Open Access Article
Open Access ArticleSolvent-free synthesis and chiroptical properties of a C–N axially chiral cruciform dimer of benzo[b]phenoxazine†
Shuhei
Ishikawa
a,
Daisuke
Sakamaki
 *a,
Masayuki
Gon
*a,
Masayuki
Gon
 b,
Kazuo
Tanaka
b,
Kazuo
Tanaka
 b and
Hideki
Fujiwara
b and
Hideki
Fujiwara
 *a
*a
aDepartment of Chemistry, Graduate School of Science, Osaka Metropolitan University, Sumiyoshi-ku, Osaka 558-8585, Japan. E-mail: sakamaki@omu.ac.jp; hfuji@omu.ac.jp
bDepartment of Polymer Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510, Japan
First published on 12th April 2024
Abstract
A novel C–N axially chiral molecule composed of two tert-butyl-substituted benzo[b]phenoxazine (BPO) was synthesized via solvent-free reactions. The absolute configurations of the enantiomers were determined by X-ray single-crystal analysis. The enantiomers had a sufficiently high racemization barrier to ignore racemization at room temperature (149 ± 20 kJ mol−1), and the solutions exhibited dual circularly polarized emissions stemming from fluorescence and phosphorescence of |gCPL| = ca. 1 × 10−3.
In recent years, heteroatom-embedded polyaromatic hydrocarbons (PAHs) have attracted increasing interest for use in functional organic materials, such as luminescent materials for organic light-emitting diodes (OLEDs).1,2 Heteroacenes with amino nitrogen atoms, such as benzo[b]phenoxazine (BPO) and benzo[b]phenothiazine (BPT), are not only utilized in dye-sensitized solar cells3,4 owing to their good electron-donating and photophysical properties but are also gaining renewed interest as organic photoredox catalysts.5–8 In 2015, we developed a straightforward method for the synthesis of double heterohelicenes, which comprised two heteroacenes with an NH group, such as 6,13-dihydro-6,13-diazapentacene (DHDAP),9BPO,10 dibenzo[b,i]phenoxazine (DBPO),9,10 phenothiazine,11BPT,12 and phenoselenazine.13 The key intermediate compounds in this process are the cruciform dimers formed via oxidative C–N coupling between the two azaacenes (Fig. 1). Oxidative C–N coupling between symmetric azaacenes, such as DBPO and DHDAP, gives achiral cruciform dimers with a mirror plane in the molecule (Fig. 1a). In contrast, oxidative C–N coupling between asymmetric azaacenes, such as BPO, BPT, and 5,12-dihydro-5,12-diazatetracene (DHDAT),14 gives chiral cruciform dimers with C–N axial chirality (Fig. 1b). Although there have been numerous examples of axially chiral C–C molecules represented by biaryl compounds,15 including circularly polarized luminescence (CPL) emitters,16–19 C–N axially chiral molecules have been less explored, probably because of the lower rotational barrier around the C–N bond compared to that around the C–C bond.20 Among the axially chiral cruciform dimers of azaacenes, we hypothesize that the dimer of BPO (1) could be an interesting material because BPO is easily obtained on the gram scale by solid-state condensation of cheap compounds (2-aminophenol and 2,3-dihydroxynaphthalene). However, the optical resolution of 1 is difficult because of its low solubility, and the properties of its enantiomers remain unknown. In addition, the dimerization reaction of BPO requires a large amount of solvent owing to the low solubility of BPO. In this study, we designed a novel cruciform dimer 2, which is a tert-butyl-substituted analogue of 1, to achieve optical resolution and investigate the properties of the enantiomers by increasing their solubility. Furthermore, we aimed to synthesize 2 using mechanochemical oxidative coupling between BPO substituted with a tert-butyl group at the 2-position (tBuBPO) to achieve completely solvent-free synthesis of 2 from commercially available inexpensive chemicals.
 | ||
| Fig. 1 (a) Achiral cruciform dimer formed by dimerization of symmetric azaacenes and (b) chiral cruciform dimers formed by dimerization of asymmetric azaacenes. | ||
Scheme 1 shows the synthesis of rac-2. tBuBPO was synthesized via a solid-state condensation reaction between 2-amino-4-tert-butylphenol and 2,3-dihydroxynaphthalene at 230 °C, which is similar to the synthesis of BPO. Oxidation of tBuBPO with DDQ in dichloromethane afforded dimer rac-2 in 73% yield. The dimerization of tBuBPO was also performed using a solvent-free mechanochemical oxidative coupling.21–23 In a 50 mL stainless-steel jar of a mixer mill, tBuBPO (500 mg, 1.73 mmol) and DDQ (236 mg, 0.6 eq.) were placed with twelve stainless steel balls (10 mm ϕ) and milled at 30 Hz for 30 min. After purification by silica gel chromatography, 389 mg (78% yield) of compound rac-2 was obtained. Rac-2 is highly soluble in common organic solvents, in contrast to the very low solubility of 1. For example, the solubility of rac-2 in dichloromethane is ∼45 mg mL−1, which is nearly 100 times higher than that of 1 (ca. 0.5 mg mL−1).
Recrystallization of rac-2 by slow diffusion of methanol vapor into the toluene solution gave good single crystals suitable for X-ray crystal structure analysis. Fig. 2 shows the crystal structure of rac-2. In 2, two tBuBPO units were almost planar, and the dihedral angle between them (D) was 83°. The unit cell contained one pair of enantiomers, and these molecules formed a centrosymmetric dimer by facing one tBuBPO moiety of 2 (Fig. 2c). In addition to π–π interactions, two intermolecular N–H/O hydrogen bonds (2.16 Å) were observed in the dimer. The enantiomer colored in light blue in Fig. 2c and d is the (R)-enantiomer, and the enantiomer colored in red is the (S)-enantiomer, according to the Cahn–Ingold–Prelog priority rules. However, the labeling of (R)- and (S)- of BPO dimers can be inverted, depending on the substituents on the BPO units (see ESI†). Therefore, for this class of cruciform dimers of azaacenes, we defined the enantiomer that gives (M,M)-double heterohelicenes upon intramolecular C–N bond formation as the (M)-enantiomer and the enantiomer that gives (P,P)-double heterohelicenes as the (P)-enantiomer, such that the configuration of the BPO skeletons and labeling always correspond, regardless of the substituents. According to this rule, the (R)-enantiomer (light blue in Fig. 2c and d) is defined as the (M)-enantiomer and the (S)-enantiomer (red in Fig. 2c and d) is defined as the (P)-enantiomer.
 | ||
| Fig. 2 (a) and (b) ORTEP representations of (M)-2 in the racemic-crystal. (c) and (d) Packing structures of rac-2. Red and light blue in (c) and (d) represent the (P)- and (M)-isomers, respectively. | ||
DFT calculations were performed for model compound of 2, in which the tert-butyl groups were replaced by methyl groups. Structural optimization of model compound 2′ reproduced the molecular structure of 2 in the crystal well. Fig. S4 (ESI†) shows the frontier Kohn–Sham molecular orbitals (MOs) from HOMO−1 to LUMO+1, and their energies. The HOMO and LUMO are localized on different BPO units, similar to the cruciform dimer of 5,12-dihydro-5,12-diazatetracene.14 The HOMO−1 and LUMO are distributed on the BPO unit with an NH group and the HOMO and LUMO+1 are distributed on the other BPO unit. The HOMO–LUMO transition was predicted to be nearly forbidden (f = 0.0001) because of the spatial separation of these MOs. The intramonomer transitions (HOMO−1 to LUMO and HOMO to LUMO+1) were predicted to have higher oscillator strengths.
The photophysical properties of rac-2 and tBuBPO were then compared. The UV-vis absorption spectra of 2 and tBuBPO in dichloromethane were very similar (Fig. S5, ESI†). Both 2 and tBuBPO exhibited strong absorption peaks at approximately 370 nm, and their spectral shapes were similar. The band at 370 nm of tBuBPO was attributed to the HOMO–LUMO transition, and that of 2 was attributed to HOMO−1 to LUMO and HOMO to LUMO+1, according to the TD-DFT calculations. The peak corresponding to the HOMO–LUMO transition of 2 was not observed, as expected from TD-DFT calculations. Fig. 3a shows the emission spectra of 2 and tBuBPO in various solvents at room temperature. Both 2 and tBuBPO exhibited small bathochromic shifts with increasing solvent polarity, and the emission peaks of 2 were slightly red-shifted compared with those of tBuBPO in each solvent. The red-shifted emission of 2 can be explained by the contribution of the HOMO–LUMO transition with an intermonomer transition character to the S1 → S0 transition. The solutions of rac-2 and tBuBPO in all solvents tested exhibited blue emission owing to small solvatochromism, and the emission quantum yields of 2 ranged from 40% (in THF) to 17% (in dichloromethane), comparable to those of tBuBPO (Fig. 3b).
We measured the temperature dependence of the emission spectra of rac-2 and tBuBPO in 2-MeTHF upon cooling from room temperature (Fig. S6, ESI†). 2 exhibited broad emission band at approximately 410 nm at 293 K. Upon cooling from 293 K, vibronic patterns appeared gradually without significant changes in the emission wavelength. Upon cooling below 93 K, new emission bands appeared in the longer-wavelength region at approximately 500 nm (Fig. 3c). The newly appearing emission was attributed to phosphorescence, based on the long afterglow that could be observed in green with the naked eye for approximately 10 s, and the lifetime at 520 nm was 2.8 s (Fig. 3d). The temperature dependence of the emission of 2 was similar to that of tBuBPO; however, the phosphorescence lifetime of 2 was longer than that of tBuBPO (2.2 s).
Optical resolution of the cruciform BPO dimer without tert-butyl groups (1) was not achieved owing to its low solubility in the mobile phase of chiral column chromatography. In contrast, the enantiomers of 2 were successfully separated using a chiral column equipped with high-performance liquid chromatography (HPLC) owing to their increased solubility (Fig. S7, ESI†). Single crystals of each enantiomer suitable for X-ray analysis were successfully obtained. X-ray single crystal analysis revealed that the faster eluting enantiomer was the (M)-isomer, and the slowly eluting enantiomer was the (P)-isomer. In the crystals, each enantiomer formed a dimer by facing the tBuBPO units, similar to rac-2; however, in this case, the two tert-butyl groups of the stacked tBuBPO units were on the same side (Fig. S9, ESI†). Two intermonomer N–H/O hydrogen bonds (2.30 and 2.02 Å) were observed in the dimer.
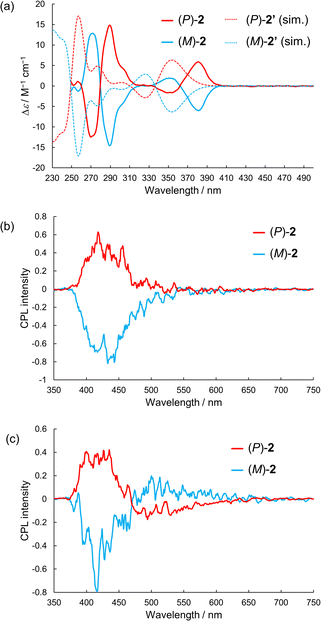
We measured the circular dichroism (CD) spectra of the enantiomers of 2 in dichloromethane. As shown in Fig. 4a, the enantiomers exhibited mirror-image CD spectra. The (P)-enantiomer exhibited the first positive Cotton effect, and the observed spectra were well reproduced by TD-DFT calculations at the PBE0/6-31G(d) level of theory, except for systematic overestimation of the transition energies. Interestingly, the sign of the first Cotton band of (P)-2 was opposite to that of the (P,P)-enantiomer of double hetero[5]helicene (BPO-DH) composed of two BPO obtained by ring fusion of (P)-1;10 that is, the conversion from a cruciform dimer to a double hetero[5]helicene by intramolecular C–N bond formation inverted the signs of the first Cotton band. The |gCD| value of 2 was 1.4 × 10−3 at 385 nm, which is comparable to those of previously reported axially chiral CPL emitters.16,18,19
To evaluate the racemization barrier of 2, we monitored temporal changes in the CD intensity of the enantiomer of 2 in toluene at 92.5, 95, 97.5, and 100 °C. The CD intensity slowly decreased with first-order kinetics (Fig. S10, ESI†) and the rate constants were determined for each temperature. The racemization barrier of 2 was estimated to be 149 ± 20 (standard error) kJ mol−1 from the Arrhenius plot using the obtained rate constants. This value is sufficiently high to ignore racemization at room temperature.
Next, we measured the CPL spectra of the enantiomers of 2. Fig. 4b shows the CPL spectra in dichloromethane at 298 K. Mirror-image CPL spectra were obtained at approximately 420 nm, which can be attributed to fluorescence. The (P)-enantiomer exhibited a positive CPL, which was in accordance with the first Cotton effect of the (P)-enantiomer in the CD spectrum. The experimental |gCPL| value was approximately 6 × 10−4. TD-DFT calculation for the optimized S1 structures reproduced the signs of gCPL (Table S4, ESI†). Interestingly, TD-DFT calculations suggested that the gCPL values could be significantly affected by the dihedral angle between the two tBuBPO units (Table S5, ESI†). The calculated gCPL value of (P)-2 at the optimized S1 geometry (dihedral angle: D = 99.7°) was +2.0 × 10−2; however, the magnitude of gCPL decreased with increasing D. Upon decreasing D, the sign of gCPL was inverted between D = 95° and 92.5°. Next, we measured the CPL spectra of the enantiomers of 2 in frozen solutions of 2-MeTHF at 83 K, because 2 exhibited phosphorescence upon cooling. New CPL peaks at approximately 500 nm, with signs opposite to those of CPL fluorescence at 420 nm at 83 K (Fig. 4c). Based on the emission lifetime of 2.8 s at 520 nm, the newly appeared CPL at 83 K was attributed to phosphorescence. The |gCPL| value of phosphorescence was ca. 1 × 10−3 at 550 nm (Fig. S11, ESI†).
In summary, we synthesized a novel C–N axially chiral compound composed of two tert-butyl-substituted benzo[b]phenoxazines, which can be obtained using only solvent-free reactions from commercially available compounds. The introduction of tert-butyl groups significantly increased the solubility of the cruciform dimer in common organic solvents, and we succeeded in the optical resolution of this class of dimers for the first time. We demonstrated that the enantiomers of 2 have a sufficiently high racemization barrier even when the NH group is not substituted with a bulky substituent. 2 exhibited moderate emission quantum yields in various solvents and long-lived phosphorescence upon cooling. The enantiomers of 2 showed clear CPL at room temperature and dual CPL originating from fluorescence and phosphorescence at liquid-nitrogen temperature. We believe that this study will lead to novel design concepts for chiral emitters and catalysts for enantioselective syntheses that can be easily obtained via solvent-free reactions.
This work was supported by a Grant-in-Aid for Scientific Research (17H04874, 18K05264, 20H02726) from the Japan Society for the Promotion of Science (JSPS), a Grant-in-Aid for Transformative Research Areas (A) “Condensed Conjugation” (JSPS KAKENHI Grant Number JP20H05866) from MEXT, Ogasawara Toshiaki Memorial Foundation, Shorai Foundation for Science and Technology, The Foundation for the Promotion of Ion Engineering, and Izumi Science and Technology Foundation. Computation time for the theoretical calculations was provided by Research Center for Computational Science, Okazaki, Japan.
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777–2781 CrossRef CAS PubMed.
- M. Mamada, M. Hayakawa, J. Ochi and T. Hatakeyama, Chem. Soc. Rev., 2024, 53, 1624–1692 RSC.
- M. Watanabe, H. Hagiwara, A. Iribe, Y. Ogata, K. Shiomi, A. Staykov, S. Ida, K. Tanaka and T. Ishihara, J. Mater. Chem. A, 2014, 2, 12952–12961 RSC.
- A. F. Buene and D. M. Almenningen, J. Mater. Chem., 2021, 9, 11974–11994 CAS.
- S. Dadashi-Silab, X. Pan and K. Matyjaszewski, Chem. – Eur. J., 2017, 23, 5972–5977 CrossRef CAS PubMed.
- F. Seyfert and H.-A. Wagenknecht, Synlett, 2021, 582–586 CAS.
- S. Shibutani, T. Kodo, M. Takeda, K. Nagao, N. Tokunaga, Y. Sasaki and H. Ohmiya, J. Am. Chem. Soc., 2020, 142, 1211–1216 CrossRef CAS PubMed.
- S. Halder, S. Mandal, A. Kundu, B. Mandal and D. Adhikari, J. Am. Chem. Soc., 2023, 145, 22403–22412 CrossRef CAS PubMed.
- D. Sakamaki, D. Kumano, E. Yashima and S. Seki, Angew. Chem., Int. Ed., 2015, 54, 5404–5407 CrossRef CAS PubMed.
- D. Sakamaki, S. Tanaka, K. Tanaka, M. Takino, M. Gon, K. Tanaka, T. Hirose, D. Hirobe, H. Yamamoto and H. Fujiwara, J. Phys. Chem. Lett., 2021, 9283–9292 CrossRef CAS PubMed.
- D. Sakamaki, D. Kumano, E. Yashima and S. Seki, Chem. Commun., 2015, 51, 17237–17240 RSC.
- S. Tanaka, D. Sakamaki, N. Haruta, T. Sato, M. Gon, K. Tanaka and H. Fujiwara, J. Mater. Chem., 2023, 11, 4846–4854 CAS.
- D. Sakamaki, Y. Inoue, K. Shimomura, D. Taura, E. Yashima and S. Seki, Tetrahedron Lett., 2023, 114, 154294 CrossRef CAS.
- K. Tanaka, D. Sakamaki and H. Fujiwara, Chem. – Eur. J., 2021, 27, 4430–4438 CrossRef CAS PubMed.
- G. Bringmann, A. J. Price Mortimer, P. A. Keller, M. J. Gresser, J. Garner and M. Breuning, Angew. Chem., Int. Ed., 2005, 44, 5384–5427 CrossRef CAS PubMed.
- K. Hassan, K.-I. Yamashita, K. Hirabayashi, T. Shimizu, K. Nakabayashi, Y. Imai, T. Matsumoto, A. Yamano and K.-I. Sugiura, Chem. Lett., 2015, 44, 1607–1609 CrossRef CAS.
- K. Takaishi, R. Takehana and T. Ema, Chem. Commun., 2018, 54, 1449–1452 RSC.
- K. Takaishi, S. Hinoide, T. Matsumoto and T. Ema, J. Am. Chem. Soc., 2019, 141, 11852–11857 CrossRef CAS PubMed.
- K. Takaishi, T. Matsumoto, M. Kawataka and T. Ema, Angew. Chem., Int. Ed., 2021, 60, 9968–9972 CrossRef CAS PubMed.
- Z.-S. Liu, P.-P. Xie, Y. Hua, C. Wu, Y. Ma, J. Chen, H.-G. Cheng, X. Hong and Q. Zhou, Chem. – Eur. J., 2021, 7, 1917–1932 CAS.
- S. Grätz, D. Beyer, V. Tkachova, S. Hellmann, R. Berger, X. Feng and L. Borchardt, Chem. Commun., 2018, 54, 5307–5310 RSC.
- S. Grätz, M. Oltermann, E. Troschke, S. Paasch, S. Krause, E. Brunner and L. Borchardt, J. Mater. Chem. A, 2018, 6, 21901–21905 RSC.
- H. Sada, D. Sakamaki, M. Gon, K. Tanaka, T. Hirose and H. Fujiwara, ChemRxiv, 2024 DOI:10.26434/chemrxiv-2024-sbv85.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, spectroscopic and computational data. CCDC 2332276–2332278. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc00977k |
| This journal is © The Royal Society of Chemistry 2024 |