore, a remarkably large surface area (2324 m2 g−1) was determined using the Brunner–Emmett–Teller (BET) analysis (Fig. S2b, ESI†) with main pore size of 2.1 nm (Fig. S2c, ESI†). The presence of dense but uniform distributed nanorods on UCOF not only contributes significantly to the large surface area but also provides an excellent support matrix for the subsequent growth of SERS-active AgNPs. In stead of directly growing AgNPs, which can be challenging to control their distribution and shape, we initially generated AuNPs (∼10 nm) on the nanorods of UCOF as seeds. This was achieved by utilizing the reducible vinyl and imino groups present in the UCOF structure, without the need for any additional reductant (Fig. 1c). The Au seeds were uniformly distributed on the UCOF, whose density can be effectively adjusted by varying the amount of AuCl4−. (Fig. S3, ESI†). As the concentration of AuCl4− increased (<10 mmol L−1), the number of Au seeds increased. However, when the concentration exceeded 10 mmol L−1, fewer but larger AuNPs were formed. Therefore, 10 mmol L−1 was chosen as the optimal concentration, considering both the density and uniformity of the Au seeds. Upon doping AuNPs into the UCOF, a weak peak appeared at 38.20° in the XRD pattern (Fig. S4, ESI†), corresponding to the crystallization peak of the (111) crystal plane of AuNPs (JCPDS # 04-0784).26 However, we observed a shift (0.2°) in the position of the diffraction peak to a smaller angle, which may be attributed to the distortion of the surface lattice of UCOF by the loading of AuNPs.
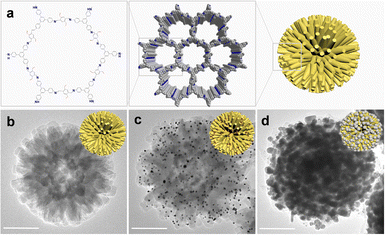 | ||
| Fig. 1 (a) The structure of the UCOF. (b)–(d) The TEM image of UCOF (b), UCOF–Au (c), and UCOF–Au@Ag (d). Scale bar 200 nm. | ||
Subsequently, a SERS-active Ag shell was grown on the Au seeds by adding Ag+ ions and sodium citrate. After reduction, most resulting AgNPs with a size in the range of 20–30 nm could be clearly observed (Fig. 1d and Fig. S5, ESI†). It can be seen that AgNPs are densely distributed, and there are many gaps between the particles smaller than 10 nm, which is favorable for generating electromagnetic field hot spots that enhance SERS signals. The characteristic XRD peak corresponding to the (111) crystal plane of Ag (38.16°) (JCPDS#04-0763) was also observed, indicating the successful encapsulation of Ag on the Au seeds.27 No obvious crystallization peak of the UCOF was observed. This might be due to the dense AgNPs shell that potentially obscured the UCOFs signal during detection. Elemental mapping results confirmed the uniform distribution of both Au and Ag within the composite (Fig. S6, ESI†). The high-resolution TEM (HR-TEM) images revealed a lattice spacing of 0.23 nm, and the diffraction ring radius in reciprocal space was measured to be 4.34 nm−1. These values are consistent with the expected parameters of AgNPs. The particle size of AgNPs can be fine tuned by controlling the concentration of Ag+. Increasing the Ag+ concentration resulted in larger AgNPs, which in turn decreased the distance between adjacent particles (Fig. S7, ESI†). However, when the Ag+ concentration became too high, the larger AgNPs tended to come into contact with each other, forming a continuous Ag shell on the UCOF surface. This continuous shell reduced the distribution of hot spots, potentially impacting the SERS performance.
The SERS performance of the UCOF–Au@Ag structures were evaluated using 4-mercaptobenzoic acid (4-MBA) as a probe, which has a high affinity to AgNPs due to its –SH group.28 The characteristic peaks of 4-MBA at 1075 cm−1 and 1583 cm−1 corresponding to C–H deforming vibration and the aromatic ring (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) breathing mode were observed, respectively. The SERS signal intensity first increased when the molar ratio of AuCl4− to Ag+ increased, but then decreased at higher ratios. The best signal enhancement effect was received with ratio of 1
C) breathing mode were observed, respectively. The SERS signal intensity first increased when the molar ratio of AuCl4− to Ag+ increased, but then decreased at higher ratios. The best signal enhancement effect was received with ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (Fig. S8, ESI†), which is consistent with the trends of hot spots formation as discussed above. Moreover, we compared the SERS performance of UCOF–Au, UCOF–Ag, and UCOF–Au@Ag with the same total molar of Au and Ag added (Fig. S9, ESI†). The results showed that the enhancement of UCOF–Au and UCOF–Ag was weaker than that of UCOF–Au@Ag, indicating that the core–shell structure was conducive to the SERS enhancement. Meanwhile, poorer reproducibility was received when silver reduction was directly performed on UCOF, further demonstrating the important role of the Au seeds. Its detection performance for different concentrations of 4-MBA was shown in Fig. S10a (ESI†). The LOD for 4-MBA was determined to be approximately 0.04 μmol L−1. SERS signals are greatly influenced by the targets located in hot spots, the uneven distribution of hot spots would lead to poor homogeneity. In our case, when the laser irradiated to the substrate, not only the target but also the UCOF were enhanced (Fig. S11, ESI†), thus normalizing the target's signal with the Raman peak signal of UCOF can effectively reduce the influence of uneven hot spot distribution on signal reproducibility (Fig. S10b, ESI†). By utilizing the characteristic Raman peak of UCOF at 1400 cm−1 as an internal standard, and the quantification of 4-MBA was achieved using the ratio of I1583/I1400. The logarithmic concentration of 4-MBA showed a linear relationship in the range of 0.05–50 μmol L−1. The analytical SERS enhanced factor of UCOF–Au@Ag for 4-MBA was calculated to be 7.0 × 107 (Fig. S10c, ESI†). The 3D-FDTD (finite difference time domain) simulation results for the optimized UCOF–Au@Ag composite further confirmed its excellent SERS enhancing ability. As shown in Fig. S10d (ESI†), the simulation reveals the presence of hot spots with intense electromagnetic fields, achieving an enhancement factor of up to 35. Considering to the relationship of E4 to the overall enhancement, 1.5 × 106 times of enhancement can be received. These hot spots are generated due to the inter-particle coupling of the AgNPs, which creates regions of highly localized electromagnetic fields. It is worth noting that after a period of time of storage (up to one month), UCOF–Au@Ag can still maintain excellent SERS activity (Fig. S12, ESI†).
100 (Fig. S8, ESI†), which is consistent with the trends of hot spots formation as discussed above. Moreover, we compared the SERS performance of UCOF–Au, UCOF–Ag, and UCOF–Au@Ag with the same total molar of Au and Ag added (Fig. S9, ESI†). The results showed that the enhancement of UCOF–Au and UCOF–Ag was weaker than that of UCOF–Au@Ag, indicating that the core–shell structure was conducive to the SERS enhancement. Meanwhile, poorer reproducibility was received when silver reduction was directly performed on UCOF, further demonstrating the important role of the Au seeds. Its detection performance for different concentrations of 4-MBA was shown in Fig. S10a (ESI†). The LOD for 4-MBA was determined to be approximately 0.04 μmol L−1. SERS signals are greatly influenced by the targets located in hot spots, the uneven distribution of hot spots would lead to poor homogeneity. In our case, when the laser irradiated to the substrate, not only the target but also the UCOF were enhanced (Fig. S11, ESI†), thus normalizing the target's signal with the Raman peak signal of UCOF can effectively reduce the influence of uneven hot spot distribution on signal reproducibility (Fig. S10b, ESI†). By utilizing the characteristic Raman peak of UCOF at 1400 cm−1 as an internal standard, and the quantification of 4-MBA was achieved using the ratio of I1583/I1400. The logarithmic concentration of 4-MBA showed a linear relationship in the range of 0.05–50 μmol L−1. The analytical SERS enhanced factor of UCOF–Au@Ag for 4-MBA was calculated to be 7.0 × 107 (Fig. S10c, ESI†). The 3D-FDTD (finite difference time domain) simulation results for the optimized UCOF–Au@Ag composite further confirmed its excellent SERS enhancing ability. As shown in Fig. S10d (ESI†), the simulation reveals the presence of hot spots with intense electromagnetic fields, achieving an enhancement factor of up to 35. Considering to the relationship of E4 to the overall enhancement, 1.5 × 106 times of enhancement can be received. These hot spots are generated due to the inter-particle coupling of the AgNPs, which creates regions of highly localized electromagnetic fields. It is worth noting that after a period of time of storage (up to one month), UCOF–Au@Ag can still maintain excellent SERS activity (Fig. S12, ESI†).
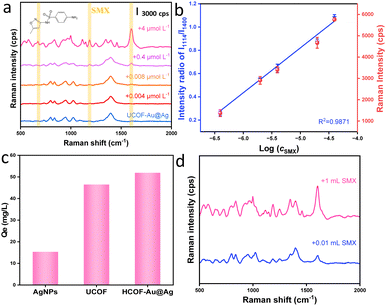
The utilization of UCOF–Au@Ag for the ECs sensing was initially validated in detecting of antibiotics SMX. The indiscriminate use of antibiotics, both for both humans and poultry/livestock, has led to significant issues, including the emergence of drug-resistant bacteria and disturbances in ecological equilibrium.29 As depicted in Fig. 2a, distinctive characteristic peaks associated with SMX were observed. Specifically, peaks at 540 cm−1 and 580 cm−1 correspond to the bending vibration of C–S, while peaks at 670 cm−1 and 1597 cm−1 are attributed to the stretching vibration of the benzene ring (the assignment of the characteristic peaks are presented in Table S1, ESI†). Remarkably, UCOF–Au@Ag exhibits high sensitivity as a SERS substrate for SMX detection, achieving nmoL−1 level detection. As shown in Fig. 2a, the LOD is approximately 0.008 μmol L−1. Quantification using the peak at 1600 cm−1, revealed a strong linear relationship between intensity and concentration (R2 = 0.9871) within the range of 0.4–40 μmol L−1 (Fig. 2b). In comparison to other reported SERS-based methods for sulphonamides (Table S2, ESI†), our approach exhibits excellent sensitivity. Such phenomenon is believed to not solely attributed to the dense distribution of hot spots, but also to the remarkable adsorption capacity of UCOF. Good homogeneity and reproducibility has been received as shown in Fig. S13 (ESI†), the calculated relative standard deviation (RSD) between batches are only 6.04% for three batches of substrate (totally 30 times of detection). As shown in Fig. 2c, the adsorption capacities of AgNPs, UCOF, and UCOF–Au@Ag for SMX were determined to 15.37, 46.50, and 51.94 mg g−1, respectively. This demonstrated the synergistic adsorption effect achieved by the combination of UCOF and AgNPs. Moreover, the exceptional adsorption ability of UCOF–Au@Ag was further exploited for enriching low concentration SMX by increasing the sample volume. As evident in Fig. 2d, the signal intensity of the SMX was considerably stronger for the sample volume of 1 mL compared to 0.01 mL, when the same amount of substrate was used. The identification ability using the fingerprint spectral can also be applied for the detection of other sulfonamide antibiotics. As shown in Fig. S14 (ESI†), despite the structural similarity among the three targets (SMX, sulfadiazine and sulfamethazine), significant difference can be noticed in peak positions and Raman intensities in their fingerprint spectra, demonstrating the capability of sensing other kinds of antibiotics.
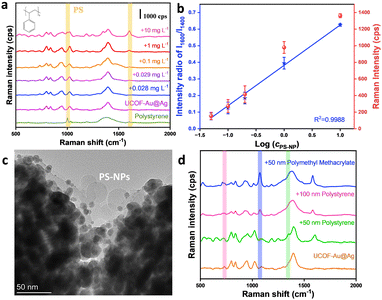
Unlike traditional dissolved pollutants, nanoplastics dispersed across various environmental media.30 Currently, the separation and quantitative detection of highly dispersible nanoplastics remains challenging. The UCOF–Ag@Au presented in this paper exhibits robust enrichment properties, we have further investigated the potential of this material in the analysis of nanoplastics. The sensing performance of the synthetic polystyrene particles (∼30 nm), has been thoroughly validated. As depicted in Fig. 3a, characteristic peaks are observed at 1600 cm−1 and 1002 cm−1, which can be attributed to the stretching vibration of the benzene ring, confirming the identity of the polystyrene nanoparticles (Table S3, ESI†).31 The LOD was determined to 0.029 mg L−1, significantly surpassing reported IR and Raman-based methods, and even rivaling mass spectrometry-based techniques (Table S4, ESI†). As for as we know, such high sensitivity and capability to detect such minute particles of polystyrene have not been previously reported. Quantification was also achieved in a concentration range of 0.05–10 mg L−1, exhibiting a strong linear relationship between the normalized intensity and its logarithmic mass concentration (R2 = 0.9988) (Fig. 3b). The enrichment capability of the substrate was validated through TEM imaging. As depicted in Fig. 3c, it is evident that the 30 nm polystyrene nanoplastics were adsorbed onto the surface of UCOF–Au@Ag, effectively shortening the distance between nanoplastics and AgNPs through the adsorption. Such properties enable trace detection of nanoplastics smaller than 100 nm. The applicability for the detection of polystyrene nanoplastics with other particle sizes (50 nm and 100 nm) and other kinds of nanoplastics (50 nm PMMA) were also validated (Fig. 3d), proving the applicability of other nanoplastics. Zeta potential results showed that electrostatic interaction only contributed partially to the adsorption. (Fig. S15, ESI†). By exploring the performance of SERS at different pH values and temperatures (Fig. S16, ESI†), it was found that the influence of temperature and pH on SERS performance was negligible. These results prove that our substrate material still retains excellent SERS activity in different detection conditions, and it has a wide range of application prospects.
In summary, the urchin-like COFs, featuring reducing groups such as vinyl and imino functionalities, were utilized to successfully anchor AuNPs onto the UCOF surface. These 10 nm AuNPs served as seeds for the uniform growth of AgNPs, leading to the formation of a dense distribution of hot spots, crucial for SERS detection. The large surface area and remarkable adsorption performance of the COFs significantly contributed to the enrichment of target analytes. The resulting UCOF–Au@AgNPs composite was employed for the trace detection of two ECs: SMX and polystyrene nanoparticles with LODs of 0.008 μmol L−1 and 0.029 mg L−1, respectively. Given its excellent adsorption capacity, SERS enhancement ability, and ease of use, the present substrate demonstrates promising applications for the SERS detection of other ECs.
The authors acknowledge the support from National Natural Science Foundation of China (22106147), the Natural Science Foundation of Hubei Province (2024AFD399).
Data availability
The data supporting this article have been included as part of the ESI.† Raw data for each experiment is available by contacting the corresponding author.Conflicts of interest
There are no conflicts to declare.Notes and references
- X. Hu, S. Gong, Q. He, J.-L. Wu and N. Li, Trends Anal. Chem., 2023, 167, 117289 CrossRef CAS.
- R. B. Carneiro, C. A. Sabatini, Á. J. Santos-Neto and M. Zaiat, Sci. Total Environ, 2019, 678, 419 CrossRef CAS PubMed.
- L. Tan, Y. Lou and J.-J. Zhu, Chem. Commun., 2023, 59, 14443 RSC.
- A. Göbel, A. Thomsen, C. S. McArdell, A. Joss and W. Giger, Environ. Sci. Technol., 2005, 39, 3981 CrossRef PubMed.
- L. Xie, K. Gong, Y. Liu and L. Zhang, Environ. Sci. Technol., 2023, 57, 25 CrossRef CAS PubMed.
- F. Lian, Y. Han, Y. Zhang, J. Li, B. Sun, Z. Geng, Z. Wang and B. Xing, Environ. Sci. Technol., 2023, 57, 6520 CrossRef CAS PubMed.
- L. Hu, M. Chernick, D. E. Hinton and H. Shi, Environ. Sci. Technol., 2018, 52, 8885 CrossRef CAS PubMed.
- J. Ding, F. Jiang, J. Li, Z. Wang, C. Sun, Z. Wang, L. Fu, N. X. Ding and C. He, Environ. Sci. Technol., 2019, 53, 8036–8046 CrossRef CAS PubMed.
- Y. Zheng, J. Li, W. Cao, X. Liu, F. Jiang, J. Ding, X. Yin and C. Sun, Sci. Total Environ, 2019, 674, 27 CrossRef CAS PubMed.
- R. Dris, H. Imhof, W. Sanchez, J. Gasperi, F. Galgani, B. Tassin and C. Laforsch, Environ. Chem., 2015, 12, 539 CrossRef CAS.
- Y. Zhang, X. Q. Li, H. M. Li, Q. H. Zhang, Y. Gao and X. J. Li, Trends Anal. Chem., 2019, 110, 344 CrossRef CAS.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Environ. Sci. Technol., 2002, 36, 1202 CrossRef CAS PubMed.
- Z.-M. Zhou, H. Zheng, T. Liu, Z.-Z. Xie, S.-H. Luo, G.-Y. Chen, Z.-Q. Tian and G.-K. Liu, Anal. Chem., 2021, 93, 8603 CrossRef CAS PubMed.
- N. P. Ivleva, Chem. Rev., 2021, 121, 11886 CrossRef CAS PubMed.
- X. X. Han, R. S. Rodriguez, C. L. Haynes, Y. Ozaki and B. Zhao, Nat. Rev. Methods Primers, 2022, 1, 87 CrossRef.
- Y. Qin, J. Qiu, N. Tang, Y. Wu, W. Yao and Y. He, Environ. Res., 2023, 228, 115926 CrossRef CAS PubMed.
- P. Liang, Q. Guo, T. Zhao, C.-Y. Wen, Z. Tian, Y. Shang, J. Xing, Y. Jiang and J. Zeng, Anal. Chem., 2022, 94, 8466 CrossRef CAS PubMed.
- T. Zhao, P. Liang, J. Ren, J. Zhu, X. Yang, H. Bian, J. Li, X. Cui, C. Fu, J. Xing, C. Wen and J. Zeng, Anal. Chim. Acta, 2023, 1255, 341102 CrossRef CAS PubMed.
- J. Li, P. Liang, T. Zhao, G. Guo, J. Zhu, C. Wen and J. Zeng, Anal. Bioanal. Chem., 2023, 415, 545 CrossRef CAS PubMed.
- L. Ouyang, M. Wang, L. Zhu, H. Tang and Q. Shuai, Nano Res., 2023, 16, 3046 CrossRef CAS.
- Y. S. Yamamoto, Y. Ozaki and T. Itoh, J. Photochem. Photobiol., C, 2014, 21, 81 CrossRef CAS.
- Z. Xia, D. Li and W. Deng, Anal. Chem., 2021, 93, 4924 CrossRef CAS PubMed.
- Y. Su, D. Wu, J. Chen, G. Chen, N. Hu, H. Wang, P. Wang, H. Han, G. Li and Y. Wu, Anal. Chem., 2019, 91, 11687 CrossRef CAS PubMed.
- J. Guo, S. Kong, Y. Lian and M. Zhao, Chem. Commun., 2024, 60, 918 RSC.
- W. Ma, G. Li, C. Zhong, Y. Yang, Q. Sun, D. Ouyang, W. Tong, W. Tian, L. Zhang and Z. Lin, Chem. Commun., 2021, 57, 7362 RSC.
- Y. Li, M. Zhai and H. Xu, Appl. Surf. Sci., 2019, 498, 143864 CrossRef CAS.
- Y. Meng, Nanomaterials, 2015, 5, 1124 CrossRef CAS PubMed.
- F. Sun, D. D. Galvan, P. Jain and Q. Yu, Chem. Commun., 2017, 53, 4550 RSC.
- B. Luo, G. Huang, Y. Yao, C. An, P. Zhang and K. Zhao, J. Cleaner Prod., 2021, 319, 128692 CrossRef CAS.
- V. K. Sharma, X. Ma, E. Lichtfouse and D. Robert, Environ. Chem. Lett., 2023, 21, 19336 Search PubMed.
- Y. Jiang, X. Wang, G. Zhao, Y. Shi, Y. Wu, H. Yang and F. Zhao, Water Res., 2024, 255, 121444 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S16 and Table S1–S4. See DOI: https://doi.org/10.1039/d4cc02963a |
| This journal is © The Royal Society of Chemistry 2024 |