Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Inverse opals with reactive surface chemistry as sensors for aqueous pollutants†
Giulia
Magnabosco
 a,
Maria
Ochs
b,
Natalie
Bonakdar
a,
Laura
Czerwenka
b,
Annette
Andrieu-Brunsen
*b and
Nicolas
Vogel
a,
Maria
Ochs
b,
Natalie
Bonakdar
a,
Laura
Czerwenka
b,
Annette
Andrieu-Brunsen
*b and
Nicolas
Vogel
 *a
*a
aInstitute of Particle Technology Friedrich-Alexander-Universität Erlangen-Nürnberg Cauerstraße 4, 91058 Erlangen, Germany. E-mail: nicolas.vogel@fau.de
bErnst-Berl Institut für Technische und Makromolekulare Chemie Technische Universität Darmstadt Alarich-Weiss-Str. 8 64287 Darmstadt, Germany. E-mail: annette.andrieu-brunsen@tu-darmstadt.de
First published on 27th June 2024
Abstract
Inverse opal colorimetric sensors operating on wetting transitions usually rely on physical differences of the infiltrating liquid. Here, we exploit a reactive surface chemistry that changes wettability upon binding of an analyte. Upon binding of Fe3+ to a Schiff base immobilized on the porous structure, the surface becomes more hydrophilic, triggering the infiltration of the structure and causing the structural color to disappear.
The detection of dissolved species in aqueous solutions is of relevance in a wide variety of fields, ranging from water management and pollutant detection1–3 to healthcare.4,5 Typically, such analyses rely on complex, multistep procedures conducted in specialized laboratories with sophisticated infrastructure. The possibility to monitor the analyte of interest in situ is attracting growing interest as a convenient and direct point of care diagnostic.6,7 Ideally, the presence of dissolved species is directly encoded via an autonomous, detectable response of a material.
Various concepts for the solid-state detection of water pollutants have been established. For example, the change in color upon binding of an analyte to a dye immobilized on solid supports can be used for sensing.8 Colorimetric sensors based on photonic crystals enable direct modification of their structural coloration by changes triggered either in the structural arrangement or in the refractive index of their components.9,10 The structural coloration generated by thin film interference of polymeric films can be used for sensing when the film shrinks or swells in the presence of the analyte.11 3D colloidal photonic crystals, commonly referred to as opals, show structural color because of the periodic arrangement of their constituent particles. For opals embedded in polymer matrices, a color change can be observed when the matrix swells by interacting with an analyte and changes the distance between the particles.12–15
Inverse opals (IOs), three-dimensional porous networks with fully interconnected, uniform pores, exhibit structural coloration visible by the naked eye due to the periodic nature of the nanopores.16–20 When prepared using a matrix able to shrink upon interaction with an analyte, they can also act as colorimetric sensors.21 Since structural coloration relies on a refractive index contrast between pore and matrix,16 the infiltration of an IO with liquid eliminates the observable coloration because of the higher refractive index of liquids compared to air.16,18,22–25 This constitutes a simple, binary read-out of the wetting state.
The regularity and interconnected nature of the nanopores in an IO induces a well-defined wetting transition that depends on the contact angle of a given liquid with the pore walls.19,22,23,26–28 The pores remain unwetted if the contact angle θ is larger than the pore opening angle ϑ (Fig. 1).24,27 Only for θ < ϑ will the liquid be able to penetrate through the neck and propagate into the porous network. The underlying reason for this change in pore filling is a change in the shape of the meniscus the liquid forms at the necks of the pores (Fig. 1b and c).24,27
This defined wetting transition is the key for an autonomous colorimetric sensor. When the surface chemistry of the IO is adjusted in a way that the contact angles of two different liquids are above and below the neck opening angle, one liquid will infiltrate the IO, while the other one cannot infiltrate the pore network (Fig. 1b–e).27 The associated loss of structural color thus unambiguously indicates the presence of the first liquid.
This concept has enabled the wetting-based discrimination between different alcohol species (i.e. methanol, ethanol, isopropanol), different alcohol contents,22,24 or between regular gasoline and diesel.24 Recently, IOs have also been exploited to pre-screen newborns for jaundice by exploiting changes in the surface tension in urine, allowing for easy, at-home monitoring of the development of this common condition.23
To this point, however, the wetting transition has been mainly limited to triggers provided by the physical properties of the liquids themselves, such as their surface tension. Since soluble analytes generally do not influence the surface tension of the liquid, the wetting transition cannot be triggered by the presence of the analyte itself. Therefore, the change in contact angle must be induced by an in situ change of the chemistry of the surface, rather than by a change in the property of the liquid. Such a reactive surface chemistry must provide selective recognition units that trigger a change in wettability upon binding of the analyte. When introduced within the porous structure of the IO, this binding can therefore favor the wetting of the previously unwetted IO in the presence of the analyte (Fig. 1f).
Here, we develop this new sensing strategy based on a change in wettability in the surface of the IO upon interaction with an analyte, namely the chelation of a metal ion. We devise a reaction scheme to create reactive surface chemistries based on half-Salen Schiff bases, successfully functionalize the inner pore surface of IOs, investigate the change in wettability induced by the binding of Fe3+ as a model ion, and demonstrate that this change in wettability can trigger a wetting transition to successfully provide a colorimetric read-out of the binding event.
We identify Schiff bases as a candidate for such a reactive surface chemistry, as their complexation capacities to different metal ions can be tailored via their molecular structure.29–31 Salen motives, a particular type of Schiff bases containing two acidic hydroxyls26–28 and two aryl-imine groups,32 can act as bidentate ligands for a variety of metal ions through the donation of the electron lone pair of the azomethine nitrogen.32 Salen motives have already been used to develop sensors to detect a variety of metal ions,33–37 typically by exploiting a change in the photochemical behavior of the ligands. Half-Salen motives, only consisting in one acidic hydroxyl aryl-imine group, can still coordinate metals, and allow for water molecules to complete the coordination sphere of the bound metal. This may in turn increase the hydrophilicity of a surface onto which such a compound is bonded.
We start the design of the autonomous colorimetric sensors by preparing IOs as porous photonic crystals that provide the structural color-based detection signal. We use the colloidal co-assembly method18 with 340 nm polystyrene colloidal particles, which enables the fabrication of nearly crack-free, well-ordered silica IOs that exhibit visible structural coloration from the periodic arrangement of the nanopores (Fig. S1a–d, ESI†). A low number of cracks and the interconnectivity of the pores (Fig. 1a and Fig. S1a, ESI†) are the prerequisite for observing a sharp wetting transition.19,38 We calculate the pore opening angle from SEM images (Fig. S1d, ESI†) to be around 20° ± 2°.
Next, we devise a reactive surface chemistry able to trigger a change in substrate wettability upon the binding of a target analyte. We synthesize (E)-2-(((2-mercaptoethyl)imino)methyl)phenol (1), a half-Salen Schiff base. This Schiff base was selected as it shows a promising selectivity towards the binding of Fe3+ ions, as observed by the change in the UV-vis spectra upon the formation of the complex in solution (Fig. S2, ESI†).
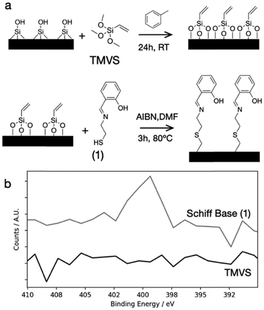
The successful surface functionalization is confirmed by X-ray photoelectron spectroscopy (XPS), where the nitrogen signal is visible after the immobilization of the Schiff-base on the surface (Fig. 2b). When a hexavalent metal is coordinated by the Schiff-base (1), it can still coordinate 4 additional water molecules, thereby increasing the water wettability of the surface (Fig. 3a).
For binding (1) to the IO silica surface, we use the thiol functionality integrated in the Schiff base as anchor group. To this end, we first create a monolayer of surface-bound vinyl-silane groups via silane chemistry. Subsequently, we employ thiol–ene chemistry to bind the Schiff base to the double bond, thus creating the reactive surface coating (Fig. 2a).39
We investigate the change in surface wettability using water contact angle (WCA) measurements on a planar glass slide (Fig. 3b, red squares). The initially hydrophilic glass surface (WCA < 20°) is rendered more hydrophobic by the introduction of the vinyl functionalities of the trimethoxyvinylsilane (TMVS) (WCA = 87° ± 7°). The binding of the Schiff base (1) lowers the hydrophobicity to a WCA of 76° ± 9 °C, reflecting the presence of the phenolic OH group.
When placed in contact with a solution of Fe3+ (60% EtOH, 5 mM, 1 hour), the hydrophilicity of the surface is significantly increased, evidenced by a decrease in WCA to 53° ± 5.
The contact angle can be further adjusted by mixing the aqueous solution with ethanol. For example, using a 20% ethanolic water solution, the CA on the Schiff base functionalized flat surface is lowered from 67° ± 4° to 42° ± 3° in the presence of Fe3+ (Fig. 3b, black squares). This control is important to match the CA with the wetting transition of the IO. Note that changing the pore structure of the inverse opal itself could be used similarly to match the wetting transition to the CAs of the reactive surface. We now incorporate the reactive surface chemistry into the porous network of the IO (Fig. 4). We first establish that a surface-functionalized IO in the absence of Fe3+ ions is not wetted by water containing 20% ethanol, as seen by the intense structural color of the sample (Fig. 4). We demonstrate the ability to trigger a wetting transition via the binding of a dissolved analyte as follows. We expose the reactive surface chemistry of the IO to a 5 mM Fe3+ solution in 60% EtOH, which has a sufficiently small CA (CA < 20°) that the liquid infiltrates the IO. After 60 min, we wash and dry the sample. When exposed to a 20% ethanol solution, this sample is now wetted, evidenced by the disappearance of the structural color (Fig. 4a and b). This simple visual observation is corroborated by reflectance spectra of the two samples, where the photonic stop band of the sample exposed to Fe3+ disappears when immersed in the 20% ethanol solution (Fig. 4c).
The wetting behavior implies that the critical contact angle for wetting is in between ∼42° (as the sample exposed to Fe3+ is wetted) and ∼67° (as the reference sample is not wetted). This experimental threshold differs from the theoretical threshold determined geometrically (Fig. 1). We ascribe these differences to (i) variability in the size of the IO pore measurement, as the value needs to be determined by a projection of a 3D surface on the image plane, (ii) intrinsic roughness of the sample, and (iii) a difference in the functionalization efficiency when performed in the confined, non-planar environment of the nanopore itself.
We assess the selectivity of the reactive surface chemistry by exposing the functionalized IOs to 5 mM solutions of Fe2+ and Fe3+ for 1 hour (Fig. 5c–f). When the system is exposed to Fe2+, it does not wet and the color is retained (Fig. 5c and d). Only when the sensor is exposed to Fe3+, the structure wets and the color disappears (Fig. 5e and f). The selectivity to Fe3+ is caused by the selective complexation of the trivalent ion, as evidenced from the decrease in contact angle. In contrast, the exposure to Fe2+ does not decrease the contact angle, indicating that the Schiff base does not complex Fe2+ (Fig. S2, ESI†). We also demonstrate the selectivity of the system towards other common metal ions. After exposure to Al3+ or Cu2+ ions (Fig. 5g–j), no color disappearance is observed.
In conclusion, we demonstrate a new concept for the fabrication of colorimetric photonic crystal sensors based on reactive surface chemistry. We control the wettability of the surface via the binding of an analyte, and exploit the wetting transition to provide a simple, unambiguous readout (color vs. no color) to detect the presence of this analyte.
In our case, the wetting transition is not triggered by differences in the physical proprieties of the infiltrating liquids, as previously shown in the literature. In this proof of principle, we trigger the wetting transition by the complexation Fe3+ ions as a model analyte. Changing the molecular structure of the underlying Schiff base may provide selectivity to more relevant target ions. The general concept is more versatile and can provide the basis for a broad range of common specific binding concepts, from crown ethers to bioconjugate chemistry for versatile, autonomous detection of different ionic, molecular or biochemical analytes.
We thank Dr Salvatore Chiera for carrying out XPS measurements. AAB and NV acknowledge funding of the German Science Foundation (Deutsche Forschungsgemeinschaft, DFG) under grant number AN 1301/5-2 and VO 1824/5-2, respectively.
AAB and NV conceptualized the research, acquired fundings, supervised and validated the results. GM, MO, AAB and NV contributed to develop the methodology of research. GM and MO contributed to the investigation and data visualization. GM wrote the original draft. All authors contributed to its review.
Data availability
The data supporting this article have been included as part of the ESI, and the raw data can be found at https://doi.org/10.5281/zenodo.10697663.†Conflicts of interest
There are no conflicts to declare.Notes and references
- R. Umapathi, S. Sonwal, M. J. Lee, G. Mohana Rani, E.-S. Lee, T.-J. Jeon, S.-M. Kang, M.-H. Oh and Y. S. Huh, Coord. Chem. Rev., 2021, 446, 214061 CrossRef CAS.
- M. I. Mead, O. A. M. Popoola, G. B. Stewart, P. Landshoff, M. Calleja, M. Hayes, J. J. Baldovi, M. W. McLeod, T. F. Hodgson, J. Dicks, A. Lewis, J. Cohen, R. Baron, J. R. Saffell and R. L. Jones, Atmos. Environ., 2013, 70, 186–203 CrossRef CAS.
- P. Kruse, J. Phys. D: Appl. Phys., 2018, 51, 203002 CrossRef.
- S. A. Walper, G. Lasarte Aragonés, K. E. Sapsford, C. W. Brown, C. E. Rowland, J. C. Breger and I. L. Medintz, ACS Sens., 2018, 3, 1894–2024 CrossRef CAS PubMed.
- J. Kim, A. S. Campbell, B. E.-F. de Ávila and J. Wang, Nat. Biotechnol., 2019, 37, 389–406 CrossRef CAS PubMed.
- A. M. López-Marzo and A. Merkoçi, Lab Chip, 2016, 16, 3150–3176 RSC.
- S. A. Jaywant and K. M. Arif, Sensors, 2019, 19, 4781 CrossRef CAS PubMed.
- N. Adarsh, M. Shanmugasundaram and D. Ramaiah, Anal. Chem., 2013, 85, 10008–10012 CrossRef CAS PubMed.
- S. Brandt, I. Pavlichenko, A. V. Shneidman, H. Patel, A. Tripp, T. S. B. Wong, S. Lazaro, E. Thompson, A. Maltz, T. Storwick, H. Beggs, K. Szendrei-Temesi, B. V. Lotsch, C. N. Kaplan, C. W. Visser, M. P. Brenner, V. N. Murthy and J. Aizenberg, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2303928120 CrossRef CAS PubMed.
- E. Armstrong and C. O’Dwyer, J. Mater. Chem. C, 2015, 3, 6109–6143 RSC.
- D. Kou, W. Ma and S. Zhang, Adv. Funct. Mater., 2021, 31, 2007032 CrossRef CAS.
- S. A. Asher, A. C. Sharma, A. V. Goponenko and M. M. Ward, Anal. Chem., 2003, 75, 1676–1683 CrossRef CAS PubMed.
- H. Jiang, Y. Zhu, C. Chen, J. Shen, H. Bao, L. Peng, X. Yang and C. Li, New J. Chem., 2012, 36, 1051 RSC.
- A. C. Arsenault, T. J. Clark, G. Von Freymann, L. Cademartiri, R. Sapienza, J. Bertolotti, E. Vekris, S. Wong, V. Kitaev, I. Manners, R. Z. Wang, S. John, D. Wiersma and G. A. Ozin, Nat. Mater., 2006, 5, 179–184 CrossRef CAS.
- C. G. Schäfer, M. Gallei, J. T. Zahn, J. Engelhardt, G. P. Hellmann and M. Rehahn, Chem. Mater., 2013, 25, 2309–2318 CrossRef.
- J. D. Joannopoulos, S. G. Johnson and J. N. Winn, Photonic Crystals: Molding the Flow of Light, Princeton University Press, Princeton, NJ, 2nd edn, 2011 Search PubMed.
- R. C. Schroden, M. Al-Daous, C. F. Blanford and A. Stein, Chem. Mater., 2002, 14, 3305–3315 CrossRef CAS.
- B. Hatton, L. Mishchenko, S. Davis, K. H. Sandhage and J. Aizenberg, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 10354–10359 CrossRef CAS PubMed.
- K. R. Phillips, G. T. England, S. Sunny, E. Shirman, T. Shirman, N. Vogel and J. Aizenberg, Chem. Soc. Rev., 2016, 45, 281–322 RSC.
- I. Papiano, S. De Zio, A. Hofer, M. Malferrari, I. Mínguez Bacho, J. Bachmann, S. Rapino, N. Vogel and G. Magnabosco, Mater. Horiz., 2023, 10, 4380–4388 RSC.
- C. Chi, F. Bai, X. Xu, P. Qu, J. Xian, L. Li and D. Zhang, Analyst, 2022, 147, 3486–3493 RSC.
- Y. Yu, S. Brandt, N. J. Nicolas and J. Aizenberg, ACS Appl. Mater. Interfaces, 2020, 12, 1924–1929 CrossRef CAS PubMed.
- N. J. Nicolas, M. A. Duffy, A. Hansen and J. Aizenberg, Adv. Healthcare Mater., 2021, 10, 2001326 CrossRef CAS PubMed.
- I. B. Burgess, N. Koay, K. P. Raymond, M. Kolle, M. Lon and J. Aizenberg, ACS Nano, 2012, 6, 1427–1437 CrossRef CAS PubMed.
- K. P. Raymond, I. B. Burgess, M. H. Kinney, M. Lončar and J. Aizenberg, Lab Chip, 2012, 12, 3666 RSC.
- T. A. Singleton, I. B. Burgess, B. A. Nerger, A. Goulet-Hanssens, N. Koay, C. J. Barrett and J. Aizenberg, Soft Matter, 2014, 10, 1325–1328 RSC.
- I. B. Burgess, L. Mishchenko, B. D. Hatton, M. Kolle, M. Lončar and J. Aizenberg, J. Am. Chem. Soc., 2011, 133, 12430–12432 CrossRef CAS PubMed.
- A. Khalil, P. Rostami, G. K. Auernhammer and A. Andrieu-Brunsen, Adv. Mater. Inter., 2021, 8, 2100252 CrossRef CAS.
- A. M. Abu-Dief and I. M. A. Mohamed, Beni-Suef Univ. J. Basic Appl. Sci., 2015, 4, 119–133 Search PubMed.
- V. K. Gupta, A. K. Singh and L. K. Kumawat, Sens. Actuators, B, 2014, 195, 98–108 CrossRef CAS.
- S. Q. Memon, N. Memon, A. Mallah, R. Soomro and M. Y. Khuhawar, Curr. Anal. Chem., 2014, 10, 393–417 CrossRef CAS.
- P. G. Cozzi, Chem. Soc. Rev., 2004, 33, 410–421 RSC.
- J. Docherty, S. Mabbott, E. Smith, K. Faulds, C. Davidson, J. Reglinski and D. Graham, Analyst, 2016, 141, 5857 RSC.
- M. Strianese, D. Guarnieri, M. Lamberti, A. Landi, A. Peluso and C. Pellecchia, Inorg. Chem., 2020, 59, 15977–15986 CrossRef CAS PubMed.
- S. Goswami, Tetrahedron Lett., 2013, 54, 5075–5077 CrossRef CAS.
- K. B. Kim, G. J. Park, H. Kim, E. J. Song, J. M. Bae and C. Kim, Inorg. Chem. Commun., 2014, 46, 237–240 CrossRef CAS.
- D. Peralta-Domínguez, M. Rodríguez, G. Ramos-Ortíz, J. L. Maldonado, M. A. Meneses-Nava, O. Barbosa-García, R. Santillan and N. Farfán, Sens. Actuators, B, 2015, 207, 511–517 CrossRef.
- K. R. Phillips, N. Vogel, I. B. Burgess, C. C. Perry and J. Aizenberg, Langmuir, 2014, 30, 7615–7620 CrossRef CAS PubMed.
- S. Li, J. M. Scheiger, Z. Wang, Z. Dong, A. Welle, V. Trouillet and P. A. Levkin, Adv. Funct. Mater., 2021, 31, 2107716 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods and further characterization. See DOI: https://doi.org/10.1039/d4cc01781a |
| This journal is © The Royal Society of Chemistry 2024 |