Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible light-mediated difluoromethylation/cyclization in batch and flow: scalable synthesis of CHF2-containing benzimidazo- and indolo[2,1-a]isoquinolin-6(5H)-ones†
Al
Hannam‡
a,
Phinyada
Kankraisri‡
a,
Karan R.
Thombare‡
b,
Prahallad
Meher
b,
Alexandre
Jean
 c,
Stephen T.
Hilton
c,
Stephen T.
Hilton
 d,
Sandip
Murarka
d,
Sandip
Murarka
 *b and
Stellios
Arseniyadis
*b and
Stellios
Arseniyadis
 *a
*a
aDepartment of Chemistry, Queen Mary University of London, Mile End Road, E1 4NS, London, UK. E-mail: s.arseniyadis@qmul.ac.uk
bDepartment of Chemistry, Indian Institute of Technology Jodhpur, Karwar-342037, Rajasthan, India. E-mail: sandipmurarka@iitj.ac.in
cIndustrial Research Centre, Oril Industrie, 13 rue Desgenétais, 76210, Bolbec, France
dUCL School of Pharmacy, University College London, 29-39 Brunswick Square, WC1N 1AX, London, UK
First published on 3rd July 2024
Abstract
We report here a practical and cost-effective method for the synthesis of CHF2-containing benzimidazo- and indolo[2,1,a]-isoquinolin-6(5H)-ones through a visible light-mediated difluoromethylation/cyclization cascade. The method, which affords functionalized multifused N-heterocyclic scaffolds in moderate to high yields under mild reaction conditions, is also easily scalable using low-cost 3D printed photoflow reactors.
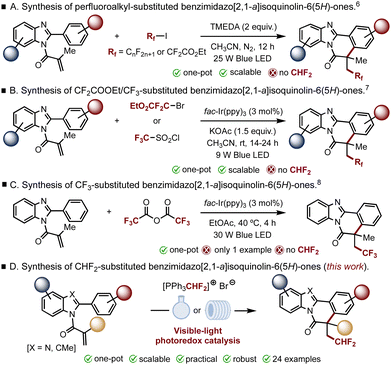
In recent years, visible light-induced synthetic transformations have played a pivotal role in the synthesis and functionalization of a variety of fused polycyclic biologically relevant nitrogen-containing heterocycles.1,2 Benzimidazo[2,1-a]isoquinolin-6(5H)-ones represent one such privileged structure featured in an array of biologically active molecules, pharmaceuticals and functional materials.3 As a result, several photoinduced approaches have been developed to access these types of compounds in a straightforward and efficient way.4 Furthermore, it is also well established that the introduction of fluorine-containing groups on a bioactive molecule can alter its properties by potentially modulating its lipophilicity and/or its pharmacokinetics.5 Accordingly, the incorporation of fluorinated functional groups on the benzimidazoiso-quinolin-6(5H)-one scaffold has gained momentum in the last few years. Lv, Yu and co-workers, for example, adopted a visible light-mediated electron-donor–acceptor (EDA) complex-driven radical cascade approach for the synthesis of perfluoroalkyl-substituted benzimidazo[2,1-a] isoquinolin-6(5H)-ones (Fig. 1A).6 He, Guan and co-workers, on the other hand, developed an iridium-catalyzed radical cascade enabling the synthesis of CF2CO2Et- and CF3-containing benzo[4,5]imidazo[2,1-a]isoquinolinones in good to excellent yields under mild reaction conditions (Fig. 1B).7 More recently, Pan and co-workers reported an iridium-catalyzed trifluoromethylation/cyclization sequence using trifluoroacetic anhydride as the trifluoromethyl radical precursor, albeit the method was only applied to one substrate (Fig. 1C).8 Surprisingly, despite significant strides in fluorination9 and trifluoromethylation chemistry,10 the incorporation of a CHF2 group has only recently started to see an increase in activity.11 This is particularly unexpected given that the CHF2 group can act as a lipophilic substitute for hydroxyl, thiol, amine, and hydroxamic acid functional groups due to its capacity to act as a weak hydrogen bond donor. Nonetheless, there is, to the best of our knowledge, no report of a radical cascade annulation combining a N-acryloyl-2-arylbenzoimidazole derivative with a CHF2 radical precursor leading to the formation of CHF2-containing benzimidazo[2,1-a]isoquinolin-6(5H)-ones. Considering the significant bioactivity of these compounds and our experience in the fields of visible light mediated radical cascades12 and difluoromethylation chemistry,13 we became interested in exploring this idea (Fig. 1D). Indeed, our proposed domino strategy would not only offer an attractive option for a sustainable synthesis of this new family of CHF2-containing heterocyclic scaffolds, it would also offer the opportunity to delve into unexplored chemical space. We report here the results of our endeavor.
 | ||
| Fig. 1 Representative syntheses of fluoroalkylated benzimidazo[2,1-a]isoquinolin-6(5H)-one derivatives. | ||
We initiated our study by conducting a first set of reactions in batch using 1a as a model substrate and difluoromethyl triphenylphosphonium bromide as the CHF2 radical precursor. The latter, first introduced by Burton,14 was found to be readily available and easy to handle compared to all the other difluoromethylation reagents. We evaluated four different photocatalysts as well as several reaction conditions as shown in Table 1. As a general trend, the best result was obtained when running the reaction in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of MeCN and DCM at rt under light irradiation (440 nm Kessil lamp) using 2 equiv. of PPh3CHF2Br, 1 equiv. of 2,6-lutidine and 2 mol% of fac-Ir(ppy)3 (68%, Table 1, entry 4). In sharp contrast, the use of Ru(bpy)3, 4CzlPN and Eosin Y under otherwise identical conditions did not provide the desired product (Table 1, entries 5–7). This disparity can be attributed to the large difference in the excited-state reduction potentials between [Ru(bpy)3Cl2] (−0.81 V vs. SCE), 4-CzIPN (−1.04 V vs. SCE), Eosin Y (−1.15 V vs. SCE) and fac[Ir(ppy)3] (−1.73 V vs. SCE),15 the latter being the only one capable of generating the CHF2 radical from PPh3CHF2Br (−1.2 V vs. SCE) via single-electron-transfer (SET). The use of other solvents, such as MeCN, THF or DMSO (Table 1, entries 1–3), or other bases, such as DIPEA, NEt3, K2CO3 or DABCO (Table 1, entries 8–11), led to the desired product, albeit in lower yields. Replacing PPh3CHF2Br by Akita's (difluoromethyl)bis(2,5-dimethylphenyl)sulfonium salt16 failed to provide the product (result not shown). Unsurprisingly, running the reaction in the absence of photocatalyst or light proved unproductive. Finally, as decreasing the amount of CHF2 radical precursor from 2 equiv. to 1.2 equiv. did not drastically impact the yield (64%, Table 1, entry 4), we maintained this stoichiometry for the rest of the study. Also, while the use of the MeCN and DCM seems counterintuitive, it proved to work well when running our oxy-difluoromethylation reactions under continuous flow using 3D printed PhotoFlow reactors,13a which we also wish to implement here.
1 mixture of MeCN and DCM at rt under light irradiation (440 nm Kessil lamp) using 2 equiv. of PPh3CHF2Br, 1 equiv. of 2,6-lutidine and 2 mol% of fac-Ir(ppy)3 (68%, Table 1, entry 4). In sharp contrast, the use of Ru(bpy)3, 4CzlPN and Eosin Y under otherwise identical conditions did not provide the desired product (Table 1, entries 5–7). This disparity can be attributed to the large difference in the excited-state reduction potentials between [Ru(bpy)3Cl2] (−0.81 V vs. SCE), 4-CzIPN (−1.04 V vs. SCE), Eosin Y (−1.15 V vs. SCE) and fac[Ir(ppy)3] (−1.73 V vs. SCE),15 the latter being the only one capable of generating the CHF2 radical from PPh3CHF2Br (−1.2 V vs. SCE) via single-electron-transfer (SET). The use of other solvents, such as MeCN, THF or DMSO (Table 1, entries 1–3), or other bases, such as DIPEA, NEt3, K2CO3 or DABCO (Table 1, entries 8–11), led to the desired product, albeit in lower yields. Replacing PPh3CHF2Br by Akita's (difluoromethyl)bis(2,5-dimethylphenyl)sulfonium salt16 failed to provide the product (result not shown). Unsurprisingly, running the reaction in the absence of photocatalyst or light proved unproductive. Finally, as decreasing the amount of CHF2 radical precursor from 2 equiv. to 1.2 equiv. did not drastically impact the yield (64%, Table 1, entry 4), we maintained this stoichiometry for the rest of the study. Also, while the use of the MeCN and DCM seems counterintuitive, it proved to work well when running our oxy-difluoromethylation reactions under continuous flow using 3D printed PhotoFlow reactors,13a which we also wish to implement here.
| Entry | Photocatalyst | Solvent | Base | Yielda (%) |
|---|---|---|---|---|
| a Determined by 19F NMR using trifluorotoluene as an internal standard. b Reaction run with 1.2 equiv. of PPh3CHF2⊕Br⊖. c Reaction run either in the dark or without photocatalyst. | ||||
| 1 | fac-[Ir(ppy)3] | MeCN | 2,6-Lutidine | 54 |
| 2 | fac-[Ir(ppy)3] | THF | 2,6-Lutidine | 34 |
| 3 | fac-[Ir(ppy)3] | DMSO | 2,6-Lutidine | 63 |
| 4 | fac-[Ir(ppy)3] | MeCN/DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2,6-Lutidine | 68 (64b) (—c) |
| 5 | Ru(bpy)3 | MeCN/DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2,6-Lutidine | — |
| 6 | 4CzlPN | MeCN/DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2,6-Lutidine | — |
| 7 | Eosin Y | MeCN/DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2,6-Lutidine | — |
| 8 | fac-[Ir(ppy)3] | MeCN/DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
DIPEA | 35 |
| 9 | fac-[Ir(ppy)3] | MeCN/DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NEt3 | 39 |
| 10 | fac-[Ir(ppy)3] | MeCN/DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
K2CO3 | 51 |
| 11 | fac-[Ir(ppy)3] | MeCN/DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
DABCO | 67 |
|
||||
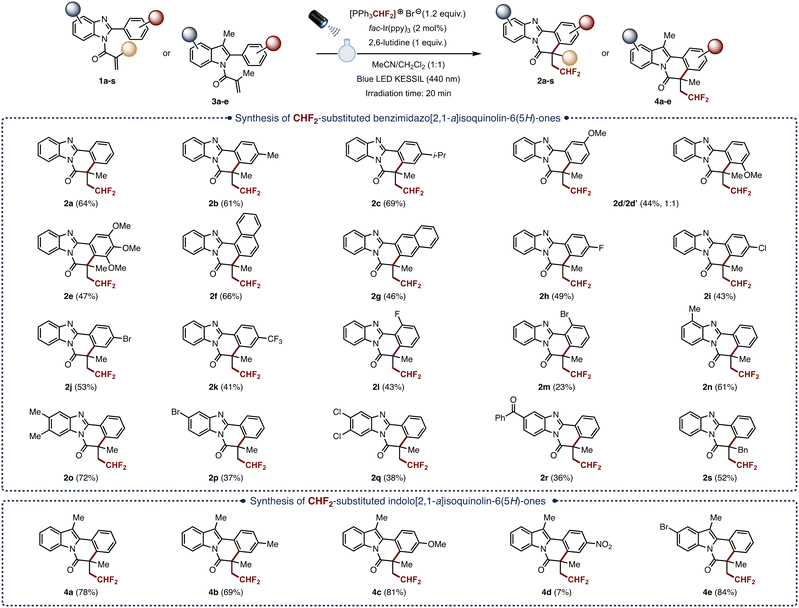
After identifying the best reaction conditions, we proceeded to examine the substrate scope by evaluating a broad range of N-substituted 2-aryl benzimidazoles (Fig. 2). As a general trend, the reaction appeared to be tolerant to substrates bearing both electron-withdrawing and electron-donating groups on the phenyl ring. Hence, the para-methyl (1b) and para-isopropyl (1c) derivatives were converted to the corresponding CHF2-containing benzimidazo[2,1-a] isoquinolin-6(5H)-ones 2b and 2c in 61% and 69% yield, respectively. Unsurprisingly, the meta-methoxy derivative 1d provided two regioisomers (2d and 2d′) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and 44% combined yield. Likewise, the tri-methoxy derivative 1e afforded the difluoromethylation/cyclization cascade product 2e in 47% isolated yield. Interestingly, the 1-naphthyl and 2-naphthyl derivatives 1f and 1g were converted in 66% and 46% yield respectively. As mentioned previously, substrates bearing an electron-withdrawing group on the phenyl ring were also readily converted. Hence, the para-fluoro (2h, 49%), para-chloro (2i, 43%), para-bromo (2j, 53%), para-trifluoromethyl (2k, 41%), ortho-fluoro (2l, 43%), and ortho-bromo (2m, 23%) derivatives were all obtained, albeit in slightly lower yields. The method was also successfully applied to 2-phenyl benzimidazoles bearing various substituents on the benzimidazole ring. Again, both electron-donating and electron-withdrawing substituents were well tolerated as showcased by the moderate to good yields ranging from 36% to 72% obtained with all the substrates tested. Comparatively, substrates bearing an electron-donating group, such as the 4-methyl (2n, 61%) and the 5,6-dimethyl (2o, 72%) derivatives, were obtained in higher yields that the ones bearing an electron-withdrawing group such as the 5-bromo (2p, 37%), the 5,6-dichloro (2q, 38%) and the 5-phenylmethanone (2r, 36%) derivatives. We also explored the scope of the reaction with regards to the substituent on the N-acryloyl moiety. Interestingly, replacing the methyl group by a benzyl group did not hamper the reaction as the corresponding product 2s was obtained in 52% yield.
1 ratio and 44% combined yield. Likewise, the tri-methoxy derivative 1e afforded the difluoromethylation/cyclization cascade product 2e in 47% isolated yield. Interestingly, the 1-naphthyl and 2-naphthyl derivatives 1f and 1g were converted in 66% and 46% yield respectively. As mentioned previously, substrates bearing an electron-withdrawing group on the phenyl ring were also readily converted. Hence, the para-fluoro (2h, 49%), para-chloro (2i, 43%), para-bromo (2j, 53%), para-trifluoromethyl (2k, 41%), ortho-fluoro (2l, 43%), and ortho-bromo (2m, 23%) derivatives were all obtained, albeit in slightly lower yields. The method was also successfully applied to 2-phenyl benzimidazoles bearing various substituents on the benzimidazole ring. Again, both electron-donating and electron-withdrawing substituents were well tolerated as showcased by the moderate to good yields ranging from 36% to 72% obtained with all the substrates tested. Comparatively, substrates bearing an electron-donating group, such as the 4-methyl (2n, 61%) and the 5,6-dimethyl (2o, 72%) derivatives, were obtained in higher yields that the ones bearing an electron-withdrawing group such as the 5-bromo (2p, 37%), the 5,6-dichloro (2q, 38%) and the 5-phenylmethanone (2r, 36%) derivatives. We also explored the scope of the reaction with regards to the substituent on the N-acryloyl moiety. Interestingly, replacing the methyl group by a benzyl group did not hamper the reaction as the corresponding product 2s was obtained in 52% yield.
Following these results, we decided to extend the method to another family of multifused N-heterocyclic scaffolds, namely the indolo[2,1-a]isoquinolin-6(5H)-ones, which are widely found in pharmaceuticals, natural products and functional materials.17 Pleasingly, the method could also be used to access these interesting compounds as showcased by the conversion of the 2-phenyl derivative 3a to the corresponding CHF2-containing indolo[2,1-a]isoquinolin-6(5H)-one 4a in 78% yield. Once again, the method tolerated both electron-donating (3b and 3c) and electron-withdrawing (3d and 3e) substituents around the phenyl and the indole rings.
To confirm the mechanism, we conducted a fluorescence quenching and a radical trapping experiment using TEMPO (Fig. 3A). The latter resulted in the formation of 36% of the CHF2-containing benzimidazo[2,1-a]isoquinolin-6(5H)-one 2a along with 34% of the TEMPO-CHF2 adduct, which strongly supports a one-electron reduction of PPh3CHF2Br and subsequent decomposition releasing the CHF2 radical. The “ON–OFF” experiment (Fig. 3B) and quantum yield calculation (Φ = 0.615) clearly show that the reaction doesn’t proceed through a radical chain mechanism (see ESI† for more details). With these results in hand, we propose the following mechanism where the excited *Ir(ppy)3 undergoes SET to the triphenyl phosphonium bromide, which leads to the release of a CHF2 radical (Fig. 3C). This radical subsequently adds onto the acryloyl moiety to form a radical intermediate, which then undergoes intramolecular cyclization, oxidation and deprotonation to form the desired product and thus complete the photocatalytic cycle.
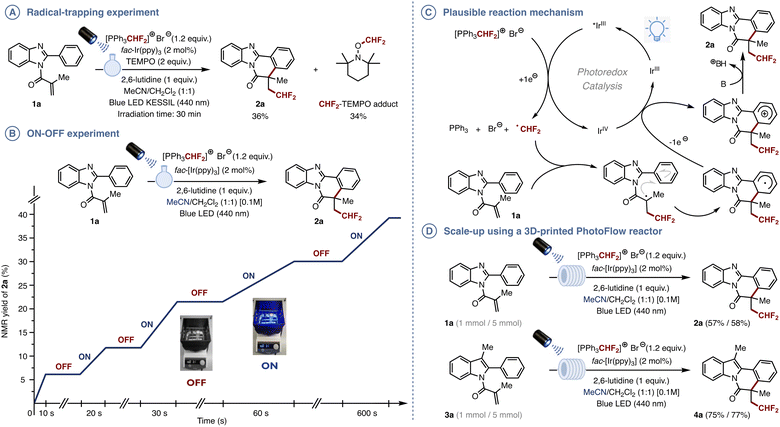 | ||
| Fig. 3 (A) Radical-trapping experiment. (B) ON–OFF experiment. (C) Plausible reaction mechanism. (D) Scale-up using a 3D-printed PhotoFlow reactor. | ||
Finally, to demonstrate the scalability of the method, a 1 and a 5 mmol scale difluoromethylation/cyclization cascade were carried out on 1a under continuous flow conditions using our standardized 3D printed PhotoFlow reactors, which offer guaranties in terms of reproducibility (Fig. 3D).18,19 The reaction proved easy to set up and the product was isolated in 57% and 58% yield, respectively. Similarly, the scale-up of the indole derivative 3a afforded the corresponding indolo[2,1-a]isoquinolin-6(5H)-one 4a in 75% and 77% isolated yield.
In summary, we have developed a scalable, operationally trivial and highly straightforward access to CHF2-containing benzimidazo[2,1,a]-isoquinolin-6(5H)-ones through a visible light-mediated difluoromethylation/cyclization cascade. The method can also be used to synthesize CHF2-containing indolo[2,1-a]isoquinolin-6(5H)-ones starting from the corresponding 2-aryl indole precursors. Interestingly, the use of standardized, low-cost, 3D printed PhotoFlow reactors facilitates reproducibility and scalability through a better control of the reaction parameters.
We would like to thank Dr Rodolphe Tamion and Dr Jean Fournier at Oril Industrie for fruitful discussions. We also would like to thank Dr Lucile Vaysse-Ludot from Oril Industrie affiliated to “Les Laboratoires Servier”, SERB [CRG/2022/000470], CSIR [02(0426)/21/EMR-II] and Queen Mary University of London for financial support, and DST-FIST [SR/FST/CS-II/2019/119(C)] for the HRMS facility at IIT Jodhpur.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- (a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (b) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC.
- (a) J. T. M. Correia, M. S. Santos, E. F. Pissinati, G. P. da Silva and M. W. Paixão, Chem. Rec., 2021, 21, 2666–2687 CrossRef CAS PubMed; (b) S. Kumar Hota, D. Jinan, S. Prakash Panda, R. Pan, B. Sahoo and S. Murarka, Asian J. Org. Chem., 2021, 10, 1848–1860 CrossRef CAS; (c) A. A. Festa, L. G. Voskressensky and E. V. Van der Eycken, Chem. Soc. Rev., 2019, 48, 4401–4423 RSC; (d) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Acc. Chem. Res., 2016, 49, 1911–1923 CrossRef CAS PubMed; (e) L. Zhou, M. Lokman Hossain and T. Xiao, Chem. Rec., 2016, 16, 319–334 CrossRef CAS PubMed; (f) B. Zhang and A. Studer, Chem. Soc. Rev., 2015, 44, 3505–3521 RSC.
- (a) M. J. Taublaender, F. Glöcklhofer, M. Marchetti-Deschmann and M. M. Unterlass, Angew. Chem., Int. Ed., 2018, 57, 12270–12274 CrossRef CAS PubMed; (b) G. Yadav and S. Ganguly, Eur. J. Med. Chem., 2015, 97, 419–443 CrossRef CAS PubMed.
- (a) F.-L. Zeng, K.-C. Xie, Y.-T. Liu, H. Wang, P.-C. Yin, L.-B. Qu, X.-L. Chen and B. Yu, Green Chem., 2022, 24, 1732–1737 RSC; (b) H.-L. Zhu, F.-L. Zeng, X.-L. Chen, K. Sun, H.-C. Li, X.-Y. Yuan, L.-B. Qu and B. Yu, Org. Lett., 2021, 23, 2976–2980 CrossRef CAS PubMed.
- (a) S. Caron, Org. Proc. Res. Dev., 2020, 24, 470–480 CrossRef CAS; (b) N. A. Meanwell, J. Med. Chem., 2018, 61, 5822–5880 CrossRef CAS PubMed; (c) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed; (d) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed.
- F.-L. Zeng, K. Sun, X.-L. Chen, X.-Y. Yuan, S.-Q. He, Y. Liu, Y.-Y. Peng, L.-B. Qu, Q.-Y. Lv and B. Yu, Adv. Synth. Catal., 2019, 361, 5176–5181 CrossRef CAS.
- L. Liu, D.-Y. Yang, Y.-H. He and Z. Guan, J. Org. Chem., 2020, 85, 11892–11901 CrossRef CAS PubMed.
- Y. Song, B. Zheng, S. Yang, Y. Li, Q. Liu and L. Pan, Org. Lett., 2023, 25, 2372–2376 CrossRef CAS PubMed.
- (a) P. A. Champagne, J. Desroches, J. D. Hamel, M. Vandamme and J. F. Paquin, Chem. Rev., 2015, 115, 9073–9174 CrossRef CAS PubMed; (b) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214–8264 CrossRef CAS PubMed; (c) T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470–477 CrossRef CAS PubMed.
- (a) J. Charpentier, N. Früh and A. Togni, Chem. Rev., 2015, 115, 650–682 CrossRef CAS PubMed; (b) E. Merino and C. Nevado, Chem. Soc. Rev., 2014, 43, 6598–6608 RSC.
- (a) J. B. I. Sap, C. F. Meyer, N. J. W. Straathof, N. Iwumene, C. W. Am Ende, A. A. Trabanco and V. Gouverneur, Chem. Soc. Rev., 2021, 50, 8214–8247 RSC; (b) Y. Zafrani, S. Saphier and E. Gershonov, Future Med. Chem., 2020, 12, 361–365 CrossRef CAS PubMed; (c) Y. Zafrani, D. Yeffet, G. Sod-Moriah, A. Berliner, D. Amir, D. Marciano, E. Gershonov and S. Saphier, J. Med. Chem., 2017, 60, 797–804 CrossRef CAS PubMed.
- (a) P. Meher, R. K. Samanta, S. Manna and S. Murarka, Chem. Commun., 2023, 59, 6092–6095 RSC; (b) S. K. Hota, S. P. Panda, S. Das, S. K. Mahapatra, L. Roy, S. De Sarkar and S. Murarka, J. Org. Chem., 2023, 88, 2543–2549 CrossRef CAS PubMed; (c) S. Das, A. Azim, S. K. Hota, S. P. Panda, S. Murarka and S. De Sarkar, Chem. Commun., 2021, 57, 13130–13133 RSC; (d) S. Das, S. K. Parida, T. Mandal, S. K. Hota, L. Roy, S. De Sarkar and S. Murarka, Org. Chem. Front., 2021, 8, 2256–2262 RSC; (e) S. Das, S. K. Parida, T. Mandal, L. Sing, S. De Sarkar and S. Murarka, Chem. – Asian J., 2020, 15, 568–572 CrossRef CAS PubMed.
- (a) J. Zhang, E. Selmi-Higashi, S. Zhang, A. Jean, S. T. Hilton, X. C. Cambeiro and S. Arseniyadis, Org. Lett., 2024, 26, 2877–2882 CrossRef CAS PubMed; (b) E. Selmi-Higashi, J. Zhang, X. C. Cambeiro and S. Arseniyadis, Org. Lett., 2021, 23, 4239–4243 CrossRef CAS PubMed; (c) N. Duchemin, R. Buccafusca, M. Daumas, V. Ferey and S. Arseniyadis, Org. Lett., 2019, 21, 8205–8210 CrossRef CAS PubMed.
- D. J. Burton, J. Fluorine Chem., 1983, 23, 339–357 CrossRef CAS.
- N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed.
- N. Noto, T. Koike and M. Akita, Chem. Sci., 2017, 8, 6375–6379 RSC.
- For selected papers, see: (a) J.-R. Zhang, H.-Y. Liu, T. Fan, Y.-Y. Chen and Y.-L. Xu, Adv. Synth. Catal., 2021, 363, 497–504 CrossRef; (b) K. Alam, S. W. Hong, K. H. Oh and J. K. Park, Angew. Chem., Int. Ed., 2017, 56, 13387–13391 CrossRef CAS PubMed.
- (a) M. R. Penny and S. T. J. Hilton, Flow. Chem., 2023, 13, 435–442 CrossRef CAS; (b) M. R. Penny, Z. X. Rao, B. F. Peniche and S. T. Hilton, Eur. J. Org. Chem., 2019, 3783–3787 CrossRef CAS.
- To obtain the 3D printed CAD designs along with the printing temperatures for polypropylene, see: (a) M. B. Montaner, M. R. Penny and S. T. Hilton, Digital Discovery, 2023, 2, 1797–1805 RSC; (b) https://github.com/vernalis/3Dprint_files .
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedures and spectroscopic data. See DOI: https://doi.org/10.1039/d4cc02557a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |