 Open Access Article
Open Access ArticleIterative click reactions using trivalent platforms for sequential molecular assembly†‡
Gaku
Orimoto
and
Suguru
Yoshida
 *
*
Department of Biological Science and Technology, Faculty of Advanced Engineering, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku, Tokyo 125-8585, Japan. E-mail: s-yoshida@rs.tus.ac.jp
First published on 9th May 2024
Abstract
A facile synthesis of multi(triazole)s by iterative click reactions is disclosed. Good functional group tolerance of sequential click assembly by sulfur–fluoride exchange (SuFEx), copper-catalyzed azide–alkyne cycloaddition (CuAAC), and thia-Michael reaction realizes the iterative click reactions. Diverse multi(triazole)-type mid-molecules can be synthesized easily from readily available modules through good chemoselective reactions without functional group transformation steps.
Iterative ligation methods have played significant roles in synthesizing diverse functional molecules including peptides and nucleic acids from simple modules in broad disciplines such as materials chemistry, pharmaceutical sciences, and chemical biology. For example, peptide synthesis is realized by iterative methods through the conjugation of protected amino acids and deprotection (Fig. 1A), which are fundamental ways to prepare bioactive mid-molecules.1 Herein, we disclose an efficient method to synthesize mid-molecules from simple modules by iterative click reactions onto trivalent platform molecules without deprotection steps.
Click reactions are gaining attention from a wide range of researchers for reliable molecular conjugation.2 We have developed a variety of trivalent platforms for efficient molecular assembly by sequential click reactions.3–5 In particular, we recently succeeded in the synthesis of trivalent platform 1a that served in the sequential click conjugation by the SuFEx reaction,6 copper-catalyzed azide–alkyne cycloaddition (CuAAC),7 and thia-Michael reaction,8 where the triazole formation remarkably enhanced the Michael reactivity of acrylamides (Fig. 1B).4c,4e It is worth noting that we succeeded in the one-pot assembly of modules onto trivalent platform 1a.4e With the orthogonality of the click reactions in mind, we conceived an idea that iterative click reactions can be accomplished by the SuFEx reaction, CuAAC, and thia-Michael reaction using linkers having two clickable groups such as an alkyne and a silyl ether moiety. The robust reliability of click reactions will allow us to synthesize diverse multi(triazole)s from simple starting materials. In this study, we synthesized various trivalent platforms bearing azide, alkene, and fluorosulfonyl groups, and divergent triazoles with a variety of functionalities (Fig. 1C, upper). On the basis of good functional group tolerance in sequential conjugations, we then developed an iterative click reaction which enabled us to synthesize tris(triazole)s from simple modules (Fig. 1C, lower).
We first synthesized a range of acrylamides 1b–1e bearing fluorosulfonyl groups from 2-azidoacrylic acid (Fig. 2). Condensation of 2-azidoacrylic acid (6) with amines 7 having an N-Boc amide group followed by deprotection of the Boc group and subsequent addition to ethenesulfonyl fluoride (8) took place smoothly to afford trivalent platforms 1b–1e leaving azide, alkene, fluorosulfonyl, secondary or tertiary amide, and iodo moieties untouched. Of note, a broad range of amines such as not only primary and secondary alkyl amines but also aromatic amines participated in the acrylamide synthesis without damaging electrophilic alkene moieties by the undesired Michael addition.
 | ||
Fig. 2 Syntheses of trivalent platforms 1. Yields for 1st, 2nd, and 3rd step were shown. See the ESI‡ for details. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Condensation was conducted with chloro-N,N,N′,N′-tetramethylformamidinium hexafluorophosphate and N-methylimidazole in MeCN at rt. PyBOP = ((1H-Benzo[d][1,2,3]triazol-1-yl)oxy)tri(pyrrolidin-1-yl)phosphonium hexafluorophosphate. Condensation was conducted with chloro-N,N,N′,N′-tetramethylformamidinium hexafluorophosphate and N-methylimidazole in MeCN at rt. PyBOP = ((1H-Benzo[d][1,2,3]triazol-1-yl)oxy)tri(pyrrolidin-1-yl)phosphonium hexafluorophosphate. | ||
A wide variety of highly functionalized modules 2–4 were conjugated using trivalent platform 1a by sequential click assembly (Fig. 3). In the SuFEx reaction of 1a as the first step, various aromatic alcohols reacted selectively at the fluorosulfonyl group to afford sulfonyl esters 9b–9j in high yields keeping reactive functionalities (Fig. 3A and B). It is worth noting that the SuFEx reaction proceeded with excellent chemoselectivities at the aromatic hydroxy group in the presence of other nucleophilic groups such as aliphatic alcohol, amide, or amine moieties, in which undesired Michael addition to the remaining acrylamide moiety was not observed. We succeeded in the synthesis of heteroaromatic sulfonyl esters 9i and 9j from 5-quinolinol or 2-pyridone keeping 2-azidoacrylamide moiety intact. In addition, perfluoroalcohol was efficiently conjugated to trivalent platform 1a, which will serve as a perfluoroalkyl tag moiety.9 The synthesis of alkyl sulfonate 9k also suggests great potential for applications involving ligand-directed labeling,10 activity-based proteome analyses,11 and covalent drug discovery12 based on the scission of sulfonate groups.
 | ||
Fig. 3 Sequential click assembly using platform 1a. See the ESI‡ for details. (A) General scheme. (B) SuFEx reaction of 1a. (C) Triazole formation of 9a. (D) Michael addition to 10a. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF was used as a solvent instead of CH2Cl2. b DMF was used as a solvent instead of CH2Cl2. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The reaction of 9a with 11 was performed in CH2Cl2 at rt. c The reaction of 9a with 11 was performed in CH2Cl2 at rt. c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The reaction of 10a with n-butanol and Cs2CO3 in THF was performed at rt. d The reaction of 10a with n-butanol and Cs2CO3 in THF was performed at rt. d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The reaction of 10a with diethyl malonate and t-BuOK in THF was performed at rt. TBTA = tris(benzyltriazolylmethyl)amine. The reaction of 10a with diethyl malonate and t-BuOK in THF was performed at rt. TBTA = tris(benzyltriazolylmethyl)amine. | ||
The second step by the CuAAC reaction using diverse alkynes allowed us to prepare triazoles 10b–10j bearing various functionalities in good yields, in which we chose 9a as a model substrate to simplify the analysis of products (Fig. 3A and C). In particular, 2-(triazolyl)acrylamide moieties were not damaged in the presence of reactive functionalities such as hydroxy, pyridyl, iodo, triflyloxy, formyl, chloro, and amide groups in the products under the Lewis-acidic conditions. It is worthy to note that a wide variety of biochemically significant moieties such as uridine, HaloTag ligand, biotin, and fluorescent sulforhodamine were efficiently introduced by the CuAAC reaction leaving the alkenyl group intact. Strain-promoted azide–alkyne cycloaddition of 9a with cycloalkyne 11 took place smoothly to provide triazole 10k in high yield.13
A range of nucleophiles 4 were successfully applied to the Michael additions of the resulting 2-(triazolyl)acrylamides 10a, which was selected as a model substrate to simplify the characterization of products (Fig. 3A and D). For example, aromatic and aliphatic thiols efficiently reacted at the remaining alkene moiety to provide sulfides 5b–5d by the thia-Michael reaction without damaging amide, ester, and fluorescent dansyl moieties. Not only the primary amino group of lysine methyl ester but also the endocyclic nitrogen atoms of adenine or cytosine served in the efficient Michael addition furnishing amines 5e–5g in good yields.14 Additionally, n-butanol and dimethyl malonate were also applicable in the Michael reaction under basic conditions, in which 2-(triazolyl)amide and alkoxysulfonyl groups remained unreacted. These results obviously demonstrate that a broad range of triazoles can be synthesized by assembling various starting materials having diverse reactive functional groups onto trivalent platform 1 in a modular synthetic manner.
The good functional group tolerance of the sequential click assembly realized an iterative conjugation using linkers 12 bearing ethynyl and tert-butyl(dimethyl)silyloxy groups by repeating CuAAC and SuFEx reactions (Fig. 4A). Indeed, we accomplished the selective synthesis of triazole 13a from azide 9a leaving the remaining acrylamide and silyloxy group untouched. Also, the CuAAC reaction allowed us to synthesize triazoles 13b and 13c selectively using linkers 12b and 12c with ethynyl and aromatic hydroxy groups. Since the silyloxy group survived in thia-Michael reaction of thiol 4a to triazole 13a, we succeeded in the facile preparation of triazole 14 in excellent yield by further SuFEx conjugation with trivalent platform 1a (Fig. 4B).
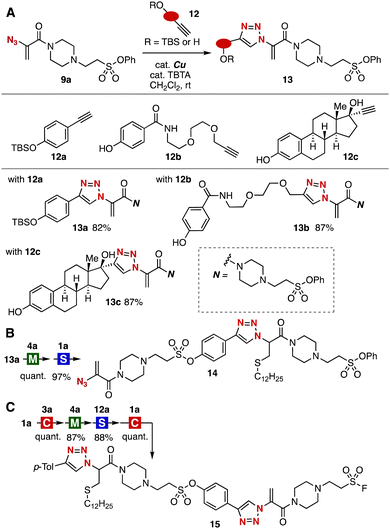 | ||
| Fig. 4 (A) Synthesis of various triazoles 13. (B) Synthesis of triazole 14. (C) Synthesis of bis(triazole) 15. Reaction conditions; M: NEt3, CH2Cl2, rt; S: DBU, CH2Cl2, rt; C: cat. (MeCN)4CuBF4, cat. TBTA, CH2Cl2, rt; see the ESI‡ for details. | ||
We found that the order of the sequential conjugation using trivalent platform 1a was switchable (Fig. 4C). For instance, after the CuAAC and thia-Michael addition of 1a with alkyne 3a and thiol 4a, respectively, keeping the fluorosulfonyl group unreacted, the SuFEx reaction with linker 12a took place smoothly without damaging the ethynyl group. The resulting alkyne reacted efficiently with trivalent platform 1a to afford bis(triazole) 15 in high yields leaving the exomethylene and fluorosulfonyl group untouched. Due to the great potential of sulfonyl fluorides in the proximity-promoted conjugation, mid-molecules having fluorosulfonyl groups will gain attention from researchers in pharmaceutical sciences.15
The synthesis of tris(triazole) 16 was achieved by the iterative click reactions resulting in the elongation of the main chain and assembly of modules 4k and 4l (Fig. 5). Indeed, the triazole formation using azide 14 with linker 12a followed by the thia-Michael reaction of thiol 4k and the SuFEx reaction with trivalent platform 1a was realized without side reactions such as undesired elimination by the retro-Michael reaction or decomposition of sulfonic acid ester moieties. Then, CuAAC with linker 12a and Michael addition of amine 4l proceeded smoothly to provide tris(triazole) 16 in high yields. Thus, we succeeded in the synthesis of tris(triazole) 16 by the iterative click reactions using thiols 4a and 4k, amine 4l, linker 12a, and trivalent platform 1a in a modular synthetic manner. This efficient chain elongation, as well as significant functional group tolerance of click reactions clarified in Fig. 3, obviously demonstrates that the iterative click reaction enables us to synthesize diverse multi(triazole)s, which are of great importance as functionalized peptide bioisosteres.16
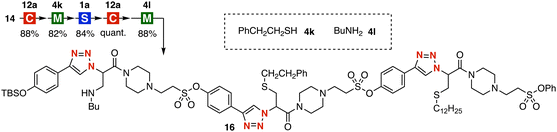 | ||
| Fig. 5 Synthesis of tris(triazole) 16. Reaction conditions; M: NEt3, CH2Cl2, rt; S: DBU, CH2Cl2, rt; C: cat. (MeCN)4CuBF4, cat. TBTA, CH2Cl2, rt; see the ESI‡ for details. | ||
In conclusion, we demonstrated the excellent functional group tolerance of the sequential click assembly by SuFEx, CuAAC, and thia-Michael reaction. Based on the chemoselective molecular conjugation methods, facile synthesis of multi(triazole)s has been developed by iterative click reactions without functional group transformation steps. Due to the wide scope of click reactions and good accessibility of modules, a huge chemical library of multi(triazole)-type mid-molecules will be constructed in a modular synthetic manner. Further studies such as one-pot click assembly of functional molecules onto trivalent platforms and applications to bioassay using diverse mid-molecules are ongoing in our laboratory.
This work was supported by JSPS KAKENHI Grant Number JP23K179200 (S.Y.) and Asahi Glass Foundation (S.Y.).
Conflicts of interest
There are no conflicts to declare.Notes and references
- For reviews of peptide synthesis, see: J. M. Palomo, RSC Adv., 2014, 4, 32658 RSC.
- J. Lahann, Click Chemistry for Biotechnology and Materials Science, John Wiley & Sons, West Sussex, 2009 Search PubMed.
- For reviews of sequential click reactions, see: (a) A.-C. Knall and C. Slugovc, Chem. Soc. Rev., 2013, 42, 5131 RSC; (b) Z.-J. Zheng, D. Wang, Z. Xu and L.-W. Xu, Beilstein J. Org. Chem., 2015, 11, 2557 CrossRef CAS PubMed; (c) S. Yoshida, Org. Biomol. Chem., 2020, 18, 1550 RSC; (d) D. Sato, Z. Wu, H. Fujita and J. S. Lindsey, Organics, 2021, 2, 161 CrossRef CAS; (e) M. S. Yadav, S. Rajkhowa, S. K. Singh, M. K. Jaiswal and V. K. Tiwari, ChemistrySelect, 2024, 9, e202400776 CrossRef CAS.
- For recent our studies on sequential click reactions using tri- or tetravalent platforms, see: (a) T. Meguro, Y. Sakata, T. Morita, T. Hosoya and S. Yoshida, Chem. Commun., 2020, 56, 4720 RSC; (b) N. Terashima, Y. Sakata, T. Meguro, T. Hosoya and S. Yoshida, Chem. Commun., 2020, 56, 14003 RSC; (c) H. Takemura, S. Goto, T. Hosoya and S. Yoshida, Chem. Commun., 2020, 56, 15541 RSC; (d) S. Yoshida, Y. Sakata, Y. Misawa, T. Morita, T. Kuribara, H. Ito, Y. Koike, I. Kii and T. Hosoya, Chem. Commun., 2021, 57, 899 RSC; (e) H. Takemura, G. Orimoto, A. Kobayashi, T. Hosoya and S. Yoshida, Org. Biomol. Chem., 2022, 20, 6007 RSC.
- For recent examples of sequential click reactions using trivalent platforms, see: (a) V. Vaněk, J. Pícha, B. Fabre, M. Buděšínský, M. Lepšík and J. Jiráček, Eur. J. Org. Chem., 2015, 3689 CrossRef; (b) B. Fabre, J. Pícha, V. Vaněk, M. Buděšínský and J. Jiráček, Molecules, 2015, 20, 19310 CrossRef CAS PubMed; (c) B. Fabre, J. Pícha, V. Vaněk, I. Selicharová, M. Chrudinová, M. Collinsová, L. Žáková, M. Buděšínský and J. Jiráček, ACS Comb. Sci., 2016, 18, 710 CrossRef CAS PubMed; (d) A.-C. Knall, M. Hollauf, R. Saf and C. Slugovc, Org. Biomol. Chem., 2016, 14, 10576 RSC; (e) T. Yokoi, H. Tanimoto, T. Ueda, T. Morimoto and K. Kakiuchi, J. Org. Chem., 2018, 83, 12103 CrossRef CAS PubMed; (f) T. Yokoi, T. Ueda, H. Tanimoto, T. Morimoto and K. Kakiuchi, Chem. Commun., 2019, 55, 1891 RSC; (g) K. Maegawa, H. Tanimoto, S. Onishi, T. Tomohiro, T. Morimoto and K. Kakiuchi, Org. Chem. Front., 2021, 8, 5793 RSC; (h) S. Sun, J. A. Homer, C. J. Smedley, Q.-Q. Cheng, K. B. Sharpless and J. E. Moses, Chem, 2023, 9, 2128 CrossRef CAS.
- For reviews of the SuFEx chemistry, see: (a) J. Dong, L. Krasnova, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2014, 53, 9430 CrossRef CAS PubMed; (b) A. S. Barrow, C. J. Smedley, Q. Zheng, S. Li, J. Dongd and J. E. Moses, Chem. Soc. Rev., 2019, 48, 4731 RSC; (c) Y.-P. Meng, S.-M. Wang, W.-Y. Fang, Z.-Z. Xie, J. Leng, H. Alsulami and H.-L. Qin, Synthesis, 2020, 673 CAS.
- (a) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef PubMed; (b) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; (c) T. R. Chan, R. Hilgraf, K. B. Sharpless and V. V. Fokin, Org. Lett., 2004, 6, 2853 CrossRef CAS PubMed; (d) A. K. Agrahari, P. Bose, M. K. Jaiswal, S. Rajkhowa, A. S. Singh, S. Hotha, N. Mishra and V. K. Tiwari, Chem. Rev., 2021, 121, 7638 CrossRef CAS PubMed.
- A. B. Lowe, Polym. Chem., 2014, 5, 4820 RSC.
- G. Park, K.-S. Ko, A. Zakharova and N. L. Pohl, J. Fluorine Chem., 2008, 129, 978 CrossRef CAS PubMed.
- T. Tamura, S. Tsukiji and I. Hamachi, J. Am. Chem. Soc., 2012, 134, 2216 CrossRef CAS PubMed.
- G. C. Adam, B. F. Cravatt and E. J. Sorensen, Cell Chem. Biol., 2001, 8, 81 CAS.
- S. Ray and A. S. Murkin, Biochemistry, 2019, 58, 5234 CrossRef CAS PubMed.
- J. Dommerholt, F. P. J. T. Rutjes and F. L. van Delft, Top. Curr. Chem., 2016, 374, 16 CrossRef PubMed.
- Aza-Michael addition has also gained attention in click chemistry. See: J. C. Worch, C. J. Stubbs, M. J. Price and A. P. Dove, Chem. Rev., 2021, 121, 6744 CrossRef CAS PubMed.
- B. Yang, H. Wu, P. D. Schnier, Y. Liu, J. Liu, N. Wang, W. F. DeGrado and L. Wan, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 11162 CrossRef CAS PubMed.
- (a) N. Agouram, E. M. El Hadrami and A. Bentama, Molecules, 2021, 26, 2937 CrossRef CAS PubMed; (b) A. Staśkiewicz, P. Ledwoń, P. Rovero, A. M. Papini and R. Latajka, Front. Chem., 2021, 9, 674705 CrossRef PubMed.
Footnotes |
| † Dedicated to Professor Koichi Narasaka with gratitude on the occasion of his 80th birthday (Sanju). |
| ‡ Electronic Supplementary Information (ESI) available: Experimental procedures, characterization for new compounds including NMR spectra. See DOI: https://doi.org/10.1039/d4cc01177e |
| This journal is © The Royal Society of Chemistry 2024 |

