Open Access Article
Open Access ArticleSynthesis of 18F-labelled aryl trifluoromethyl ketones with improved molar activity†
Lukas
Veth
 *,
Albert D.
Windhorst
*,
Albert D.
Windhorst
 and
Danielle J.
Vugts
and
Danielle J.
Vugts
Dept. of Radiology & Nuclear Medicine Amsterdam UMC, Location Vrije Universiteit Amsterdam De Boelelaan, 1117, Amsterdam, The Netherlands. E-mail: l.r.veth@amsterdamumc.nl
First published on 10th June 2024
Abstract
A method for the radiosynthesis of 18F-labelled aryl trifluoromethyl ketones starting from widely available Weinreb amides using [18F]fluoroform is presented. The method uses potassium hexamethyldisilazane as base and delivers products in high molar activity (up to 24 GBq μmol−1) and excellent radiochemical conversions. The applicability for PET tracer synthesis is demonstrated by the radiosynthesis of ten (hetero)aryl trifluoromethylketones, bearing electron-withdrawing and -donating substituents including a derivative of bioactive probenecid.
Positron emission tomography (PET) is a non-invasive imaging technique of increasing importance for the diagnosis of several diseases and the development of new drug molecules.1–3 It relies on the use of radiolabelled tracers comprising short-lived radioisotopes, the most prominent being fluorine-18 (18F, t1/2 = 110 min). Fluorine-18 is routinely produced with medical cyclotrons and can be obtained as either [18F]fluorine (carrier-added, c.a.) or [18F]fluoride (no-carrier added, n.c.a.). Therefore, the great advances for fluorination reactions in organic chemistry using fluorine-19, often relying on the use of specialised fluorination reagents, cannot be easily translated to radiochemistry.1,3–9 An additional challenge lies in the requirement of high molar activity (Am), i.e., the synthesis of the product without significant isotopic dilution with fluorine-19 required for the tracer principle.10,11 This is especially true for the synthesis of [18F]CF3-containing radiotracers.
Trifluoromethyl ketones (TFMKs) are valuable structural motifs in bioactive compounds.12–16 The radiosynthesis of their 18F-isotopologues is therefore of great interest with regard to possible applications for PET. Prakash and co-workers reported the first synthesis of 18F-labelled TFMKs (Scheme 1A).17 By reacting c.a. [18F]F2 with difluorinated silyl enol ethers, the target compounds could be obtained in modest radiochemical yields (RCY, up to 28%). However, since [18F]F2 is carrier-added, i.e., diluted with its isotopologue [19F]F2, the molar activity obtained (up to 0.02 GBq μmol−1) proved to be too low for applications in PET.
Inspired by this work, Szabó and co-workers recently developed an alternative approach (Scheme 1B).18 Instead of using difluorinated silyl enol ethers as precursors, they were further reacted to the corresponding bromo- or iododifluoromethyl ketones. These were then reacted with [18F]TBAF, a source of nucleophilic [18F]F−. This reaction proceeds through a halogen exchange of C–Br to C–18F, commonly referred to as “halex reaction”. Despite the use of a n.c.a. 18F-source, the molar activity remained relatively low (up to 1.27 GBq μmol−1 when starting with ca. 5 GBq [18F]F−). This can be explained by isotopic dilution resulting from 18F-19F-exchange reactions and represents a common issue when using halex reactions for the synthesis of [18F]CF3-containing compounds. In addition, both methods suffer from long synthetic routes to access the required precursors.
In recent years, the direct incorporation of [18F]CF3-groups into target compounds from suitable synthetic building blocks has been established as a viable strategy for the radiosynthesis of [18F]CF3-containing compounds.19–25 Despite requiring additional reaction steps, such building blocks have proven useful for expanding the radiochemical space of 18F-labelled structural motifs for applications in PET while often providing higher molar activities. Among them, [18F]fluoroform has demonstrated to be of particular value. Several approaches have been reported for the synthesis of [18F]fluoroform from [18F]fluoride in high molar activity (up to 163 GBq μmol−1).20,26,27 On the one hand, [18F]fluoroform can be used to access other synthetic building blocks such as [18F]CuCF320–22,28 and [18F]Ruppert–Prakash reagent,23,29 which have enabled access to previously inaccessible radiochemical space. On the other hand, it can be used to directly incorporate a [18F]CF3-group into suitable precursors through a base-promoted nucleophilic substitution reaction.20,25,30 Very recently, Telu, Pike, and co-workers reported the synthesis of 11C- and 18F-labelled TFMKs from their corresponding methyl esters using radiolabelled fluoroform (Scheme 1C).31 Although mainly focusing on [11C]fluoroform, the applicability to [18F]fluoroform is demonstrated with three examples. Unfortunately, the molar activity obtained for the 18F-labelled TFMKs was not reported. Herein, we describe the synthesis of 18F-labelled aryl trifluoromethyl ketones starting from their corresponding Weinreb amide analogues with improved molar activity using [18F]fluoroform as trifluoromethylation reagent.
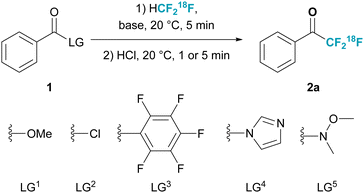
We commenced our studies with the evaluation of the appropriate precursor 1. The synthesis of TFMKs employing non-radioactive fluoroform has been reported to proceed with esters,32–36 and acyl chlorides.34 In addition, the use of Ruppert–Prakash reagent, also proceeding through a nucleophilic substitution reaction, has enabled the synthesis from more motifs.37–42 We evaluated the following five activated carbonyl compounds: methyl esters, acyl chlorides, pentafluorophenyl esters, imidazoles, and Weinreb amides (Table 1, entries 1–5) using potassium hexamethyldisilazane (KHMDS) as base. To our delight, we found that both methyl ester and Weinreb amide (entries 1 and 5) delivered the desired product in 81–91% radiochemical conversion (RCC). In contrast, all others did not deliver any product (entries 2–4). Using a weaker base like KOtBu delivered the product in significantly lower RCC or did not deliver any product (entries 6 and 7). Further investigation (entries 8 and 9) revealed that a lower amount of KHMDS (50 μmol) improved the reaction performance to deliver 2a in 99 ± 2% RCC. A screen of commonly employed solvents showed that the reaction proceeded as well in THF (95 ± 1% RCC, entry 10), however the use of toluene diminished the reaction performance (34 ± 5% RCC, entry 11). For all experiments discussed above, KHMDS was added as freshly prepared 0.5 M solution in DMF. It was also possible to use commercially obtainable KHMDS solution (1M in THF) delivering 2a in quantitative RCC (entry 12). Lastly, it was possible to reduce the amount of precursor to only 30 μmol while maintaining the same reaction performance (entry 13). Importantly, control reactions without base or precursor 1 showed no formation of 2a (entries 14 and 15).
| Entry | LG | Base [μmol] | Solvent [μL] | RCCb |
|---|---|---|---|---|
| a Standard reaction conditions: (1) 1a (50 μmol), base, [18F]fluoroform (ca. 25–50 MBq), solvent, 20 °C, 5 min; (2) HCl (conc, 100 μL), 20 °C, 1 or 5 min. b Radiochemical conversion based on HPLC analysis starting from [18F]fluoroform, average ± standard deviation, n = 3. c n = 1. d 30 μmol 1, n = 2. e Without 1. For details on the synthesis of [18F]fluoroform, see ESI. | ||||
| 1 | LG1 | KHMDS (100) | DMF (300) | 81 ± 2% |
| 2 | LG2 | KHMDS (100) | DMF (300) | n.d.c |
| 3 | LG3 | KHMDS (100) | DMF (300) | n.d.c |
| 4 | LG4 | KHMDS (100) | DMF (300) | n.d.c |
| 5 | LG5 | KHMDS (100) | DMF (300) | 91 ± 3% |
| 6 | LG1 | KOtBu (100) | DMF (300) | 49 ± 2% |
| 7 | LG5 | KOtBu (50) | DMF (200) | n.d. |
| 8 | LG5 | KHMDS (25) | DMF (150) | 70 ± 5% |
| 9 | LG5 | KHMDS (50) | DMF (650) | 99 ± 2% |
| 10 | LG5 | KHMDS (50) | THF (650) | 95 ± 1% |
| 11 | LG5 | KHMDS (50) | PhMe (650) | 34 ± 5% |
| 12 | LG 5 | KHMDS (50) | DMF/THF (600/50) | 100% |
| 13d | LG5 | KHMDS (30) | DMF/THF (620/30) | 96 ± 6% |
| 14 | LG5 | none | DMF (100) | n.d. |
| 15e | None | KHMDS (50) | DMF (200) | n.d. |
Having established reaction conditions for a successful synthesis of 18F-labelled TFMK 2a, we sought out to investigate the scope of the reaction (Scheme 2). We found the reaction to be applicable to substrates bearing a range of different functional groups. The reaction tolerated the presence of electron-withdrawing functional groups, like trifluoromethyl- and chloro-substituents (2b and 2c), resulting in quantitative RCC and proved to be applicable to a naphthalene as well (2d). In addition, electron-donating substituents were tolerated (2e and 2f), although a 4-methoxy-substituent led to a diminished RCC (2f), due to the lower reactivity towards nucleophilic trifluoromethylation. Remarkably, heterocyclic substrates bearing N-, O- and S-atoms reacted smoothly to deliver the corresponding products in 90–100% RCC (2g–2i). Unfortunately, both alkenyl- and alkyl-substituted substrates 2j and 2k were found to be unreactive and did not deliver any product. This is in contrast to a recent report using methyl ester precursors (Scheme 1c) and could be caused by the stronger basicity of KHMDS compared to KOtBu.
 | ||
Scheme 2 Scope of 18F-labelled trifluoromethyl ketones. Standard reaction conditions: (1) substrate 1a–j (50 μmol), KHMDS (50 μmol), [18F]fluoroform (25–50 MBq), DMF/THF (600/50 μL), 5 min, 20 °C, (2) conc. HCl (100 μL), 1 min, 20 °C. Radiochemical conversion based on HPLC analysis starting from [18F]fluoroform, average ± standard deviation.a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n = 2, for detailed data, see ESI.† n = 2, for detailed data, see ESI.† | ||
In order to demonstrate the applicability of the developed method to PET tracer synthesis and evaluate the molar activity that can be obtained, full batches of [18F]fluoroform (2.2 ± 0.6 GBq) were used for the radiofluorination of 2l, a derivative of probenecid. The product was then isolated using semi-preparative high performance liquid chromatography (HPLC). Using the standard conditions with 30 μmol precursor 1l-a, the product could be obtained in 91 ± 3% RCY, >99% RCP and Am = 24.4 ± 2.4 GBq μmol−1 (for more detailed data, see ESI†). The molar activity obtained here is >19-times higher compared to previous reports (see Scheme 1) which represents a significant improvement and is within the range of clinical utility. It should be noted that the synthesis was started from 8.8 ± 3.0 GBq [18F]fluoride. Starting with higher activities, 50–500 GBq are common for PET tracer productions, will in principle lead to even higher molar activities.26 As shown in Table 1, the use of methyl esters delivers the products in slightly lower yield. This is undermined by the probenecid example. Although leading to the formation of product in comparable RCC, experiments with full batches of [18F]fluoroform (starting from 4.9 ± 0.3 GBq [18F]fluoride) led to the isolation of 2l in diminished yield (for more detailed data, see ESI†). The Am was found to be comparable when taking into account the lower starting activity.
In summary, we have developed a novel method for the synthesis of 18F-labelled aryl trifluoromethyl ketones from their corresponding Weinreb amides using [18F]fluoroform as trifluoromethylation reagent. The method possesses a good functional group tolerance as demonstrated by the scope of the reaction. Further, its applicability to PET tracer development was demonstrated by the synthesis of 2l, a derivative of probenecid, using 2.2 ± 0.6 GBq of [18F]fluoroform. In contrast to previous reports on the synthesis of 18F-labelled TFMKs, the target compounds can be obtained with a molar activity of 24.4 ± 2.4 GBq μmol−1 (>19-times higher).
L. V. is supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) with a Walter-Benjamin fellowship (project no. 519969214).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- S. M. Ametamey, M. Honer and P. A. Schubiger, Chem. Rev., 2008, 108, 1501–1516 CrossRef CAS PubMed.
- A. A. M. Van Der Veldt, E. F. Smit and A. A. Lammertsma, Front. Oncol., 2013, 3, 208 Search PubMed.
- J. Rong, A. Haider, T. E. Jeppesen, L. Josephson and S. H. Liang, Nat. Commun., 2023, 14, 3257 CrossRef CAS PubMed.
- L. Cai, S. Lu and V. W. Pike, Eur. J. Org. Chem., 2008, 2853–2873 CrossRef CAS.
- A. F. Brooks, J. J. Topczewski, N. Ichiishi, M. S. Sanford and P. J. H. Scott, Chem. Sci., 2014, 5, 4545–4553 RSC.
- M. G. Campbell, J. Mercier, C. Genicot, V. Gouverneur, J. M. Hooker and T. Ritter, Nat. Chem., 2017, 9, 1–3 CrossRef CAS PubMed.
- X. Deng, J. Rong, L. Wang, N. Vasdev, L. Zhang, L. Josephson and S. H. Liang, Angew. Chem., Int. Ed., 2019, 58, 2580–2605 CrossRef CAS PubMed.
- R. Halder and T. Ritter, J. Org. Chem., 2021, 86, 13873–13884 CrossRef CAS PubMed.
- I. N.-M. Leibler, S. S. Gandhi, M. A. Tekle-Smith and A. G. Doyle, J. Am. Chem. Soc., 2023, 145, 9928–9950 CrossRef CAS PubMed.
- Z. Liu, Y. Li, J. Lozada, P. Schaffer, M. J. Adam, T. J. Ruth and D. M. Perrin, Angew. Chem., Int. Ed., 2013, 52, 2303–2307 CrossRef CAS PubMed.
- M. Sergeev, M. Lazari, F. Morgia, J. Collins, M. R. Javed, O. Sergeeva, J. Jones, M. E. Phelps, J. T. Lee, P. Y. Keng and R. M. Van Dam, Commun. Chem., 2018, 1, 10 CrossRef PubMed.
- C. A. Veale, P. R. Bernstein, C. M. Bohnert, F. J. Brown, C. Bryant, J. R. Damewood, R. Earley, S. W. Feeney, P. D. Edwards, B. Gomes, J. M. Hulsizer, B. J. Kosmider, R. D. Krell, G. Moore, T. W. Salcedo, A. Shaw, D. S. Silberstein, G. B. Steelman, M. Stein, A. Strimpler, R. M. Thomas, E. P. Vacek, J. C. Williams, D. J. Wolanin and S. Woolson, J. Med. Chem., 1997, 40, 3173–3181 CrossRef CAS PubMed.
- D. L. Boger, H. Sato, A. E. Lerner, B. J. Austin, J. E. Patterson, M. P. Patricelli and B. F. Cravatt, Bioorg. Med. Chem. Lett., 1999, 9, 265–270 CrossRef CAS PubMed.
- R. R. Frey, C. K. Wada, R. B. Garland, M. L. Curtin, M. R. Michaelides, J. Li, L. J. Pease, K. B. Glaser, P. A. Marcotte, J. J. Bouska, S. S. Murphy and S. K. Davidsen, Bioorg. Med. Chem. Lett., 2002, 12, 3443–3447 CrossRef CAS PubMed.
- E. Muraglia, S. Altamura, D. Branca, O. Cecchetti, F. Ferrigno, M. V. Orsale, M. C. Palumbi, M. Rowley, R. Scarpelli, C. Steinkühler and P. Jones, Bioorg. Med. Chem. Lett., 2008, 18, 6083–6087 CrossRef CAS PubMed.
- J. M. Ontoria, S. Altamura, A. Di Marco, F. Ferrigno, R. Laufer, E. Muraglia, M. C. Palumbi, M. Rowley, R. Scarpelli, C. Schultz-Fademrecht, S. Serafini, C. Steinkühler and P. Jones, J. Med. Chem., 2009, 52, 6782–6789 CrossRef CAS PubMed.
- G. K. Surya Prakash, M. M. Alauddin, J. Hu, P. S. Conti and G. A. Olah, J. Labelled Compd. Radiopharm., 2003, 46, 1087–1092 CrossRef.
- D. N. Meyer, M. A. Cortés González, X. Jiang, L. Johansson-Holm, M. Pourghasemi Lati, M. Elgland, P. Nordeman, G. Antoni and K. J. Szabó, Chem. Commun., 2021, 57, 8476–8479 RSC.
- D. Van Der Born, A. Pees, A. J. Poot, R. V. A. Orru, A. D. Windhorst and D. J. Vugts, Chem. Soc. Rev., 2017, 46, 4709–4773 RSC.
- B. Y. Yang, S. Telu, M. B. Haskali, C. L. Morse and V. W. Pike, Sci. Rep., 2019, 9, 14835 CrossRef PubMed.
- D. van der Born, C. Sewing, J. K. D. M. Herscheid, A. D. Windhorst, R. V. A. Orru and D. J. Vugts, Angew. Chem., Int. Ed., 2014, 53, 11046–11050 CrossRef CAS PubMed.
- P. Ivashkin, G. Lemonnier, J. Cousin, V. Grégoire, D. Labar, P. Jubault and X. Pannecoucke, Chem. – Eur. J., 2014, 20, 9514–9518 CrossRef CAS PubMed.
- A. Pees, M. J. W. D. Vosjan, N. Vasdev, A. D. Windhorst and D. J. Vugts, Chem. Commun., 2021, 57, 5286–5289 RSC.
- M. Huiban, M. Tredwell, S. Mizuta, Z. Wan, X. Zhang, T. L. Collier, V. Gouverneur and J. Passchier, Nat. Chem., 2013, 5, 941–944 CrossRef CAS PubMed.
- E. Carbonnel, T. Besset, T. Poisson, D. Labar, X. Pannecoucke and P. Jubault, Chem. Commun., 2017, 53, 5706–5709 RSC.
- A. Pees, A. D. Windhorst, M. J. W. D. Vosjan, V. Tadino and D. J. Vugts, Eur. J. Org. Chem., 2020, 1177–1185 CrossRef CAS.
- A. Pees, M. J. W. D. Vosjan, J. Y. Chai, H. Cha, D. Y. Chi, A. D. Windhorst and D. J. Vugts, J. Labelled Compd. Radiopharm., 2021, 64, 466–476 CrossRef CAS PubMed.
- S. Telu, S. Jana, M. B. Haskali, B. Yeun Yang, J. E. Jakobsson, Q. Zhao, K. M. Ramos-Torres, P. Brugarolas and V. W. Pike, Chem. – Eur. J., 2023, 29, e202204004 CrossRef CAS PubMed.
- L. Veth, A. D. Windhorst and D. J. Vugts, Angew. Chem., Int. Ed., 2024, e202404278 Search PubMed.
- D. Van Der Born, J. D. M. Herscheid, R. V. A. Orru and D. J. Vugts, Chem. Commun., 2013, 49, 4018 RSC.
- S. Jana, S. Telu, J. E. Jakobsson, B. Y. Yang and V. W. Pike, Chem. Commun., 2024, 60, 4589–4592 RSC.
- J. Russell and N. Roques, Tetrahedron, 1998, 54, 13771–13782 CrossRef CAS.
- G. K. S. Prakash, P. V. Jog, P. T. D. Batamack and G. A. Olah, Science, 2012, 338, 1324–1327 CrossRef CAS PubMed.
- Y. Zhang, M. Fujiu, H. Serizawa and K. Mikami, J. Fluorine Chem., 2013, 156, 367–371 CrossRef CAS.
- Z. Han, S. Chen, Y. Tu, X. Lian and G. Li, Eur. J. Org. Chem., 2019, 4658–4661 CrossRef CAS.
- Y. Fujihira, Y. Liang, M. Ono, K. Hirano, T. Kagawa and N. Shibata, Beilstein J. Org. Chem., 2021, 17, 431–438 CrossRef CAS PubMed.
- M. S. Said, N. S. Khonde, P. Kumar and J. M. Gajbhiye, Org. Lett., 2023, 25, 1094–1098 CrossRef CAS PubMed.
- D. M. Rudzinski, C. B. Kelly and N. E. Leadbeater, Chem. Commun., 2012, 48, 9610 RSC.
- X. Liu, L. Liu, T. Huang, J. Zhang, Z. Tang, C. Li and T. Chen, Org. Lett., 2021, 23, 4930–4934 CrossRef CAS PubMed.
- R. Krishnamurti, D. R. Bellew and G. K. S. Prakash, J. Org. Chem., 1991, 56, 984–989 CrossRef CAS.
- X. Ispizua-Rodriguez, S. B. Munoz, V. Krishnamurti, T. Mathew and G. K. S. Prakash, Chem. Eur. J., 2021, 27, 15908–15913 CrossRef CAS PubMed.
- J. Wiedemann, T. Heiner, G. Mloston, G. K. S. Prakash and G. A. Olah, Angew. Chem., Int. Ed., 1998, 37, 820–821 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc01776e |
| This journal is © The Royal Society of Chemistry 2024 |