CO2 capture by imidazolium-based deep eutectic solvents: the effect of steric hindrance of N-heterocyclic carbenes†
Mingzhe
Chen
,
Yi
Zhou
,
Qing
Lu
and
Dezhong
Yang
 *
*
School of Science, China University of Geosciences, Beijing 100083, China. E-mail: yangdz@cugb.edu.cn
First published on 14th June 2024
Abstract
CO2 capture by deep eutectic solvents (DESs) formed between 1,3-bis(isopropyl)imidazolium 1,2,4-triazolide ([IiPim][Triz]) and ethylene glycol (EG) is investigated in this study. [IiPim][Triz]-EG DESs exhibit a capacity of ∼1.0 mol CO2 per mol DES at 1.0 atm and 25 °C. Surprisingly, mechanistic results disclose that CO2 reacts with EG but does not bind with the C-2 site of the [IiPim]+ cation, which may be due to the high steric hindrance of the C-2 site of the N-heterocyclic carbene IiPim present in [IiPim][Triz]-EG DESs.
The climate change mainly caused by the unexpected amount of anthropogonic greenhouse gas emissions has been a global critical issue. As the major component of greenhouse, CO2 is mainly emitted from the combustion of fossil fuels. Reducing CO2 emissions plays a key role in mitigating climate change and preventing detrimental effects of CO2 on ecosystems.1 Carbon capture technology is one of the most promising methods to curb CO2 emissions, and various carbon capture technologies have been developed. The current benchmark industrial technology for carbon capture is the amine-based process using aqueous alkanolamines as absorbents.2 Nevertheless, this method suffers from inherent shortcomings, including degradation of amines and intensive energy cost for regenerating absorbents.3
In the past decade, deep eutectic solvents (DESs) have drawn a great deal of interest because of their unique characteristics, such as low volatility, easy synthesis procedures, and tunable structures.4 Most DESs are formed by combining hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs) due to the intermolecular hydrogen bonds between HBAs and HBDs.5 The applications of DESs have been explored in various fields,6,7 and DESs are also emerging as promising candidates for CO2 capture.8–10 DESs which capture CO2 only through physical interactions are called physical-based DESs,11–14 such as DESs formed by ammonium/phosphonium halide salts as HBAs and alcohols or carboxylic acids as HBDs.15–18 The CO2 capacity of physical-based DESs is low because of the weak interactions between CO2 and solvents. With the aim of improving the CO2 capacity of DESs, chemical-based or functionalized DESs are developed, which can chemically capture CO2 through reactions between CO2 and components of the DESs.19 Most of the chemical-based DESs are synthesized by introducing amino groups or basic anions into components of DESs. The DESs containing amino groups can chemically capture CO2 by forming carbamate species.20–24 Anion-functionalized DESs exhibit promising chemisorption performances for CO2,25–28 such as azolide-based DESs29,30 and phenolate-based DESs.31,32 The CO2 capture mechanisms of anion-functionalized DESs present various reaction pathways depending on the structures of the components of the DESs.8
Chemical-based DESs containing imidazolium ionic liquids are also used for carbon capture. The C-2 site of the imidazolium cation can be deprotonated by basic anions to form N-heterocyclic carbene (NHC), which can react with CO2 resulting in the formation of carboxylate species.33,34 Zhang et al. reported CO2 capture by DES DBN–BmimCl–Im (DBN: 1,5-diazabicyclo[4.3.0]non-5-ene; BmimCl: 1-butyl-3-methylimidazolium chloride; Im: imidazole), and the results suggest that the Bmim carbene formed through the deprotonation of Bmim+ by the imidazolide anion [Im]− reacts with CO2.35 Lee et al. investigated CO2 capture by DESs composed of an imidazolium ionic liquid [Emim][2-CNpyr] ([Emim][2-CNpyr]: 1-ethyl-3-methylimidazolium 2-cyanopyrrolide) and ethylene glycol (EG), and the Emim carbene present in [Emim][2-CNpyr]-EG DESs is also found to bind with CO2.36
Herein, we investigate CO2 capture by DESs consisting of EG and 1,3-bis(isopropyl)imidazolium 1,2,4-triazolide ([IiPim][Triz]) to gauge the role of the steric hindrance of the C-2 site of imidazolium cations. Interestingly, the mechanistic studies disclose that CO2 is not attached to the C-2 site of the [IiPim]+ cation present in DESs, which may be due to the high steric hindrance of the IiPim carbene (IiPim: 1,3-bis(isopropyl)imidazol-2-ylidene). These findings of our work may provide deep insights into the structure effect of components on CO2 absorption by imidazolium-based DESs.
Fig. 1 presents CO2 capture by DESs [IiPim][Triz]-EG. [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), (1
3), (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), and (1
4), and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) could capture 0.96, 0.98, and 0.99 mol CO2 per mol DES, respectively. As reported in the literature, the carbene derived from the [Emim]+ cation in [Emim][2-CNpyr]-EG DESs can react with CO2 to form a carboxylate Emim+–COO−.36 Similarly, in [IiPim][Triz]-EG solvents, the acid–base reaction between the cation [IiPim]+ and the anion [Triz]− can also form a carbene IiPim. Therefore, it is reasonable if one assumes that the carbene IiPim present in [IiPim][Triz]-EG can also react with CO2 to form a carboxylate species. Unexpectedly, mechanism results of [IiPim][Triz]-EG with and without CO2 indicate that the carbene IiPim in DESs does not react with CO2, which is shown in the following sections.
5) could capture 0.96, 0.98, and 0.99 mol CO2 per mol DES, respectively. As reported in the literature, the carbene derived from the [Emim]+ cation in [Emim][2-CNpyr]-EG DESs can react with CO2 to form a carboxylate Emim+–COO−.36 Similarly, in [IiPim][Triz]-EG solvents, the acid–base reaction between the cation [IiPim]+ and the anion [Triz]− can also form a carbene IiPim. Therefore, it is reasonable if one assumes that the carbene IiPim present in [IiPim][Triz]-EG can also react with CO2 to form a carboxylate species. Unexpectedly, mechanism results of [IiPim][Triz]-EG with and without CO2 indicate that the carbene IiPim in DESs does not react with CO2, which is shown in the following sections.
The reaction between CO2 and [IiPim][Triz] was first studied using nuclear magnetic resonance (NMR) spectra. [IiPim][Triz] is solid at room temperature, so the interactions between [IiPim][Triz] and CO2 are studied in DMSO-d6 solvent. As shown in Fig. 2a, after capture, the hydrogen peaks related to the IiPim+–COO− carboxylate can be observed, which are at 7.86 (H-4′), 5.29 (H-6′), and 1.41 (H-7′) ppm. The carbon peaks (Fig. 2b) related to the IiPim+–COO− carboxylate are found at 142.2 (C-2′), 117.9 (C-4′), 50.6 (C-6′) and 155.3 (C-d′) ppm. The C-d’ peak is the carbonyl carbon of the IiPim+–COO− carboxylate. Moreover, the carbonyl carbon of [Triz]-based carbamate is found at 144.2 (C-9) ppm.36,37 These new hydrogen and carbon peaks detected in the [IiPim][Triz] + CO2 system suggest that CO2 reacts with both the IiPim carbene and [Triz]− anion present in IL [IiPim][Triz].
The NMR results of the [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) system are shown in Fig. 3. In the 1H NMR spectra (Fig. 3a), new peaks at 3.51 (H-b) and 3.80 (H-c) ppm can be found, and the proton (H-8) of the [Triz]− shifts downfield from 7.80 to 8.19 ppm upon CO2 absorption. In the 13C NMR spectra (Fig. 3b), there are new peaks at 61.0 (C-b), 66.2 (C-c), and 157.7 (C-d) ppm. These new hydrogen and carbon signals are attributed to the hydrogens and carbons of the EG-based carbonate species,25,27 demonstrating that CO2 reacts with EG in the [IiPim][Triz]:EG (1
3) system are shown in Fig. 3. In the 1H NMR spectra (Fig. 3a), new peaks at 3.51 (H-b) and 3.80 (H-c) ppm can be found, and the proton (H-8) of the [Triz]− shifts downfield from 7.80 to 8.19 ppm upon CO2 absorption. In the 13C NMR spectra (Fig. 3b), there are new peaks at 61.0 (C-b), 66.2 (C-c), and 157.7 (C-d) ppm. These new hydrogen and carbon signals are attributed to the hydrogens and carbons of the EG-based carbonate species,25,27 demonstrating that CO2 reacts with EG in the [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solvent. The C-d signal is ascribed to the carbonyl carbon of O–COO−.29,36 Importantly, the hydrogen or carbon peaks related to the carboxylate IiPim+–COO− are not detected in the NMR spectra of [IiPim][Triz]:EG (1
3) solvent. The C-d signal is ascribed to the carbonyl carbon of O–COO−.29,36 Importantly, the hydrogen or carbon peaks related to the carboxylate IiPim+–COO− are not detected in the NMR spectra of [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) after CO2 capture, and the carbonyl carbon of [Triz]-based carbamate is also not observed. Similarly, in the NMR spectra of the [IiPim][Triz]:EG (1
3) after CO2 capture, and the carbonyl carbon of [Triz]-based carbamate is also not observed. Similarly, in the NMR spectra of the [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (Fig. S1, ESI†) and [IiPim][Triz]:EG (1
4) (Fig. S1, ESI†) and [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (Fig. S2, ESI†) systems after CO2 uptake, the peaks of carboxylate and carbamate species are also not observed. Therefore, the above NMR results confirm that the IiPim carbene in [IiPim][Triz]-EG DESs does not react with CO2.
5) (Fig. S2, ESI†) systems after CO2 uptake, the peaks of carboxylate and carbamate species are also not observed. Therefore, the above NMR results confirm that the IiPim carbene in [IiPim][Triz]-EG DESs does not react with CO2.
Fourier transform infrared (FTIR) spectroscopy is also used to study interactions between CO2 and [IiPim][Triz]-based absorbents. Fig. 4 presents the FTIR spectra of the DMSO-d6 solution of [IiPim][Triz] before and after CO2 capture. New peaks at 1738, 1665, and 1273 cm−1 can be clearly seen. The peak at 1738 cm−1 is ascribed to the asymmetrical stretching band of the COO− group of [Triz]-based carbamate,38,39 while the peak at 1665 cm−1 is the asymmetrical stretching band of the COO− group of carbene-based carboxylate.40 These two peaks again reveal that CO2 is attached to both the anion [Triz]− and the cation [IiPim]+ present in IL [IiPim][Triz]. The peak that falls at around 1273 cm−1 can be attributed to the combination band of the ring stretching and N–H bending of 1,2,4-triazole,41,42 which can be observed as well in the spectra of 1,2,4-triazole (Fig. S3, ESI†). The FTIR spectra of [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) before and after capture are seen in Fig. 5. The new peak at 1638 cm−1 is attributed to the –COO− asymmetrical stretching of EG-based carbonate, and the peak appearing at 1285 cm−1 is the O–COO− stretching band.25,27,29,36 The combination band of 1,2,4-triazole ring stretching and N–H bending is observed at 1274 cm−1. Therefore, the new peaks in Fig. 5 suggest that the reaction occurs between CO2 and EG. The FTIR spectra of the [IiPim][Triz]:EG (1
3) before and after capture are seen in Fig. 5. The new peak at 1638 cm−1 is attributed to the –COO− asymmetrical stretching of EG-based carbonate, and the peak appearing at 1285 cm−1 is the O–COO− stretching band.25,27,29,36 The combination band of 1,2,4-triazole ring stretching and N–H bending is observed at 1274 cm−1. Therefore, the new peaks in Fig. 5 suggest that the reaction occurs between CO2 and EG. The FTIR spectra of the [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) + CO2 (Fig. S4, ESI†) and [IiPim][Triz]:EG (1
4) + CO2 (Fig. S4, ESI†) and [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) + CO2 (Fig. S5, ESI†) systems show similar peaks to that of [IiPim][Triz]:EG (1
5) + CO2 (Fig. S5, ESI†) systems show similar peaks to that of [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) + CO2. However, the peaks around 1738 and 1665 cm−1 are not detected in the spectra of [IiPim][Triz]-EG solvents after CO2 absorption, indicating once more that the reaction between CO2 and the cation [IiPim]+ or the anion [Triz]− can be neglected.
3) + CO2. However, the peaks around 1738 and 1665 cm−1 are not detected in the spectra of [IiPim][Triz]-EG solvents after CO2 absorption, indicating once more that the reaction between CO2 and the cation [IiPim]+ or the anion [Triz]− can be neglected.
The possible CO2 capture mechanism by [IiPim][Triz]-EG solvents used can be proposed based on the above spectral data, as shown in Scheme 1. EG is deprotonated by the [Triz]− anion with the formation of the EG-based alkoxide anion, which can bind with CO2 to form a carbonate. The reason why CO2 is not attached to the C-2 site of the imidazolium ring in [IiPim][Triz]-EG DESs may be mainly because of the high steric hindrance of the C-2 site of the IiPim carbene with two isopropyl groups.43 The steric hindrance of the –OH group of EG is lower in comparison with that of the C-2 site of the IiPim ring, so CO2 is bonded to the O atom of EG rather than the C-2 site of the IiPim ring.
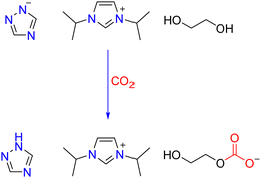 | ||
| Scheme 1 The possible reaction mechanism between CO2 and [IiPim][Triz]-EG DESs studied in this work. | ||
The regeneration of [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) is also investigated. The absorbed CO2 can be released at a low temperature of 60 °C (Fig. S6, ESI†). During five absorption-desorption cycles (Fig. S7, ESI†), the decrease of CO2 capacity is low, suggesting the good reversibility of [IiPim][Triz]:EG (1
3) is also investigated. The absorbed CO2 can be released at a low temperature of 60 °C (Fig. S6, ESI†). During five absorption-desorption cycles (Fig. S7, ESI†), the decrease of CO2 capacity is low, suggesting the good reversibility of [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) DESs. In addition, [IiPim][Triz]:EG (1
3) DESs. In addition, [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and (1
4) and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) can also release captured CO2 at 60 °C. The comparison between [IiPim][Triz]-EG and other DESs for CO2 capture is shown in Table S1 (ESI†).
5) can also release captured CO2 at 60 °C. The comparison between [IiPim][Triz]-EG and other DESs for CO2 capture is shown in Table S1 (ESI†).
In summary, [IiPim][Triz]-EG DESs can efficiently capture CO2 with a capacity of ∼1.0 mol CO2 per mol DES. The mechanism results suggest that CO2 is not bonded to the C-2 site of the [IiPim]+ cation, which can be due to the higher steric hindrance of the C-2 site in comparison with the –OH group of EG. Moreover, CO2 captured by [IiPim][Triz]-EG can be released at a low temperature of 60 °C. The findings in this work reveal that the steric hindrance can be a pivotal factor in tuning the CO2 capture behaviors of imidazolium-based DESs.
This work is supported by the National Natural Science Foundation of China (No. 21503196) and the Fundamental Research Funds for the Central Universities (No. 265QZ2022003 and 2652019111).
Data availability
The data associated with this work can be found in the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- R. E. Siegel, S. Pattanayak and L. A. Berben, ACS Catal., 2023, 13, 766–784 CrossRef CAS.
- Z. Zhang, Y. Zheng, L. Qian, D. Luo, H. Dou, G. Wen, A. Yu and Z. Chen, Adv. Mater., 2022, 34, 2201547 CrossRef CAS PubMed.
- F. Zhao, C. Cui, S. Dong, X. Xu and H. Liu, Sep. Purif. Technol., 2023, 304, 122091 CrossRef CAS.
- K. A. Omar and R. Sadeghi, J. Mol. Liq., 2023, 384, 121899 CrossRef CAS.
- D. Yu, Z. Xue and T. Mu, Cell Rep. Phys. Sci., 2022, 3, 100809 CrossRef CAS.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- J. Wang, Y. Lyu, R. Zeng, S. Zhang, K. Davey, J. Mao and Z. Guo, Energy Environ. Sci., 2024, 17, 867–884 RSC.
- J. Ruan, L. Chen and Z. Qi, Green Chem., 2023, 25, 8328–8348 RSC.
- Y. Liu, Z. Dai, Z. Zhang, S. Zeng, F. Li, X. Zhang, Y. Nie, L. Zhang, S. Zhang and X. Ji, Green Energy Environ., 2021, 6, 314–328 CrossRef CAS.
- R. Biswas, Chem. Eng. Technol., 2024, 47, 20–35 CrossRef CAS.
- J. Sun, Y. Sato, Y. Sakai and Y. Kansha, J. Cleaner Prod., 2023, 414, 137695 CrossRef CAS.
- K. Kumar, S. Keshri, A. Bharti, S. Kumar and S. Mogurampelly, Ind. Eng. Chem. Res., 2022, 61, 4659–4671 CrossRef CAS.
- A. Alhadid, J. Safarov, L. Mokrushina, K. Müller and M. Minceva, Front. Chem., 2022, 10, 864663 CrossRef CAS PubMed.
- Y. Zhang, X. Ji and X. Lu, Renewable Sustainable Energy Rev., 2018, 97, 436–455 CrossRef CAS.
- R. B. Leron, A. Caparanga and M.-H. Li, J. Taiwan Inst. Chem. Eng., 2013, 44, 879–885 CrossRef CAS.
- G. Li, D. Deng, Y. Chen, H. Shan and N. Ai, J. Chem. Thermodyn., 2014, 75, 58–62 CrossRef CAS.
- H. Ghaedi, M. Ayoub, S. Sufian, A. M. Shariff, S. M. Hailegiorgis and S. N. Khan, J. Mol. Liq., 2017, 243, 564–571 CrossRef CAS.
- Y. Cui, X. Wang, X. Zhang, S. Chen, Y. Liu, J. Zhang, L. Dong, L. Shi and X. Zhang, Int. J. Thermophys., 2023, 44, 98 CrossRef CAS.
- Y. Xu, R. Zhang, Y. Zhou, D. Hu, C. Ge, W. Fan, B. Chen, Y. Chen, W. Zhang, H. Liu, G. Cui and H. Lu, Chem. Eng. J., 2023, 463, 142298 CrossRef CAS.
- Y. Gu, Y. Hou, S. Ren, Y. Sun and W. Wu, ACS Omega, 2020, 5, 6809–6816 CrossRef CAS PubMed.
- W. Qian, J. Hao, M. Zhu, P. Sun, K. Zhang, X. Wang and X. Xu, J. CO2 Util., 2022, 59, 101955 CrossRef CAS.
- S. K. Shukla, D. Nikjoo and J.-P. Mikkola, Phys. Chem. Chem. Phys., 2020, 22, 966–970 RSC.
- M. Chen and J. Xu, Molecules, 2023, 28, 5461 CrossRef CAS PubMed.
- S. Wen, T. Wang, X. Zhang, X. Hu and Y. Wu, J. Environ. Chem. Eng., 2024, 12, 112533 CrossRef CAS.
- H. Yan, L. Zhao, Y. Bai, F. Li, H. Dong, H. Wang, X. Zhang and S. Zeng, ACS Sustainable Chem. Eng., 2020, 8, 2523–2530 CrossRef CAS.
- H. Fu, X. Wang, H. Sang, J. Liu, X. Lin and L. Zhang, J. CO2 Util., 2021, 43, 101372 CrossRef CAS.
- G. Cui, Y. Xu, D. Hu, Y. Zhou, C. Ge, H. Liu, W. Fan, Z. Zhang, B. Chen, Q. Ke, Y. Chen, B. Zhou, W. Zhang, R. Zhang and H. Lu, Chem. Eng. J., 2023, 469, 143991 CrossRef CAS.
- M. Chen, W. Xiong, W. Chen, S. Li, F. Zhang and Y. Wu, AIChE J., 2024, 70, e18319 CrossRef CAS.
- G. Cui, M. Lv and D. Yang, Chem. Commun., 2019, 55, 1426–1429 RSC.
- R. Dikki, V. Khokhar, M. Zeeshan, S. Bhattacharjee, O. K. Coskun, R. Getman and B. Gurkan, Green Chem., 2024, 26, 3441–3452 RSC.
- M.-N. Nie, Z. Wang, Q.-H. Niu, J.-X. Dai, Q.-Q. Wang, J.-S. Peng and P. Ji, J. Org. Chem., 2023, 88, 5368–5376 CrossRef CAS PubMed.
- Z. Wang, M. Chen, B. Lu, S. Zhang and D. Yang, ACS Sustainable Chem. Eng., 2023, 11, 6272–6279 CrossRef CAS.
- R. Zhang, Q. Ke, Z. Zhang, B. Zhou, G. Cui and H. Lu, Int. J. Mol. Sci., 2022, 23, 11401 CrossRef CAS PubMed.
- X. Suo, Y. Fu, C.-L. Do-Thanh, L.-Q. Qiu, D.-E. Jiang, S. M. Mahurin, Z. Yang and S. Dai, J. Am. Chem. Soc., 2022, 144, 21658–21663 CrossRef CAS PubMed.
- N. Zhang, Z. Huang, H. Zhang, J. Ma, B. Jiang and L. Zhang, Ind. Eng. Chem. Res., 2019, 58, 13321–13329 CrossRef CAS.
- Y.-Y. Lee, D. Penley, A. Klemm, W. Dean and B. Gurkan, ACS Sustainable Chem. Eng., 2021, 9, 1090–1098 CrossRef CAS.
- T. R. Gohndrone, T. Bum Lee, M. A. DeSilva, M. Quiroz-Guzman, W. F. Schneider and J. F. Brennecke, ChemSusChem, 2014, 7, 1970–1975 CrossRef CAS PubMed.
- T. R. Gohndrone, T. Song, M. A. DeSilva and J. F. Brennecke, J. Phys. Chem. B, 2021, 125, 6649–6657 CrossRef CAS PubMed.
- G. Cui, N. Zhao, J. Wang and C. Wang, Chem. – Asian J., 2017, 12, 2863–2872 CrossRef CAS PubMed.
- M. Thomas, M. Brehm, O. Hollóczki, Z. Kelemen, L. Nyulászi, T. Pasinszki and B. Kirchner, J. Chem. Phys., 2014, 141, 024510 CrossRef PubMed.
- F. Billes, H. Endrédi and G. Keresztury, J. Mol. Struct. Theochem, 2000, 530, 183–200 CrossRef CAS.
- R. Y. Jin, X. H. Sun, Y. F. Liu, W. Long, W. T. Lu and H. X. Ma, J. Mol. Struct., 2014, 1062, 13–20 CrossRef CAS.
- K. Mei, X. He, K. Chen, X. Zhou, H. Li and C. Wang, Ind. Eng. Chem. Res., 2017, 56, 8066–8072 CrossRef CAS.
Footnote |
† Electronic supplementary information (ESI) available: Experimental section, NMR and FTIR spectra of DES systems, and the reusability of DESs [IiPim][Triz]:EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). See DOI: https://doi.org/10.1039/d4cc02422b 3). See DOI: https://doi.org/10.1039/d4cc02422b |
| This journal is © The Royal Society of Chemistry 2024 |





