Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mesoporous supraparticles with a tailored solid–liquid–gas interface for visual indication of H2 gas and NH3 vapours†
Andreas
Zink‡
 a,
Jakob
Reichstein‡
a,
Jakob
Reichstein‡
 a,
Nico
Ruhland
a,
Nina
Stockinger
a,
Boris S.
Morozov
a,
Nico
Ruhland
a,
Nina
Stockinger
a,
Boris S.
Morozov
 b,
Carlos
Cuadrado Collados
c,
Matthias
Thommes
c,
Evgeny A.
Kataev
b,
Carlos
Cuadrado Collados
c,
Matthias
Thommes
c,
Evgeny A.
Kataev
 b,
Susanne
Wintzheimer
b,
Susanne
Wintzheimer
 ad and
Karl
Mandel
ad and
Karl
Mandel
 *ad
*ad
aDepartment of Chemistry and Pharmacy, Inorganic Chemistry, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Egerlandstraße 1, D-91058 Erlangen, Germany. E-mail: karl.mandel@fau.de
bDepartment of Chemistry and Pharmacy, Organic Chemistry, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Nikolaus-Fiebiger-Str. 10, 91058 Erlangen, Germany
cInstitute of Separation Science and Technology, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Egerlandstraße 3, D-91058 Erlangen, Germany
dFraunhofer-Institute for Silicate Research ISC, Neunerplatz 2, D-97082 Würzburg, Germany
First published on 6th May 2024
Abstract
Dual-gasochromic supraparticles that undergo rapid eye-readable and gas-specific colour changes upon reaction with hydrogen or ammonia are reported. This functionality is achieved by tailoring the solid–liquid–gas interface within the mesoporous framework of supraparticles via spray-drying.
Gasochromic materials that visually indicate the presence of toxic, flammable, or explosive gases through a change in their absorbance become increasingly relevant. Their optical readout facilitates remote and spatially resolved gas detection without a power supply. In addition, optical signal transduction makes an operation in explosive atmospheres possible by eliminating electrical contacts, i.e., the risk of sparks.1 Hydrogen (H2) gas and ammonia (NH3) vapours are currently relevant as targets for gasochromic detectors. They are considered promising energy carriers to foster the decarbonisation of our energy system. However, both gases pose danger to humans due to the high flammability range of H2/air mixtures1 and the toxicity of NH3.2,3 Therefore, any unintended release during the production, transport, or usage of both gases must be immediately detected and the leakage precisely localized.
A gasochromic particle that can be incorporated as an additive into diverse materials and indicate the presence of both gases through rapid, eye-readable, and gas-specific colour changes at every point of use, i.e., a dual-gasochromic particle, has the potential to meet this demand.
Despite the large variety of gasochromic materials available for H2 gas4,5 and NH3 vapours,6–8 respectively, no dual-gasochromic particle for these two species has been reported to the best of our knowledge. It is important to mention that electrical dual-gas detectors for H2 and NH39 or multi-gas detector arrays7,10 were established but not in the form of one flexibly applicable, gasochromic particle. Most materials for visual H2 detection are based on the activation of H2 molecules at catalytically active noble metal surfaces and the subsequent reduction of organic11 or inorganic chromophores.4,12 NH3 vapours are typically detected by impregnating organic pH indicator dyes into porous host materials and exploiting the basic nature of NH3 as a proton acceptor.8 Both types of gasochromic materials incorporate functional chromophores into porous support materials. Recently, we introduced supraparticles (SPs) as a new class of (meso)porous support materials for gasochromic indicators.13 SPs are hierarchical assemblies of nanoparticle (NP) building blocks with a consistent structural motif,14 offering a highly customizable material design.13,15–17 The established gasochromic SPs exploit (ir)reversible colour changes of incorporated Reichardt's betaine dye or resazurin (RES) for the detection of NH3 vapours13 and H2 gas,15–18 respectively. However, the NH3-indicator SPs cannot detect H2, and vice versa.18 Thus, the development of a SP for the visual detection of both gases is still an open challenge.
Herein, we report a pioneering example of a dual-gasochromic SP for H2 gas and NH3 vapours. Aiming for such a SP, we elaborated a suitable material design that involves the selection of appropriate building blocks and the adjustment of the chemical microenvironment within the solid–liquid–gas interface, provided by the porous framework of the SP. We found that a suitable dye species must undergo distinguishable colour change reactions upon reduction and deprotonation, respectively. Furthermore, we demonstrate that a suitable microenvironment can be created by spray-drying of a mixed dispersion containing SiO2 NPs, Pt NPs, the chosen dye molecules, and an acid (H2SO4), functioning as an additional key component. The resulting SPs achieve the targeted functionality through the interaction of H2 with the incorporated Pt NPs and NH3 with the acidic liquid phase within the gas-accessible pores of the SP. Both reactions at the solid–gas and liquid–gas interface induce a gas-specific colour change of the comprised dye molecules via reduction or deprotonation, respectively. From a broader perspective, the confined space within such mesoporous SPs provides a reaction environment similar to solution chemistry with the following advantages: exotic precursor combinations can be united almost independently of their surface-chemistry using forced assembly. The resulting SP powders can be easily incorporated as active material into sensor devices19 or as pigment in coatings and clothing.18
The first step to create a dual-gasochromic SP for H2 gas and NH3 vapours was the identification of suitable chromophore systems and reaction conditions. Therefore, we probed the colour response of various indicator dye solutions by mixing them with NH3 solution or H2 gas (Fig. S1, ESI†). The main findings can be summarized as follows: Pt NPs with a size of roughly 5–10 nm (dynamic light scattering, DLS, Fig. S2, ESI†) are required for the H2-induced reduction of dye molecules by providing active hydrogen at their surface.11 Many dyes require an acid (e.g., the presence of H2SO4) to realize a strong colour change upon reaction with NH3 solution. An indicator dye species suitable for the envisaged SPs must undergo distinguishable colour responses upon deprotonation, induced by an increase in the pH value of its surrounding medium, and additionally also due to the reduction by active hydrogen. Among all tested indicator dyes (see ESI†), resazurin (RES) and methyl red (MR) are most suitable for the visual indication of both H2 and NH3.
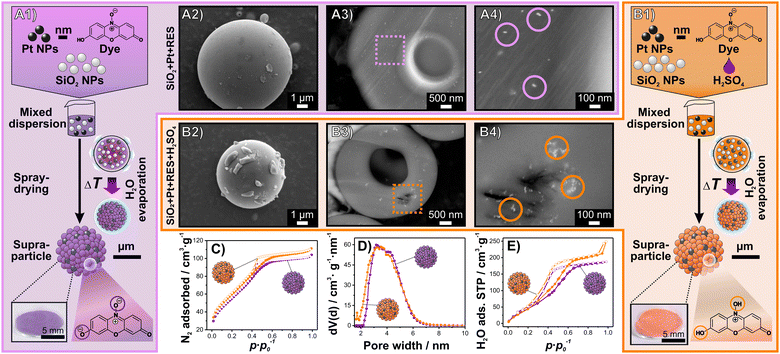
In the second step, we created a SP that provides the required chemical microenvironment by forced assembly of the identified key elements, i.e., Pt NPs, dye molecules, H2SO4, and framework-building SiO2 NPs (≈5–15 nm, DLS, Fig. S3, ESI†) via spray-drying (Fig. 1A1 and A2). In this process, the different building blocks are united in a mixed dispersion, fed through a nozzle and atomized into small droplets. These droplets enter a hot chamber, which induces the evaporation of the solvent thereby forcing the assembly of all components at the liquid–gas interface, yielding a SP entity. Spray-drying is a continuous, high-throughput and industrially established process, which makes its scalability possible.20 Herein, we achieved a SP production capacity of 12 g h−1 using a lab-scale spray dryer.
For a proof-of-concept study, we exemplary used RES as an indicator dye as it is well studied by others11 and us.15–18 We focused in our study on how the addition of H2SO4 to the mixed dispersion prior to spray-drying influences the morphology, structure, texture and functionality of the resulting SPs. The mixed dispersion of the reference SP system containing SiO2 NPs, Pt NPs and RES had a pH ≈ 10 due to the NaOH-stabilisation of the SiO2 NP dispersion. The other mixed dispersion had a pH ≈ 2 due to the addition of H2SO4. The performed spray-drying processes of the two mixed dispersions yielded homogenous, free-flowing powders with a purple and orange colour, respectively (Fig. 1A1 and B1). These colours correspond to the deprotonated (purple) and protonated species of RES (RES-H+, orange), respectively,21 matching the colours and UV-vis spectra of the aqueous solutions of RES (Fig. S1, ESI†). Scanning electron microscopy (SEM) imaging (Fig. 1A2 and B2) and laser diffraction measurements (Fig. S4, ESI†) revealed that neither the morphology nor the size distribution of the SPs are significantly altered by the addition of H2SO4. Both types of SPs show a spherical or doughnut-like morphology and a size distribution ranging from ≈2 to ≈8 μm. As the Pt NPs are essential for the detection of H2,16 we studied their distribution within the SP framework via SEM imaging of SP cross-sections using back-scattered electron detecting to obtain elemental contrast. Without the addition of H2SO4, the Pt NPs are well dispersed throughout the entire SP (Fig. 1A3) and partially form small agglomerates (Fig. 1A4). In contrast, the addition of H2SO4 to the mixed dispersion results in the formation of larger Pt NPs agglomerates within the SP that, in turn, are distributed throughout the entire SP (Fig. 1B3 and B4). The enhanced agglomeration of the citric acid stabilised Pt NPs is attributed to the H2SO4-induced destabilisation of the colloidal dispersion.22
Similarly, the interstitial pores between the assembled NPs of the SP framework are essential for the targeted functionality.15 The mesopores are intended to provide the required micro-environment within the solid–liquid–gas interface that consists of solid NPs, liquid pore water that is adsorbed in humid atmospheres15 and the surrounding gas atmosphere. Therefore, we investigate the effect of the addition of H2SO4 to the mixed dispersion prior to spray-drying on the textural properties and the water adsorption capacity of the resulting SPs. Details of the conducted N2 ad-/desorption at 77 K and H2O ad-/desorption measurements at 298 K are provided in the ESI† (Fig. S5), while the main findings can be summarized as follows: both SPs are mesoporous, which is indicated by their almost identically shaped type IV N2 isotherms (Fig. 1C).23 Their non-local density functional theory (NLDFT) pore size distribution is narrow (≈2–6 nm) with minor differences likely associated with the colloidal destabilization induced by H2SO4 (Fig. 1D). Additionally, the presence of H2SO4 in the SPs increases the total water adsorption capacity and causes a small shift of the relative pressure range at which water condensation occurs to smaller values (Fig. 1E). We attribute these changes mainly to the hygroscopic nature of H2SO4.24
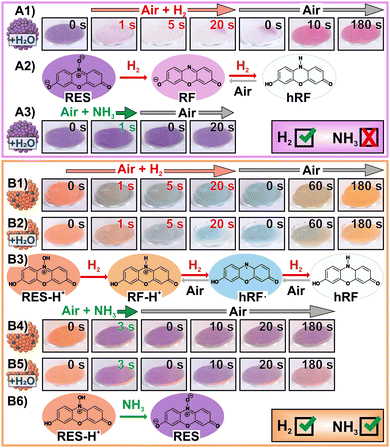
Next, we probed the gasochromic functionality of the two SP powder samples by exposing the SP powders to H2 gas for ≈ 20 s and NH3 vapours for ≈3 s, respectively – optionally, also after ex situ H2O dosing in a climate chamber (1 h, 30 °C, 98% r.h.) to increase the amount of water in the pores.15 The SP powder without H2SO4 showed the previously reported rapid, eye-readable, two-step (ir)reversible colour change upon H2 dosing (Fig. 2A1, after ex situ H2O dosing).15–17 First exposure to H2 leads to the irreversible reduction of deprotonated RES (purple) to deprotonated resorufin (RF, pink), followed by a reversible reduction to hydroresorufin (hRF, colourless) upon further H2 dosing (Fig. 2A2). The condensed water in the mesopores grants the mobility of the incorporated dye molecules. This dye mobility enables their migration to the surface of the Pt NPs, where they can be reduced by active hydrogen when H2 gas is present.15 SP powder without ex situ H2O dosing did not show a complete conversion to colourless hRF due to a lower dye mobility (Fig. S6, ESI†). However, as the RES molecules in SPs without H2SO4 are deprotonated, no colour change is observed upon dosing NH3 vapours (Fig. 2A3).
In contrast, SP powder containing Pt NPs, SiO2 NPs, RES and H2SO4 showed rapid, eye-readable, gas-specific colour change reactions upon exposure to H2 or NH3 vapours (Fig. 2B, more snapshots provided in Fig. S7, ESI†). First reaction with H2 irreversibly reduced the orange RES-H+ to the slightly brighter RF-H+ (Fig. 2B1–B3). This is confirmed by distinct changes in their absorption and fluorescence spectra (Fig. S8, ESI†). Further H2 dosing led to blue powder due to the formation of a scarcely reported stable radical state of hRF (hRF, Fig. 2B3).21 When the dye mobility was high enough, hRF· was further reduced by H2 dosing to colourless hRF (Fig. 2B2). After stopping H2 dosing, the colourless SP powder (hRF) turned blue (hRF), before reaching a final orange colour (RF-H+). It is important to mention that the colouration of these SPs occurred much slower (up to 180 s) compared to their reduction (≈5–10 s) and the colouration of SPs without H2SO4 (≈5–10 s). We attribute this slow reversibility to the stability of the blue hRF· radical in air.
In contrast to H2 dosing, NH3 vapours induced a rapid, visual colour change of SPs containing H2SO4 from orange to purple (Fig. 2B4 and B5) due to deprotonation of RES-H+ to RES (Fig. 2B6). This reaction is caused by a pH increase of the liquid phase in the mesopores of the SPs that is likely induced by the adsorption and dissolution of gaseous NH3 species. The proposed mechanisms are supported by a control SP sample containing SiO2 NPs, RES, H2SO4 but no Pt NPs, which showed no response to H2 but an identical colour change to NH3 dosing (Fig. S9, ESI†). The NH3-induced colour change of the SP powders is partially irreversible, as the initially orange colour related to RES-H+ was never fully recovered (Fig. 2B4 and B5). Comparing the reversibility of the colour change upon repeated NH3/air dosing revealed a slightly higher degree of reversibility for SPs after ex situ H2O dosing compared to pristine SPs (Fig. S10, ESI†). The enhanced amount of water after H2O dosing increases the amount of NH3 required to alter the pH of the liquid phase in the mesopores.
Besides testing separate H2 and NH3 dosing, the SPs containing Pt NPs, SiO2 NPs, RES and H2SO4 were subjected to simultaneous exposure to both target species. Details of this investigation are found in the ESI† (Fig. S11), while the main finding of this investigation can be summarized as follows: NH3 poisons the incorporated Pt NPs,25 which results in impaired H2-induced reduction of the dye species, when both NH3 and H2 are present. Therefore, upon dosing of H2 and NH3, either pink RF is formed, when H2 reacts with the SPs first, or deprotonated purple RES, when the SPs react first with NH3.
Next, we studied the adjustability of the conceptualised SPs and synthesized SPs containing MR as indicator dye. SPs with MR also showed eye-readable, gas-specific colour change reactions upon exposure to H2 gas or NH3 vapours (Fig. S12, ESI†). Preliminary quantitative in situ UV-vis measurements (Fig. S13, ESI†) demonstrate that dual-gasochromic SPs carrying RES or MR also undergo strong colour changes when they are exposed to ≈2 vol% H2 in N2 (i.e., below the lower flammability limit of 4 vol%1) or ≈ 620 ppm NH3 in N2 (i.e., below the limit at which human health issues occur2,3).
In conclusion, we synthesised and characterised the first dual-gasochromic SPs that undergo rapid, eye-readable, gas-specific colour changes upon reaction with H2 gas or NH3 vapours. We revealed the parameters that affect the visual response and characteristics of the SPs. We demonstrated that only SPs assembled from SiO2 NPs, Pt NPs, suitable dye species (RES or MR) and H2SO4, achieve the desired functionality. Thereby, the addition of H2SO4 caused just minor changes to the morphology and texture of the SPs but improved the water adsorption capability and ultimately made the desired two gas-specific colour changes via scarcely reported reactions possible. The functionality of the SPs results from the synergistic interplay of all four types of building blocks within the solid–liquid–gas interface of their mesoporous framework (Fig. S14, ESI†). This interplay includes the adsorption and condensation of water in the mesopores, the dissociation of H2 at the surface of Pt NPs and the mobility of incorporated indicator dye molecules in the liquid phase. The later move through and react with the surface of the solid framework, e.g., via hydrogenation. Furthermore, we herein demonstrated the possibility of adjusting the pH of the liquid phase in the mesopores during SP synthesis via H2SO4 addition as well as afterward through the interaction with NH3 vapours. The dual-gasochromic SPs are therefore a pioneering example for the emergence of new functionalities by exploiting the customisation of the solid–liquid–gas interface within the mesopores of a micron-sized SP.
The contribution of all authors according to the CRediT system is listed in the ESI.† All authors have approved the final version of the manuscript.
This work was financially supported by the BMBF (NanoMatFutur grant 03XP0149 and project IDcycLIB 03XP0393C). J. R. acknowledges his scholarship funding from the German Federal Environmental Foundation (DBU). The authors thank BÜCHI Labortechnik AG for providing the spray dryer equipment.
Conflicts of interest
There are no conflicts to declare.Notes and references
- T. Hübert,
et al.
, Sens. Actuators, B, 2011, 157, 329 CrossRef
.
- J. Pauluhn, Regul. Toxicol. Pharmacol., 2013, 66, 315 CrossRef CAS PubMed
.
- L. Silverman,
et al.
, J. Ind. Hyg. Toxicol., 1949, 31, 74 CAS
.
- Y.-A. Lee,
et al.
, Sens. Actuators, B, 2017, 238, 111 CrossRef CAS
.
-
(a) H. G. Girma,
et al.
, ACS Sens., 2023, 8, 3004 CrossRef CAS PubMed
; (b) X. Sun, et al. , Appl. Surf. Sci., 2022, 599, 153878 CrossRef CAS
.
- S. Sutthasupa,
et al.
, Food Chem., 2021, 362, 130151 CrossRef CAS PubMed
.
- L. Engel,
et al.
, Sens. Actuators, B, 2021, 330, 129281 CrossRef CAS
.
- A. T. Hoang,
et al.
, Sens. Actuators, B, 2016, 230, 250 CrossRef CAS
.
-
(a) L. Du,
et al.
, Sens. Actuators, B, 2023, 375, 132873 CrossRef CAS
; (b) D. D. Roy, et al. , Int. J. Hydrogen Energy, 2023, 48, 4931 CrossRef CAS
.
-
(a) S. H. Lim,
et al.
, Nat. Chem., 2009, 1, 562 CrossRef CAS PubMed
; (b) J. Chen, et al. , ACS Nano, 2018, 12, 6079 CrossRef CAS PubMed
; (c) J. Zhang, et al. , Sens. Actuators, B, 2021, 326, 128822 CrossRef CAS
; (d) Y. Zhang and L.-T. Lim, Sens. Actuators, B, 2018, 255, 3216 CrossRef CAS
.
- M. E. Smith,
et al.
, Anal. Chem., 2020, 92, 10651 CrossRef CAS PubMed
.
- S. Hwan Cho,
et al.
, Chem. Eng. J., 2022, 446, 136862 CrossRef CAS
.
- S. Wintzheimer,
et al.
, Part. Part. Syst. Charact., 2019, 36, 1900254 CrossRef CAS
.
-
(a) S. Wintzheimer,
et al.
, ACS Nano, 2018, 12, 5093 CrossRef CAS PubMed
; (b) J. Reichstein, et al. , Adv. Mater., 2023, 35, e2306728 CrossRef PubMed
.
- J. Reichstein,
et al.
, Adv. Funct. Mater., 2022, 32, 2112379 CrossRef CAS
.
- K. Zhang,
et al.
, Chem. Mater., 2023, 35, 6808 CrossRef CAS
.
- K. Zhang,
et al.
, J. Chem. Phys., 2023, 158, 134722 CrossRef CAS PubMed
.
- J. Reichstein,
et al.
, Adv. Mater. Technol., 2024, 2400441 CrossRef
.
- C. Pannek,
et al.
, Sens. Actuators, B, 2020, 306, 127572 CrossRef CAS
.
-
(a) D. P. Debecker,
et al.
, Chem. Soc. Rev., 2018, 47, 4112 RSC
; (b) S. Wintzheimer, et al. , Adv. Mater., 2023, 35, e2306648 CrossRef PubMed
.
- S. Khazalpour and D. Nematollahi, RSC Adv., 2014, 4, 8431 RSC
.
- G. Marzun,
et al.
, Langmuir, 2014, 30, 11928 CrossRef CAS PubMed
.
- M. Thommes,
et al.
, Pure Appl. Chem., 2015, 87, 1051 CrossRef CAS
.
- K. B. Kiradjiev,
et al.
, Ind. Eng. Chem. Res., 2020, 59, 4802 CrossRef CAS
.
- D. A. Finkelstein,
et al.
, J. Phys. Chem. C, 2015, 119, 9860 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of experimental section, material characterization. See DOI: https://doi.org/10.1039/d4cc01247j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |