 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rational design of pH-responsive near-infrared spirocyclic cyanines: the effects of substituents and the external environment†
Akihiro
Sakama‡
 a,
Hyemin
Seo‡
a,
Joji
Hara
a,
Yutaka
Shindo
bc,
Yuma
Ikeda
a,
Kotaro
Oka
bcde,
Daniel
Citterio
a,
Hyemin
Seo‡
a,
Joji
Hara
a,
Yutaka
Shindo
bc,
Yuma
Ikeda
a,
Kotaro
Oka
bcde,
Daniel
Citterio
 a and
Yuki
Hiruta
a and
Yuki
Hiruta
 *a
*a
aDepartment of Applied Chemistry, Faculty of Science and Technology, Keio University, Hiyoshi 3-14-1, Kohoku-ku, Yokohama, Kanagawa 223-8522, Japan. E-mail: hiruta@applc.keio.ac.jp
bDepartment of Biosciences and Informatics, Faculty of Science and Technology, Keio University, 3-14-1 Hiyoshi, Kohoku-ku, Yokohama, Kanagawa 223-8522, Japan
cSchool of Frontier Engineering, Kitasato University, 1-15-1 Kitasato, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan
dWaseda Research Institute for Science and Engineering, Waseda University, 2-2 Wakamatsu-cho, Shinjuku-ku, Tokyo 162-8480, Japan
eCollege of Medicine, Kaohsiung Medical University, Kaohsiung City 80708, Taiwan
First published on 13th May 2024
Abstract
pH-responsive spirocyclic cyanine dyes were designed and synthesized. The equilibrium constant for cyclization (pKcycl) could be rationally controlled by changing the nucleophilic moiety and the side chains. Encapsulation in polymeric micelles inhibited the H-aggregation of the dye, and the pKcycl could be shifted according to the amphiphilic polymer employed.
Fluorescent dyes have been gaining much attention in the field of bioimaging due to their high brightness and biocompatibility. While autofluorescence and high background signals are conventional drawbacks of fluorescence imaging, it is effective to use fluorescence in the near-infrared (NIR, λ = 650–900 nm) region to selectively visualize the target tissue, while suppressing autofluorescence from normal tissue, especially in in vivo deep tissue imaging.1 Although several types of NIR fluorescent dyes have been developed as bioimaging probes, most of them could not differentiate the target cells or tissue from normal ones or blood vessels. This issue can be overcome by imparting responsiveness to a stimulus, such as pH change, to the dyes. The pH level in a living organism is strictly controlled and is closely related to biological functions. Therefore, the difference in pH provides clues for understanding biological phenomena and diagnosing diseases.2 pH-responsive fluorescent probes emitting visible light have been developed based on xanthene or boron dipyrromethene scaffolds;3,4 however, only limited examples of pH-responsive NIR probes have been reported.
Heptamethine cyanines are NIR fluorescent dyes known to possess high potential for cancer imaging. Indocyanine green (ICG) has been approved for clinical use in sentinel lymph node (SLN) mapping during surgery due to its low toxicity.5 One limitation of ICG is its short imaging time because of its poor stability. IR-780, another popular heptamethine cyanine dye, has improved fluorescence properties compared with ICG and long retention time for in vivo imaging,6 and has great potential as a bioimaging agent. However, most heptamethine cyanine dyes lack targetability to the cells/tissues of interest. Attempts have been made to add sensing ability to cyanine dyes. Among them, pH-responsive cyanines have been actively investigated. One of them possesses a basic nitrogen atom in the dye structure. Cyanines lacking side chains on one or both indolenine nitrogen atoms exhibit long-wavelength absorption and fluorescence under acidic conditions, while under basic conditions the absorption shifts to shorter wavelengths and little fluorescence is emitted.7 The weak point of this type of pH-responsive cyanines is the difficulty in finely adjusting the range of pH response according to the target pH. Aminocyanine derivatives bearing a diamine moiety on the central carbon of the polymethine chain were developed.8,9 Fluorescence quenching by photo-induced electron transfer (PeT) has also been employed for controlling the fluorescence of heptamethine cyanines.10 However, the highest occupied molecular orbital (HOMO) level of NIR dyes is rather high for sufficient fluorescence quenching by PeT.11 Lin's group and Miki's group had their eye on spirocyclization as the mechanism for pH-responsive ON/OFF switching of the fluorescence. They designed heptamethine cyanines bearing a nucleophilic moiety, which causes nucleophilic attack to the electron-deficient polymethine chain to form a cycle (spirocyclization).12,13 Xie and co-workers also developed fluorescent pH turn-on oxazinoindoline dyes utilizing spirocyclization.14 Spirocyclization has been applied mainly to fluorescein and rhodamine derivatives.15,16 The conversion between open and closed forms causes a drastic change in the conjugated system, connecting and breaking up, resulting in a large change in absorbance and an associated distinct OFF/ON change in fluorescence. The value of the equilibrium constant between the open and closed form of the dye (pKcycl) is dependent on the structure of the nucleophilic moiety and the electron density of the fluorophore;17,18 thus, the pH response range can be tuned according to the purpose. However, there are few previous reports revealing a relationship between pKcycl and the structure of heptamethine cyanine dyes.
We decided to clarify how pKcycl is altered by changing the nucleophilic moiety and the hydrophobicity of a spirocyclic cyanine, and the environment in which the dye is placed. In this context, we synthesized pH-responsive spirocyclic cyanine dyes having hydroxymethyl, methylcarbamoyl, or arylcarbamoyl groups as the nucleophilic subunit (Fig. 1). To evaluate the effect of the hydrophobicity of the dye on pKcycl, several substituents with different polarity were introduced into the side chains on the indolenine nitrogens.
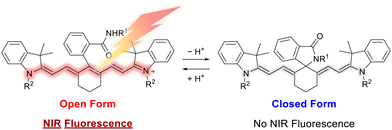 | ||
| Fig. 1 Molecular design strategy for pH-responsive cyanine dye: the closed form is converted to the open form via protonation. | ||
Heptamethine cyanine dyes can generally easily undergo self-aggregation due to the wide π-conjugated system. Encapsulation with lipids or micelles can prevent self-aggregation and improve the photostability, biocompatibility, fluorescence intensity, and water-solubility.19 In this work, the dyes were encapsulated into polymeric micelles, and their pH responsiveness in the encapsulated environment was evaluated.
A spirocyclic cyanine dye with a hydroxymethyl group (IR-HM) was firstly synthesized (Fig. 2a), because the hydroxymethyl group has been frequently employed in previously reported spirocyclic dyes such as rhodamine derivatives, and IR-HM can be synthesized by a single step from commercially available IR-780. To evaluate the pH responsiveness of IR-HM, absorption and fluorescence spectra (Fig. S2a and d in the ESI†) were measured at different pH.
At pH 9.0, IR-HM has an absorption peak at 761 nm and the fluorescence emission peak was measured to be at 785 nm, which is also in the NIR region. IR-HM should exist solely as the open form at this pH. On the other hand, the absorption peak shifted to 400 nm in basic environments (pH 12.5), and there was almost no absorbance at 760 nm, resulting in a 360 nm shift from the NIR to the UV region, which indicated that a spirocyclization reaction occurred. As the case of the rhodamine derivative shows, spirocyclization causes such a dramatic absorption change that the dye changes from colorless to colored. The interruption/connection of the central conjugated chain upon cyclization/ring opening equilibrium causes a large shift in absorption wavelength, and more drastic OFF/ON switching of the fluorescence in the NIR region is achieved, compared with pH-responsive cyanine dyes based on simple protonation equilibrium.7–9 The structural change upon the spirocyclization equilibrium was reflected also in the chemical shifts in the 1H NMR spectra and the appearance of the sample solutions (Fig. S3 in the ESI†). Additionally, the quantum chemical calculations of frontier orbital energy levels confirmed the change in the HOMO–LUMO energy gap depending on the spirocyclization equilibrium (Fig. S4 in the ESI†). The molecular design of spirocyclic dyes using a hydroxymethyl group was confirmed to be applicable to cyanine dyes and to provide a sharp pH response. However, the pKcycl [10.6/10.8, determined based on absorbance (Abs) and fluorescence intensity (FI), respectively] was too high for the dye to work in the physiological pH range (Fig. 2c and e), so it was necessary to change the nucleophilic moiety. It was previously reported that unsubstituted carbamoyl groups are applicable as the nucleophile for spirocyclic heptamethine cyanine dyes and provide a pH-responsive change in fluorescence intensity in the broad range of pH 4–11.12 We designed a cyanine dye possessing a methylcarbamoyl group (IR-MA), aiming at enhancement of the nucleophilicity of the carbamoyl group by an electron-donating methyl group (Fig. 2a). Its pKcycl was successfully lowered to 7.9 (Abs)/8.2 (FI) (Fig. S2b, e in the ESI† and Fig. 2c, e). By installing a more acidic phenyl amide20 moiety [IR-PAH and IR-PAM (Me)] as shown in Fig. 2a, the pKcycl was further shifted to 7.0 (Abs)/7.2 (FI) and 7.1 (Abs)/7.3 (FI), respectively (Fig. S2c, f in the ESI† and Fig. 2b–e).
IR-PAM derivatives were chosen to evaluate the effect of the side chains on pKcycl. To increase the water solubility of IR-PAM (Me), IR-PAM with carboxylic acid moieties [IR-PAM (COOH)] and its PEG amide [IR-PAM (PEG)] were synthesized (Fig. 3a). Their pKcycl values were determined to be 10.9 (Abs)/11.2 (FI) and 10.2 (Abs)/10.8 (FI), respectively, larger than those of IR-PAM (Me) (Fig. S5a, b, e, f in the ESI† and Fig. 3b). Conversely, when introducing hydrophobic substituents [IR-PAM (COOt-Bu) and IR-PAM (n-Hex)], as shown in Fig. 3a, the pKcycl shifted to the acidic side (Fig. S5c, d, g, h in the ESI† and Fig. 3b). This demonstrates that the dye with a more hydrophilic chromophore part has a higher pKcycl. In other words, the open form of the hydrophilic dye is more stable than that of the hydrophobic dye in aqueous solution of the same pH. As the open form is positively charged, it is stabilized by hydration, and polar substituents increase the energetic gain by hydration. On the other hand, hydrophobic substituents hinder hydration of the dye, decreasing the stability of the open form.
Judging from the shape of the absorbance spectra (Fig. S3c and d in the ESI†), the dyes with hydrophobic substituents were considered to form H-aggregates, which results in a shorter peak wavelength than the original maximum wavelength of the dyes (∼760 nm). To prevent H-aggregation, IR-PAM (n-Hex) was encapsulated in amphiphilic polymeric micelles (Fig. S6 in the ESI†). Pluronic F127 (PEG-PPO-PEG) and PEG-b-PCL were employed as polymers because of their high biocompatibility. The center of those amphiphilic polymeric micelles has a hydrophobic core coated by a hydrophilic shell, and hence hydrophobic dyes would be localized in the hydrophobic core. The encapsulation efficiency of IR-PAM (n-Hex)@F127 was 95.8%. The absorption spectra of IR-PAM (n-Hex)@F127 were measured, and less H-aggregate peaks were observed (Fig. 4a). This result indicates that the aggregation was prevented by encapsulation. A pH-responsive change in absorbance was also observed. Fluorescence intensity increased from pH 8.5 to 5.8 as the pH was lowered, but decreased below pH 5.5 (Fig. 4b). This is considered to be caused by the inner filter effect, in which the emitted fluorescence is absorbed by the surrounding dye molecules and quenched, due to the high concentration of dye in the micelle. The pKcycl of IR-PAM (n-Hex)@PEG-b-PCL was located on the more basic side compared to that of IR-PAM (n-Hex)@F127 [8.1 (Abs)/8.3 (FI) and 6.2 (Abs)/6.8 (FI), respectively] as shown in Fig. S7 in the ESI† and Fig. 4c and d. This is because PEG-b-PCL micelles have a more hydrophobic core than F127, based on the evaluation of the polarity of the core of each micelle using the solvatofluorochromic dye KSD-321 (Fig. S8 in the ESI†). The more hydrophobic the environment of the dye, the more stably the dye can exist as the open form, which explains the shift of pKcycl towards the basic side. The response curves obtained using either type of polymeric micelles exhibited a wider pH-response range compared to those observed in the aqueous solution of the dye alone. This finding is consistent with prior reports indicating that within polymeric micelles, the dyes exhibited multiple apparent pKa values depending on their localization (from the center of the core to the interface with the aqueous solution), thus resulting in a broad pH-response range.22,23
Since we succeeded in developing pH-responsive cyanine dye-encapsulated polymeric micelles that work in the physiological pH range, their application to cellular pH imaging was investigated. The extracellular pH of cancer cells is around 6.5 and shows a rather acidic environment compared with normal cells (pH 7.4).24 To evaluate the applicability of IR-PAM (n-Hex)@F127 to visualize an acidic environment, we incubated HeLa cells, whose intracellular pH was intentionally changed to pH 6.4 and 7.4 by using nigericin, with IR-PAM (n-Hex)@F127 (Fig. 4e and f). The measured fluorescence intensities at pH 6.4 were approximately twice as high as those at pH 7.4 (Fig. 4g). IR-PAM (n-Hex)@F127 appeared to be mainly localized to the lipid membranes of organelles, and its localization did not change with pH. Thus, it can be concluded that IR-PAM (n-Hex)@F127 is applicable for NIR fluorescence imaging of cell acidity.
In summary, we have developed spirocyclic cyanine dyes, and demonstrated that changing their chemical structure resulted in a different pH response. By changing the nucleophile moiety from a hydroxy group (IR-HM) to an amide moiety [IR-MA, IR-PAH and IR-PAM (Me)], the pKcycl was lowered from 11 to around 7–8. Next, IR-PAM (COOH), IR-PAM (PEG), IR-PAM (COOt-Bu), and IR-PAM (n-Hex) were synthesized, and the effect of the polarity of the side chains on pKcycl was evaluated. The pH-responsiveness of these derivatives showed that a more hydrophilic dye structure leads to an increase in the pKcycl. IR-PAM (n-Hex) was encapsulated into amphiphilic polymers to suppress the aggregation and improve the solubility in water. The pKcycl of IR-PAM (n-Hex) in the micelles shifted to reflect the hydrophobicity of the polymer chain. NIR laser irradiation of HeLa cells stained with IR-PAM (n-Hex)@F127 showed a 2-fold difference in fluorescence emission at intracellular pH of 6.4 and 7.4. Thus, we demonstrated the effects of substituents and the external environment on the pH-responsiveness of spirocyclic cyanine dyes and provided guidelines for the regulation of it. Through further sensitization of pH response and tuning of pKcycl, these spirocyclic cyanines will offer potential applications for in vivo imaging or biomedical tools, such as cancer diagnosis and photothermal therapy.
Akihiro Sakama: formal analysis, writing – original draft. Hyemin Seo: investigation, writing – original draft. Joji Hara: investigation. Yutaka Shindo: investigation. Yuma Ikeda: investigation. Kotaro Oka: supervision, resources. Daniel Citterio: writing – review & editing, supervision. Yuki Hiruta: conceptualization, methodology, writing – review & editing, supervision, project administration, funding acquisition.
This study was supported by Grant-in Aid for Early-Career Scientists (Grant no. 19K16339), and Scientific Research (C) (Grant no. 21K06495) from the Japan Society for the Promotion of Science (JSPS), AMED under Grant Number JP20lm0203012, 21lm0203004j0005, and 22ym0126806j0001, Nakatani Foundation Grant Program for Biomedical Engineering Research, Kowa Life Science Foundation, and the Program for the Advancement of Next Generation Research Projects (Type C) at Keio to Y. H. The authors thank Dr Naoko Iwasawa, Mr Masato Ishizeki, and Ms Yuzuka Kuronuma of Keio University for the advice on synthesizing cyanine dyes.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Wu, Z. Shi, L. Zhu, J. Li, X. Han, M. Xu, S. Hao, Y. Fan, T. Shao, H. Bai, B. Peng, W. Hu, X. Liu, C. Yao, L. Li and W. Huang, Adv. Opt. Mater., 2022, 10, 2102514 CrossRef CAS.
- J. Yin, Y. Hu and J. Yoon, Chem. Soc. Rev., 2015, 44, 4619–4644 RSC.
- R. J. Iwatate, M. Kamiya and Y. Urano, Chem. Eur. J., 2016, 22, 1696–1703 CrossRef CAS PubMed.
- Y. Urano, D. Asanuma, Y. Hama, Y. Koyama, T. Barrett, M. Kamiya, T. Nagano, T. Watanabe, A. Hasegawa, P. L. Choyke and H. Kobayashi, Nat. Med., 2009, 15, 104–109 CrossRef CAS PubMed.
- M. Mihara, H. Hara, J. Araki, K. Kikuchi, M. Narushima, T. Yamamoto, T. Iida, H. Yoshimatsu, N. Murai, K. Mitsui, T. Okitsu and I. Koshima, PLoS One, 2012, 7, e38182 CrossRef CAS PubMed.
- C. Zhang, S. Wang, J. Xiao, X. Tan, Y. Zhu, Y. Su, T. Cheng and C. Shi, Biomaterials, 2010, 31, 1911–1917 CrossRef CAS PubMed.
- R. C. Gilson, R. Tang, A. Som, C. Klajer, P. Sarder, G. P. Sudlow, W. J. Akers and S. Achilefu, Mol. Pharmaceutics, 2015, 12, 4237–4246 CrossRef CAS PubMed.
- T. Myochin, K. Kiyose, K. Hanaoka, H. Kojima, T. Terai and T. Nagano, J. Am. Chem. Soc., 2011, 133, 3401–3409 CrossRef CAS PubMed.
- A. Kurutos, Y. Shindo, Y. Hiruta, K. Oka and D. Citterio, Dyes Pigm., 2020, 181, 108611 CrossRef CAS.
- B. Tang, F. Yu, P. Li, L. Tong, X. Duan, T. Xie and X. Wang, J. Am. Chem. Soc., 2009, 131, 3016–3023 CrossRef CAS PubMed.
- K. Kiyose, S. Aizawa, E. Sasaki, H. Kojima, K. Hanaoka, T. Terai, Y. Urano and T. Nagano, Chem. Eur. J., 2009, 15, 9191–9200 CrossRef CAS PubMed.
- L. He, W. Lin, Q. Xu, M. Ren, H. Wei and J.-Y. Wang, Chem. Sci., 2015, 6, 4530–4536 RSC.
- K. Miki, K. Kojima, K. Oride, H. Harada, A. Morinibu and K. Ohe, Chem. Commun., 2017, 53, 7792–7795 RSC.
- X. Xie, G. A. Crespo and E. Bakker, Anal. Chem., 2013, 85, 7434–7440 CrossRef CAS PubMed.
- S.-n Uno, M. Kamiya, T. Yoshihara, K. Sugawara, K. Okabe, M. C. Tarhan, H. Fujita, T. Funatsu, Y. Okada, S. Tobita and Y. Urano, Nat. Chem., 2014, 6, 681–689 CrossRef CAS PubMed.
- M. Kamiya, D. Asanuma, E. Kuranaga, A. Takeishi, M. Sakabe, M. Miura, T. Nagano and Y. Urano, J. Am. Chem. Soc., 2011, 133, 12960–12963 CrossRef CAS PubMed.
- M. Sakabe, D. Asanuma, M. Kamiya, R. J. Iwatate, K. Hanaoka, T. Terai, T. Nagano and Y. Urano, J. Am. Chem. Soc., 2013, 135, 409–414 CrossRef CAS PubMed.
- L. Yuan, W. Lin and Y. Feng, Org. Biomol. Chem., 2011, 9, 1723–1726 RSC.
- C. Yue, P. Liu, M. Zheng, P. Zhao, Y. Wang, Y. Ma and L. Cai, Biomaterials, 2013, 34, 6853–6861 CrossRef CAS PubMed.
- F. G. Bordwell, J. A. Harrelson, Jr. and T. Y. Lynch, J. Org. Chem., 1990, 55, 3337–3341 CrossRef CAS.
- Y. Ando, Y. Homma, Y. Hiruta, D. Citterio and K. Suzuki, Dyes Pigm., 2009, 83, 198–206 CrossRef CAS.
- X. Xie, J. Zhai, Z. Jarolímová and E. Bakker, Anal. Chem., 2016, 88, 3015–3018 CrossRef CAS PubMed.
- Q. Chen, J. Zhai, J. Li, Y. Wang and X. Xie, Nano Res., 2022, 15, 3471–3478 CrossRef CAS.
- B. A. Webb, M. Chimenti, M. P. Jacobson and D. L. Barber, Nat. Rev. Cancer, 2011, 11, 671–677 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, absorption and fluorescence spectra, and 1H and 13C NMR spectra of the new compounds. See DOI: https://doi.org/10.1039/d4cc01484g |
| ‡ A. S. and H. S. contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |



