Ruthenium-infused nickel sulphide propelling hydrogen generation via synergistic water dissociation and Volmer step promotion†
Nasrin Banu
G.
a,
Rama Prakash
M.
a,
Anantharaj
Sengeni
 b and
Bernaurdshaw
Neppolian
b and
Bernaurdshaw
Neppolian
 *a
*a
aEnergy & Environmental Remediation Laboratory, Department of Chemistry, Faculty of Engineering & Technology, SRM Institute of Science and Technology, Kattankulathur, Chennai, Tamilnadu 603203, India. E-mail: neppolib@srmist.edu.in; neppolianb@gmail.com
bDepartment of Chemistry, Indian Institute of Technology, Kanpur, Uttar Pradesh 208 016, India
First published on 29th July 2024
Abstract
The inclusion of ruthenium (Ru) to decorate nickel sulphide (Ru@NiS/Ni foam) resulted in a highly efficient electrocatalyst for the alkaline HER by enhancing water dissociation at the interface and reducing the energy barrier of the Volmer step. This strategic fusion significantly boosts the catalyst's performance in facilitating hydrogen production.
The accelerating consumption of fossil fuels, coupled with their rapid depletion and the worsening environmental consequences of CO2 emissions, underscores the urgent need for a shift towards a hydrogen economy.1–6 Hydrogen, a versatile energy carrier, can be generated from various source materials via methods such as photolysis, electrolysis, thermolysis, etc.7–11 Presently, the predominant industrial method relies on hydrocarbons, which, despite cost-effectiveness, pollute the atmosphere with CO2.12,13 Electrolysis of water offers an environmentally friendly alternative, but faces challenges like sluggish kinetics.14–22 Pt-based materials dominate the hydrogen evolution reaction (HER) but are hindered by cost, driving the need for efficient non-noble metal electrocatalysts. Transition metal chalcogenides (TMCs) have emerged as promising substitutes due to their exceptional catalytic properties and tunability.23–27
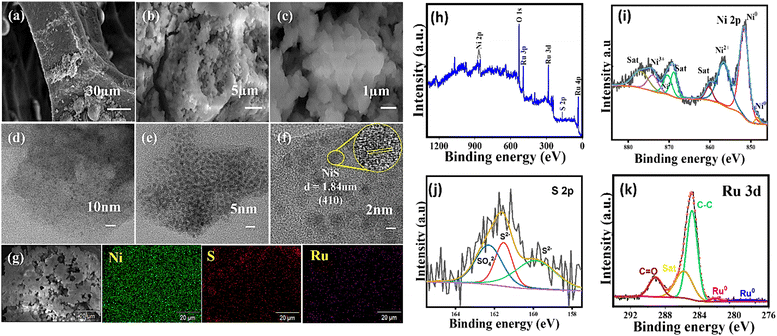
Among TMCs, nickel sulfide (NiS) exhibits notable characteristics for HER catalysis, including phase structure optimization, good electronic conductivity, active surface sites, and tunable surface chemistry.28–32 Ru and Pt are renowned for their effectiveness in catalyzing the Volmer step of the HER in alkaline conditions when combined with 3d transition metal oxides or hydroxides.33–35 This established synergy has led us to explore a similar catalytic system by selecting Ru but pairing it with NiS. During the HER process, the NiS undergoes a surface transformation into nickel hydroxide (Ni(OH)2). This newly formed Ni(OH)2 surface, in conjunction with Ru, creates a highly favorable environment that significantly enhances the water dissociation step, a critical phase of the HER. This synergistic effect, resulting from the dynamic surface reconstruction, has been demonstrated by numerous prior studies to effectively boost the overall efficiency of the HER.36–39 The synthesis of Ru-infused NiS (Ru@NiS/Ni foam) was achieved through a hydrothermal approach as represented in Fig. S1 (ESI†), enabling the introduction of Ru and the subsequent sulphurization process. The XRD patterns of NiS/Ni foam and Ru@NiS/Ni foam were acquired to confirm the phase and effect of Ru inclusion. Fig. S2 (ESI†) reveals the XRD patterns of NiS/Ni foam and Ru@NiS/Ni foam, which clearly depicts the predominant presence of metallic nickel from the Ni foam substrate by (111), (200) and (220) peaks. Formation of NiS is confirmed by the presence of a (300) peak at 30.79° with a notable intensity, while accompanying other weak peaks ((001), (220), (410), and (321)) at 21.45°, 37.48°, 49.79° and 54.98°, which matches with the JCPDS number 01-1286. The XRD pattern of Ru@NiS/Ni foam shows no noticeable peak shifts induced by Ru doping, likely attributed to the low Ru content. The introduction of ruthenium in NiS leads to a reduction in the intensity of the NiS peaks, indicating that Ru influences the crystal structure leading to smaller particle sizes or increased defects and results in less crystallinity.40,41 To elucidate the structural transformations induced by ruthenium incorporation, a comprehensive analysis of NiS/Ni foam and Ru@NiS/Ni foam was initiated through FESEM. As depicted in Fig. S3(a)–(c) (ESI†), the FESEM images of NiS/Ni foam, captured at increasing magnifications, unveil the emergence of sea sponge structured NiS microspheres, exhibiting an average size of 3 μm. This observation indicates a pronounced sulfidation process during the hydrothermal synthesis. The transition from distinct microspheres to agglomerated particles is evident from Fig. 1(a)–(c), signifying the impact of Ru inclusion on the structural characteristics. The FESEM images of Ru@NiS/Ni foam at the corresponding magnifications display a noteworthy change in sample morphologies. Fig. 1(d) and (e) display the TEM images of Ru@NiS/Ni foam at increasing magnifications, revealing dispersed NiS particles. The HRTEM image of Ru@NiS/Ni foam at higher magnification, shown in Fig. 1(f), clearly displays lattice fringes. The measured distance between adjacent lattice fringes corresponds to the (410) plane of NiS with a d-spacing of 1.84 nm, matching the JCPDS number 01-1286. This result aligns well with the XRD results, supporting our prediction of structural reorganization due to Ru doping. EDS mapping confirmed the elemental distribution of Ni (green), S (red), and Ru (pink) in Ru@NiS/Ni foam, as shown in Fig. 1(g). This evidence demonstrates that the introduction of Ru has increased the defects in NiS, thereby enhancing the HER performance. The Ru@NiS/Ni foam was analysed using XPS to study the elemental composition and chemical states within the material. In the survey spectrum (Fig. 1h), the distinct peaks affirm the presence of Ni, S and Ru, along with oxygen due to surface oxidation of the sample. In Fig. 1i, metallic Ni is identified at 848.5 eV & 851.66 eV and the coexistence of Ni2+ and Ni3+ is revealed at 870.51, 874.1 and 856.7 eV, respectively.
These observations signify a profound sulfidation process, leading to the formation of NiS/Ni. The accompanying satellite peaks at 860.07, 868.03 and 876.8 eV provide added information about the chemical environment surrounding Ni. The sulphur component is scrutinized in Fig. 1j depicting the S 2p spectrum. Here, the presence of S2− and SO42− is unequivocally validated, with binding energy values in concordance with expectations and corroborated by previous reports. This insight points towards the predominate existence of stoichiometric millerite (NiS) in the NiS formation, complemented by the presence of surface oxidized sulfate moieties.42,43 Turning attention to the ruthenium spectrum (Fig. 1k) two peaks at 278.74 and 282.1 eV, corresponding to Ru0 and a satellite peak at 285.54 eV collectively affirm the successful integration of Ru. These findings align with established literature, validating the efficacy of the sulfidation of Ni and Ru addition into the composite material.44
The Ru@NiS/Ni foam prepared in this study underwent a thorough assessment of its HER activity in 1.0 M KOH, compared with pristine Ni foam and NiS/Ni foam. The iR corrected LSVs presented in Fig. 2a illustrate the superior HER performance of Ru@NiS/Ni foam, attributable to an increased dissociation of water at the interface. Ni foam, NiS/Ni foam, Ru@NiS/Ni foam and Pt/C 20 wt%/Ni foam exhibited overpotentials of 287, 177, 69 and 20 mV, respectively, to attain a HER current density of −10 mA cm−2. This noteworthy reduction of 108 mV in the HER overpotential highlights the advantageous impact of implementing Ru into NiS/Ni foam. The Tafel slopes, as depicted in Fig. 2b, further emphasize the decreased energy barrier for the Volmer step and enhanced kinetics of the HER after Ru doping. The calculated value of Ru@NiS/Ni foam (74 mV dec−1) suggests that the catalytic interfaces followed the Volmer–Heyrovsky mechanism, with the first electron transfer being the rate-determining step. The Nyquist plots in Fig. 2c, studied at −1.1 V vs. Hg/HgO (−0.164 V vs. RHE), show distinct charge transfer resistances. Specifically, Ni foam exhibited the highest Rct at 1.46 Ω, while NiS/Ni foam and Ru@NiS/Ni foam displayed lower values of 0.78 Ω and 0.618 Ω, respectively. This indicates that the sulfidation of Ni foam and the introduction of Ru in NiS/Ni foam contributed to improved charge transfer capabilities under HER conditions. Furthermore, the Cdl values were calculated from scan rate-dependent cyclic voltammograms (CVs) obtained in a non-faradaic potential window (−1.0 to −0.85 V vs. Hg/HgO) just before the onset of the HER. The plot in Fig. 2d, representing the double layer charging current density difference against scan rate, reveals slope values (2Cdl) of 12, 19 and 26 μF cm−2 for Ni foam, NiS/Ni foam and Ru@NiS/Ni foam, respectively. This correlation aligns with the observed HER activity trend, underscoring the significance of the electrochemical performance enhancements induced by the addition of Ru.
The Bode plots in Fig. 2e reinforce this trend, highlighting the positive impact of Ru doping on the HER performance of NiS/Ni foam by reducing its Rct under catalytic turnover conditions. Analysis of the Bode phase angle plot reveals values consistently lower than 45°, indicating that the reaction at this potential is primarily controlled by charge transfer. Additionally, the plot shows that the time constant is relatively lower for Ni foam and NiS/Ni foam, suggesting that they require more time to facilitate the desired charge transfer process, i.e., the HER. It is standard practice to compare charge transfer resistance (Rct) and electrochemical surface area (ECSA), which is indirectly measured as Cdl, under consistent electrochemical conditions. In this investigation, along with Rct and Cdl values, we have examined admittance values extrapolated at the lowest frequency in Fig. 2f. These values depict the intersection of the linear line emerging from Ru and levelling off at Ru + Rct in Bode absolute impedance plots. Notably, the admittance value calculated at the lowest frequency directly corresponds to the Cdl value of the electrode under investigation. These admittance values, derived from EIS measurements conducted under catalytic turnover conditions, where the catalytic interfaces were actively engaged in the HER, provide a relatively precise reflection of ECSA compared to ECSA values obtained through CV measurements in a non-faradaic region.
The inclusion of Ru in the NiS/Ni foam electrode enhances its activity by slightly increasing the time constant, as seen in both Nyquist and Bode plots. Improved charge transfer and increased time constants are among several factors contributing to the observed activity enhancement. To gauge the catalytic performance of the electrocatalysts, ECSA indirectly measured from Cdl serves as a valuable metric. The admittance values extracted from the Bode absolute impedance plot in Fig. 2f, at the lowest frequency, are 0.37 S, 0.63 S, and 0.81 S for Ni foam, NiS/Ni foam, and Ru@NiS/Ni foam, respectively. The notably higher admittance of the Ru@NiS/Ni foam electrode underscores its superior catalytic ability. The comparison in Fig. S6 (ESI†), encompassing admittance, phase angle, and angular frequency of Ni foam, NiS/Ni foam, and Ru@NiS/Ni foam, clearly demonstrates that Ru@NiS/Ni foam is the superior catalyst for the HER. To evaluate the endurance of the Ru@NiS/Ni foam electrode under harsh conditions, CA analysis was performed at an overpotential of −1.2 V and −2 V vs. Hg/HgO, respectively. Chronoamperometry tests indicated significant attenuation in stability at −1.2 V, and this issue was exacerbated at a higher potential of −2 V, where a substantial decline in stability was observed. These results underscore a critical challenge in achieving long-term stability for Ru@NiS/Ni foam during the HER. To address this issue, we are actively engaged in research aimed at optimizing the material's composition and structure to enhance its stability and ensure more reliable performance in alkaline HER applications.
To track the structural changes that might have occurred during the harsh-condition endurance test, we performed a set of post-CA analyses. The XRD pattern (Fig. S9(a), ESI†) of Ru@NiS/Ni foam after the reaction retains almost all the intense peaks of NiS and metallic nickel from the Ni foam. SEM (Fig. S10(a)–(c), ESI†) and TEM (Fig. S10(d), ESI†) analyses of the spent Ru@NiS/Ni foam electrode revealed no apparent changes in surface morphology, testifying to the robustness of the Ru@NiS/Ni foam HER electrode. Further insights were obtained from HRTEM analysis (Fig. S10(e), ESI†), which displayed an interplanar spacing of 2.75 nm, indexed to the (300) crystal plane in pure NiS (JCPDS 01-1286), aligning well with the XRD results.
The EDS elemental colour maps (Fig. S10(f), ESI†) and composition analysis (Fig. S9(c), ESI†) confirmed the presence of all elements, including oxygen, due to the KOH electrolyte and the formation of oxides. The XPS survey spectra depicted in Fig. S9(b) (ESI†) revealed the presence of Ni, S, Ru, O, and K. Subsequent Ni 2p3/2 analysis (Fig. S11(a), ESI†) post-CA exhibited a consistent chemical profile, notably lacking the NiO peak corresponding to Ni foam. The O 1s spectrum (Fig. S11(b), ESI†) indicated negligible changes, suggesting the preservation of a similar oxygen-containing chemical environment characteristic of Ni–O and Ni–OH. Additionally, in Fig. S11(c) (ESI†), a new peak in the S 2p spectrum emerged, attributed to SO42− formation due to excessive oxidation of S2−. In the Ru 3d XPS spectra (Fig. S11(d), ESI†), the Ru0 peak was accompanied by the appearance of Ru3+ and Ru4+ peaks at 284.84 and 281.59 eV, respectively, due to the oxidation of Ru.
These comprehensive post-CA characterizations affirm the structural integrity of the Ru@NiS/Ni foam electrode, endorsing its suitability as a HER electrode in alkaline water electrolysis. In this pioneering electrocatalysis study, we enhanced the performance of NiS, a renowned non-noble metal electrocatalyst for the HER, by strategically integrating Ru. This integration assisted increased water dissociation and decreased the energy barrier in the Volmer step, resulting in enhanced electrochemical activity. The introduction of Ru yielded a synergistic effect, markedly improving the charge transfer characteristics, reducing the overpotential, and enhancing the catalytic efficiency. The mechanistic insights gained not only advance NiS-based catalyst understanding but also inspire future research for sustainable hydrogen evolution reactions, contributing to renewable energy advancements.
The authors express their sincere thanks to Prof. Wonyong Choi, Korea Institute of Energy Technology, South Korea and Prof. Masaya Matsuoka, Osaka Prefecture University, Japan for their scientific support in characterization studies. The authors are grateful for the financial support received from the Ministry of Human Resource Development, Scheme for Promotion of Academic and Research Collaboration (SPARC) [Award No.: SPARC/2019-2020/P2197/SL] and the Department of Science and Technology, Promotion of University Research and Scientific Excellence (DST-PURSE) [Award No.: SR/PURSE/2021/65].
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- M. van der Spek, C. Banet, C. Bauer, P. Gabrielli, W. Goldthorpe, M. Mazzotti, S. T. Munkejord, N. A. Røkke, N. Shah, N. Sunny, D. Sutter, J. M. Trusler and M. Gazzani, Energy Environ. Sci., 2022, 15, 1034–1077 RSC.
- D. Guan, B. Wang, J. Zhang, R. Shi, K. Jiao, L. Li, Y. Wang, B. Xie, Q. Zhang, J. Yu, Y. Zhu, Z. Shao and M. Ni, Energy Environ. Sci., 2023, 16, 4926–4943 RSC.
- P. J. Feibelman, Phys. Today, 2005, 58, 13–14 CrossRef.
- H. B. Gray, Nat. Chem., 2009, 1, 7 CrossRef PubMed.
- G. Glenk and S. Reichelstein, Nat. Energy, 2019, 4, 216–222 CrossRef.
- D. Parra, L. Valverde, F. J. Pino and M. K. Patel, Renewable Sustainable Energy Rev., 2019, 101, 279–294 CrossRef.
- S. K. Lakhera, A. Rajan, R. T. P. and N. Bernaurdshaw, Renew. Sustainable Energy Rev., 2021, 152, 111694 CrossRef.
- T. S. Teets and D. G. Nocera, Chem. Commun., 2011, 47, 9268 RSC.
- V. N. Rao, N. L. Reddy, M. M. Kumari, K. K. Cheralathan, P. Ravi, M. Sathish, B. Neppolian, K. R. Reddy, N. P. Shetti, P. Prathap, T. M. Aminabhavi and M. V. Shankar, J. Environ. Manage., 2019, 248, 109246 CrossRef PubMed.
- C. Acar, I. Dincer and C. Zamfirescu, Int. J. Energy Res., 2014, 38, 1903–1920 CrossRef.
- G. A. Naikoo, F. Arshad, I. U. Hassan, M. A. Tabook, M. Z. Pedram, M. Mustaqeem, H. Tabassum, W. Ahmed and M. Rezakazemi, Front. Chem., 2021, 9 DOI:10.3389/fchem.2021.736801.
- J. O. Ighalo and P. B. Amama, Int. J. Hydrogen Energy, 2024, 51, 688–700 CrossRef CAS.
- H. Zhang, Z. Sun and Y. H. Hu, Renew. Sustainable Energy Rev., 2021, 149, 111330 CrossRef CAS.
- S. Li, Q. Kang, J. Baeyens, H. L. Zhang and Y. M. Deng, IOP Conf. Ser.: Earth Environ. Sci., 2020, 544, 012011 Search PubMed.
- M. K. Sarmah, T. Singh, P. Kalita and A. Dewan, RSC Adv., 2023, 13, 25253–25275 RSC.
- Z. P. Ifkovits, J. M. Evans, M. C. Meier, K. M. Papadantonakis and N. S. Lewis, Energy Environ. Sci., 2021, 14, 4740–4759 RSC.
- X. Li, X. Hao, A. Abudula and G. Guan, J. Mater. Chem. A, 2016, 4, 11973–12000 RSC.
- S. Anantharaj and S. Noda, Small, 2019, 16, 1905779 CrossRef PubMed.
- I. Roger, M. A. Shipman and M. D. Symes, Nat. Rev. Chem., 2017, 1, 0003 CrossRef CAS.
- N. Dubouis and A. Grimaud, Chem. Sci., 2019, 10, 9165–9181 RSC.
- N. Mahmood, Y. Yao, J.-W. Zhang, L. Pan, X. Zhang and J.-J. Zou, Adv. Sci., 2017, 5, 1700464 CrossRef PubMed.
- M. Zeng and Y. Li, J. Mater. Chem. A, 2015, 3, 14942–14962 RSC.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- J. Wang, F. Xu, H. Jin, Y. Chen and Y. Wang, Adv. Mater., 2017, 29, 1605838 CrossRef PubMed.
- J. Yu, Y. Dai, Q. He, C. Cheng, Z. Shao and M. Ni, Appl. Phys. Rev., 2020, 7, 041304 CAS.
- Y. Jin, Z. Zhang, H. Yang, P. Wang, C. Shen, T. Cheng, B. Huang and Q. Shao, SmartMat, 2022, 3, 130–141 CrossRef CAS.
- L. Yu, X. Huang, Q. Zhang and Z. Zhang, Acta Phys. Chim. Sin, 2022, 6(38), 2109020 Search PubMed.
- S. Anantharaj, S. R. Ede, K. Sakthikumar, K. Karthick, S. Mishra and S. Kundu, ACS Catal., 2016, 6, 8069–8097 CrossRef CAS.
- J. Joo, T. Kim, J. Lee, S. Choi and K. Lee, Adv. Mater., 2019, 31, 1806682 CrossRef PubMed.
- M. Wang, L. Zhang, Y. He and H. Zhu, J. Mater. Chem. A, 2021, 9, 5320–5363 RSC.
- Y. Li, X. Bao, D. Chen, Z. Wang, N. Dewangan, M. Li, Z. Xu, J. Wang, S. Kawi and Q. Zhong, ChemCatChem, 2019, 11, 5913–5928 CrossRef.
- Y. Yan, B. Yu Xia, B. Zhao and X. Wang, J. Mater. Chem. A, 2016, 4, 17587–17603 RSC.
- B. Ruqia and S. Choi, ChemSusChem, 2018, 11, 2643–2653 CrossRef CAS.
- M. Yang, J. Zhang, W. Zhang, Z. Wu and F. Gao, J. Alloys Compd., 2020, 823, 153790 CrossRef CAS.
- X. Xiao, X. Wang, X. Jiang, S. Song, D. Huang, L. Yu, Y. Zhang, S. Chen, M. Wang, Y. Shen and Z. Ren, Small Methods, 2020, 4, 1900796 CrossRef CAS.
- M. Zhong, M. Xu, S. Ren, W. Li, C. Wang, M. Gao and X. Lu, Energy Environ. Sci., 2024, 17, 1984–1996 RSC.
- Y. Wang, J. Wang, T. Xie, Q. Zhu, D. Zeng, R. Li, X. Zhang and S. Liu, Appl. Surf. Sci., 2019, 485, 506–512 CrossRef CAS.
- Q. Q. Chen, X. Yang, C.-C. Hou, K. Li and Y. Chen, J. Mater. Chem. A, 2019, 7, 11062–11068 RSC.
- H. Liu, Q. Jia, S. Huang, L. Yang, S. Wang, L. Zheng and D. Cao, J. Mater. Chem. A, 2022, 10, 4817–4824 RSC.
- Y. Wang, J. Wang, T. Xie, Q. Zhu, D. Zeng, R. Li, X. Zhang and S. Liu, Appl. Surf. Sci., 2019, 485, 506–512 CrossRef CAS.
- Y. Xu, T. Ren, K. Ren, S. Yu, M. Liu, Z. Wang, X. Li, L. Wang and H. Wang, Chem. Eng. J., 2021, 408, 127308 CrossRef CAS.
- B. Guan, Y. Liu, B. Yin, K. Liu, D. Wang, H. Zhang and C.-J. Cheng, Chem. Eng. J., 2017, 308, 1165–1173 CrossRef CAS.
- X. Zhao, F. Gong, Y. Zhao, B. Huang, D. Qian, H.-E. Wang, W. Zhang and Z. Yang, Chem. Eng. J., 2020, 392, 123675 CrossRef CAS.
- J. Cored, A. García-Ortiz, S. Iborra, M. J. Climent, L. Liu, C. Chuang, T. Chan, C. Escudero, P. Concepción and A. Corma, J. Am. Chem. Soc., 2019, 141, 19304–19311 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc01842g |
| This journal is © The Royal Society of Chemistry 2024 |