Open Access Article
Open Access ArticleSynthesis of a novel twisted π-conjugated macrocycle via double Friedel–Crafts reaction and its physical properties†
Zhiyan
Jiang
 b and
Yoichiro
Kuninobu
b and
Yoichiro
Kuninobu
 *ab
*ab
aInstitute for Materials Chemistry and Engineering, Kyushu University, 6-1 Kasugakoen, Kasuga-Shi, Fukuoka 816-8580, Japan. E-mail: kuninobu@cm.kyushu-u.ac.jp
bDepartment of Interdisciplinary Engineering Sciences, Interdisciplinary Graduate School of Engineering Sciences, Kyushu University, 6-1 Kasugakoen, Kasuga-Shi, Fukuoka 816-8580, Japan
First published on 29th June 2024
Abstract
We synthesized a cyclic molecule from diarylalkynes and Meldrum's acid derivatives as the methylenation reagent via double Friedel–Crafts reaction. Single-crystal X-ray structure analysis confirmed the twisted structure of the molecule. We also investigated their physical properties and homoconjugation by UV-Vis, photoluminescence, DFT and TD-DFT calculations.
In recent years, three-dimensional π-conjugated macrocycles exhibiting geometric structures1 have been synthesized. Besides compounds with zero Gaussian curvature, such as carbon nanotubes and cycloparaphenylenes (CPPs),2 twisted macrocycles3 are formed from combined special structures that can significantly alter their electronic, optical and chemical properties, and thus have received much attention. For example, the Möbius band is a well-known topological structure discovered independently by the German mathematicians and astronomers Möbius and Johanlestein in 1858,4 and macrocycles with the Möbius ring structure have been successfully synthesized recently.5 Besides, infinity-shaped or figure-eight molecules,6 molecular cages,7 and Penrose stairs8 have been widely reported so far. Twisted macrocycles have potential applications in various advanced technologies, such as fluorescent sensor,9 luminescent material10 and host–guest chemistry.11
The typical cyclic π-conjugated molecules are composed of sp2 carbon skeleton, allowing for the delocalization of π-electrons across the molecules.12 Generally, the introduction of sp3 carbons typically disrupts the π-conjugation. Therefore, examples where a π-conjugated macrocycle includes sp3 carbons without cleavage of the π-conjugation are rare.13 The flexibility provided by tetrahedron structure of sp3 carbons facilitates the design of a wide variety of cyclic molecular structures, and thus it is also necessary to use a method for ensuring the coherence of the π-conjugated structure over sp3 carbon atoms.
One effective approach to extend the π-conjugated system over sp3 carbons is homoconjugation,14 where the π-conjugated system is extended across σ-bond(s) via through-space interaction instead of through-bond interaction.15 To achieve effective homoconjugation, a twisted or rigid structure is often necessary for keeping the molecular configuration to obtain the largest orbital overlap.14 Furthermore, based on previous researches, diarylmethane is the classic structure for realizing homoconjugation.16 Therefore, we hypothesised that if diarylmethane moieties can bridge two rigid π-conjugated structures, it is possible to build a π-conjugated system that includes sp3 carbons without cleavage of the π-conjugation. Our recent research opens up the possibility of this assumption. We recently developed Meldrum's acid derivatives as cyclisation reagents capable of double C–C bond formation to synthesise fluorene derivatives from biaryl derivatives without using a catalyst (Fig. 1(a)).17 Owing to the existence of electron-donating amino groups, this reaction occurs at the C4–H and C4′–H positions of the biaryl, resulting in the formation of cyclic fluorene compound. We planned to synthesise a novel cyclic molecule 3 using this reaction: the diarylalkynes 1, which serve as the rigid parts,18 are bridged by sp3 carbon atoms from Meldrum's acid derivative (Fig. 1(b)). We expected that the π-conjugated structure of the entire molecule could be constructed with homoconjugation whereas the cyclic π-conjugated structure is hybridized by sp3 carbon atoms (Fig. 1(c)).
We then investigated the synthesis of twisted macrocycle using our reported reaction.17 Treatment of amino group-containing diarylalkyne 1 with 2.5 equivalents of Meldrum's acid derivative 2 in dichloroethane at 120 °C for 12 h gave cyclic molecule 3 in 69% yield (Scheme 1).
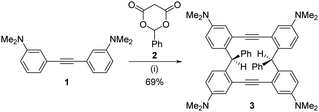 | ||
| Scheme 1 Synthesis of twisted macrocycle 3. Reagents and conditions: (i) 2 (2.5 equiv.), ClCH2CH2Cl, 120 °C, 6 h. | ||
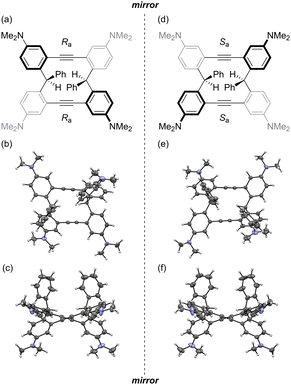
The structure of the obtained cyclic molecule 3 was confirmed using 1H nuclear magnetic resonance (NMR) spectroscopy (Fig. S14, ESI†), 13C NMR (Fig. S15, ESI†), high resolution mass spectrometry (HRMS) (ESI†), and single-crystal X-ray crystallography (Fig. 2 and Fig. S2–S4, ESI†). In 1H NMR spectrum, the methine proton of 3 is observed in downfield (7.09 ppm, Fig. S14, ESI†) compared to that of 4,4′-(phenylmethylene)bis(N,N-dimethylaniline) (Leuco Malachite Green, 5.39 ppm).19 This result is probably attributed to the deshielding effect of the alkyne moieties.
Single-crystal X-ray crystallography revealed that the crystal packing structure of 3 is composed of the pair of enantiomers (Fig. S2, ESI†). For each enantiomer, the two diarylalkyne parts have the same axial chirality, which served as the basis for naming the enantiomers (Ra,Ra)-3 and (Sa,Sa)-3 (Fig. 2 and Fig. S1, ESI†).20 Furthermore, product 3 exhibits a twisted cyclic structure. Although the two phenyl groups derived from 2 could have been in the same or opposite direction, they were found to be in the same direction (Fig. 2(c) and (f)). This finding may be attributed to steric repulsion between the phenyl group and phenyl methine moiety. The four ortho-phenylene moieties and two C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bonds were almost planar and straight, respectively.
C triple bonds were almost planar and straight, respectively.
Furthermore, the interconversion between two enantiomers of 3 was investigated by density functional theory (DFT) calculations (Method: B3LYP/6-31G(d), for the details, see ESI†). We found the interconversion between two enantiomers of 3 was a racemization which proceeds through a transient intermediate of the transition state TS-3. The energy barrier is about 7.5 kcal mol−1 (Fig. 3). Compared with previous researches,8b,c,21 this relatively lower energy barrier makes the racemization proceed easily at room temperature.22,23
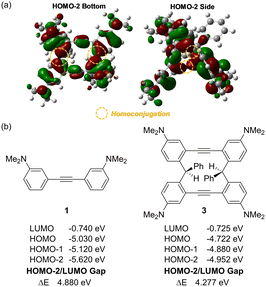
DFT calculations were performed to verify the hypothesis whether homoconjugation provided by diarylmethane moieties can construct continuous π-conjugated systems containing sp3 carbons. Interestingly, the results of the calculations show that there is an overlap of π-orbitals at the ipso-carbon atoms on the aromatic rings (yellow circle in Fig. 4(a)) and the HOMO−2/LUMO energy gap of 3 is smaller than that of 1 (Fig. 4(b)). These results indicate that a weak homoconjugation at the HOMO−2 level exists in 3.
To further validate the effect of homoconjugation on the HOMO−2 level, we measured the UV-Vis and fluorescence spectra of the twisted macrocycle 3 and its precursor 1 (Fig. S5 and Table S10, ESI†). In 0.01 mM dichloromethane solution, 3 exhibited a maximum absorption at 271 nm and small absorption peaks at 222 and 347 nm. Precursor 1 exhibited a maximum absorption at 266 nm and small absorption peaks at 219 and 338 nm. By comparing the absorption spectra of 1 and 3, a slight red shift was detected (219 to 222, 266 to 271, and 338 to 348 nm). The red shift observed in the UV-Vis spectra was also observed in the fluorescence spectra of 1 and 3 (406 to 415 nm).
TD-DFT calculations (B3LYP/6-311++G(d,p)) were carried out. Based on stimulated UV-Vis spectra of 1 (Fig. S9, ESI†) and 3 (Fig. S10, ESI†), 3 exhibited a maximum absorption at 292 nm and small absorption peaks at 370 nm. Precursor 1 exhibited a maximum absorption at 303 nm and small absorption peaks at 246 nm. The redshift was also observed in the TD-DFT calculations. Electronic transitions related to HOMO−2 were listed in Table S25 (ESI†) (precursor 1) and Table S26 (ESI†) (product 3). We found that excited states involving HOMO−2 (Fig. S9, ESI† marked in red) of precursor 1 has relatively higher oscillator strength. About product 3, excited states involving HOMO−2 (Fig. S10, ESI† marked in red) of product 3 shows medium contribution to the overall absorption. Therefore, TD-DFT calculations illustrate explicitly the significant contribution of the HOMO−2 level to the absorption spectrum. This result also supports the existence of homoconjugation at the HOMO−2 level of product 3.
In summary, we successfully synthesised a cyclic molecule with a twisted topological structure from electron-rich diarylalkynes and Meldrum's acid derivatives via a double Friedel–Crafts reaction. This is a special example of a sp3 carbon-bridged twisted cyclic π-conjugated molecule with maintaining the π-conjugated system by homoconjugation. Single-crystal X-ray structural analysis revealed that this molecule exists as a mixture of enantiomers in a single crystal unit. In the absorption and fluorescence spectra of the twisted macrocycle, a red shift was observed compared to that of the diarylalkyne precursor. The DFT and TD-DFT calculations can explain this red shift and the existence of homoconjugation. This research demonstrates the versatility and potential of sp3 carbon atoms in complex π-conjugated systems, providing a new method for construction of new π-conjugated macrocycle bridged sp3 carbon atoms by homoconjugation.
This work was supported in part by JSPS KAKENHI grant numbers JP 21H01941 and 22H05370, Tokuyama Science Foundation, and JST SPRING, grant number JPMJSP2136. We are grateful to BSc. T. Matsumoto at Evaluation Center of Materials Properties and Function, Institute for Materials Chemistry and Engineering, Kyushu University for single crystal X-ray structure analyses. We also appreciate Prof. K. Fujita at Kyushu University for measuring fluorescence spectra and fluorescent quantum yields. We also wish to express our gratitude to Dr S. Asano and Prof. J. Hayashi at Kyushu University for TGA analysis. We greatly appreciate Daicel Corporation for their assistance with optical resolution. Finally, we are also grateful to Dr A. Senno at Faculty of Pharmaceutical Sciences, Kyushu University for assistance of Circular Dichroism spectra measurements.
Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for compound 3 has been deposited at the CCDC 2335048† and can be obtained from https://www.ccdc.cam.ac.uk/.Conflicts of interest
There are no conflicts to declare.Notes and references
- Reviews: (a) J. F. Stoddarta, Chem. Soc. Rev., 2009, 38, 1521 RSC; (b) Y. Segawa, D. R. Levine and K. Itami, Acc. Chem. Res., 2019, 52, 2760 CrossRef CAS PubMed ; Books: ; (c) A. T. Balaban, From Chemical Topology to Three-Dimensional Geometry, Springer, New York, NY, 2006 Search PubMed; (d) A. Kerber, R. Laue, M. Meringer, C. Rücker and E. Schymanski, Mathematical Chemistry and Chemoinformatics, De Gruyter, Berlin, Boston, 2014 Search PubMed.
- Reviews: (a) E. J. Leonhardt and R. Jasti, Nat. Rev. Chem., 2019, 3, 672 CrossRef CAS; (b) J. Wang, X. Zhang, H. Jia, S. Wang and P. Du, Acc. Chem. Res., 2021, 54, 4178 CrossRef CAS PubMed; (c) D. Imoto, A. Yagi and K. Itami, Precis. Chem., 2023, 1, 516 CrossRef CAS.
- (a) Y. Morisaki, M. Gon, T. Sasamori, N. Tokitoh and Y. Chujo, J. Am. Chem. Soc., 2014, 136, 3350 CrossRef CAS PubMed; (b) K. C. Sahoo, M. A. Majewski, M. Stępień and H. Rath, J. Org. Chem., 2017, 82, 8317 CrossRef CAS PubMed; (c) I. Casademont-Reig, T. Woller, J. Contreras-García, M. Alonso, M. Torrent-Sucarrat and E. Matito, Phys. Chem. Chem. Phys., 2018, 20, 2787 RSC; (d) Y. Segawa, M. Kuwayama, Y. Hijikata, M. Fushimi, T. Nishihara, J. Pirillo, J. Shirasaki, N. Kubota and K. Itami, Science, 2019, 365, 272 CrossRef CAS PubMed; (e) K. J. Weiland, T. Brandl, K. Atz, A. Prescimone, D. Häussinger, T. Šolomek and M. Mayor, J. Am. Chem. Soc., 2019, 141, 2104 CrossRef CAS PubMed; (f) A. Ghosh, S. Dash, A. Srinivasan, C. H. Suresh, S. Peruncheralathan and T. K. Chandrashekar, Org. Chem. Front., 2019, 6, 3746 RSC; (g) Y. Li, A. Yagi and K. Itami, J. Am. Chem. Soc., 2020, 142, 3246 CrossRef CAS PubMed; (h) M. Rickhaus, M. Jirasek, L. Tejerina, H. Gotfredsen, M. D. Peeks, R. Haver, H.-W. Jiang, T. D. W. Claridge and H. L. Anderson, Nat. Chem., 2020, 12, 236 CrossRef CAS PubMed . Reviews: ; (i) M. Stępień, N. Sprutta and L. Latos-Grażyński, Angew. Chem., Int. Ed., 2011, 50, 4288 CrossRef PubMed; (j) M. Rickhaus, M. Mayor and M. Juríček, Chem. Soc. Rev., 2016, 45, 1542 RSC; (k) B. Szyszko, M. J. Białek, E. Pacholska-Dudziak and L. Latos-Grażyński, Chem. Rev., 2017, 117, 2839 CrossRef CAS PubMed; (l) M. A. Majewski and M. Stępień, Angew. Chem., Int. Ed., 2019, 58, 86 CrossRef CAS PubMed; (m) M. Hasegawa, Y. Nojima and Y. Mazaki, ChemPhotoChem, 2021, 5, 10421058 Search PubMed; (n) S. M. Bachrach, J. Org. Chem., 2023, 88, 7962 CrossRef CAS PubMed.
- J. H. E. Cartwright and D. L. González, Math. Intell., 2016, 38, 69 CrossRef.
- Single-stranded Möbius molecules: (a) D. Ajami, O. Oeckler, A. Simon and R. Herges, Nature, 2003, 426, 819 CrossRef CAS PubMed; (b) Y. Tanaka, S. Saito, S. Mori, N. Aratani, H. Shinokubo, N. Shibata, Y. Higuchi, Z. S. Yoon, K. S. Kim, S. B. Noh, J. K. Park, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2008, 47, 681 CrossRef CAS PubMed; (c) G. R. Schaller, F. Topić, K. Rissanen, Y. Okamoto, J. Shen and R. Herges, Nat. Chem., 2014, 6, 608 CrossRef CAS PubMed; (d) G. Naulet, L. Sturm, A. Robert, P. Dechambenoit, F. Röhricht, R. Herges, H. Bock and F. Durola, Chem. Sci., 2018, 9, 8930 RSC; (e) Y.-Y. Fan, D. Chen, Z.-A. Huang, J. Zhu, C.-H. Tung, L.-Z. Wu and H. Cong, Nat. Commun., 2018, 9, 3037 CrossRef PubMed; (f) J. S. Moore, X. Jiang, S. D. Laffoon, D. Chen, S. Pérez-Estrada, A. S. Danis, J. Rodríguez-López, M. A. Garcia-Garibay and J. Zhu, J. Am. Chem. Soc., 2020, 142, 6493 CrossRef PubMed; (g) Z. Luo, X. Yang, K. Cai, X. Fu, D. Zhang, Y. Ma and D. Zhao, Angew. Chem., Int. Ed., 2020, 59, 14854 CrossRef CAS PubMed; (h) J. Malinčík, S. Gaikwad, J. P. Mora-Fuentes, M.-A. Boillat, A. Prescimone, D. Häussinger, A. G. Campaña and T. Šolomek, Angew. Chem., Int. Ed., 2022, 61, e202208591 CrossRef PubMed . Double-stranded Möbius Molecules: ; (i) S. Nishigaki, Y. Shibata, A. Nakajima, H. Okajima, Y. Masumoto, T. Osawa, A. Muranaka, H. Sugiyama, A. Horikawa, H. Uekusa, H. Koshino, M. Uchiyama, A. Sakamoto and K. Tanaka, J. Am. Chem. Soc., 2019, 141, 14955 CrossRef CAS PubMed . Möbius carbon nanobelt: ; (j) Y. Segawa, T. Watanabe, K. Yamanoue, M. Kuwayama, K. Watanabe, J. Pirillo, Y. Hijikata and K. Itami, Nat. Synth., 2022, 1, 535 CrossRef; (k) W. Fan, T. M. Fukunaga, S. Wu, Y. Han, Q. Zhou, J. Wang, Z. Li, X. Hou, H. Wei, Y. Ni, H. Isobe and J. Wus, Nat. Synth., 2023, 2, 880 CrossRef.
- (a) Z. Zhang, W.-Y. Cha, N. J. Williams, E. L. Rush, M. Ishida, V. M. Lynch, D. Kim and J. L. Sessler, J. Am. Chem. Soc., 2014, 136, 7591 CrossRef CAS PubMed; (b) K. Senthilkumar, M. Kondratowicz, T. Lis, P. J. Chmielewski, J. Cybinska, J. L. Zafra, J. Casado, T. Vives, J. Crassous, L. Favereau and M. Stępień, J. Am. Chem. Soc., 2019, 141, 7421 CrossRef CAS PubMed; (c) A. Robert, G. Naulet, H. Bock, N. Vanthuyne, M. Jean, M. Giorgi, Y. Carissan, C. Aroulanda, A. Scalabre, E. Pouget, F. Durola and Y. Coquerel, Chem. – Eur. J., 2019, 25, 14364 CrossRef CAS PubMed; (d) L. H. Wang, N. Hayase, H. Sugiyama, J. Nogami, H. Uekusa and K. Tanaka, Angew. Chem., Int. Ed., 2020, 59, 17951 CrossRef CAS PubMed; (e) T. A. Schaub, E. A. Prantl, J. Kohn, M. Bursch, C. R. Marshall, E. J. Leonhardt, T. C. Lovell, L. N. Zakharov, C. K. Brozek, S. R. Waldvogel, S. Grimme and R. Jasti, J. Am. Chem. Soc., 2020, 142, 8763 CrossRef PubMed; (f) W. Fan, T. Matsuno, Y. Han, X. Wang, Q. Zhou, H. Isobe and J. Wu, J. Am. Chem. Soc., 2021, 143, 15924 CrossRef CAS PubMed; (g) C. Li, Z. Huang, Y. Hu, W. Liang, R. Su, M. Chen, L. Zhou, D. Wu, G. Gao and J. You, Org. Lett., 2021, 23, 3746 CrossRef CAS PubMed; (h) M. Krzeszewski, H. Ito and K. Itami, J. Am. Chem. Soc., 2022, 144, 862 CrossRef CAS PubMed; (i) M. Hasegawa, C. Hasegawa, Y. Nagaya, K. Tsubaki and Y. Mazaki, Chem. – Eur. J., 2022, 28, e2022022 CrossRef PubMed; (j) K. Wypych, M. Dimitrova, D. Sundholm and M. Pawlicki, Org. Lett., 2022, 24, 4876 CrossRef CAS PubMed; (k) L.-H. Wang, J. Nogami, Y. Nagashima and K. Tanaka, Org. Lett., 2023, 25, 4225 CrossRef CAS PubMed; (l) A. Artigas, Y. Carissan, D. Hagebaum-Reignier, H. Bock, F. Durola and Y. Coquerel, Chem. – Eur. J., 2024, e202401016 CrossRef CAS PubMed.
- (a) K. Matsui, Y. Segawa, T. Namikawa, K. Kamada and K. Itami, Chem. Sci., 2012, 4, 84 RSC; (b) T. Matsushima, S. Kikkawa, I. Azumaya and S. Watanabe, ChemistryOpen, 2018, 7, 278 CrossRef CAS PubMed; (c) N. Hayase, J. Nogami, Y. Shibata and K. Tanaka, Angew. Chem., Int. Ed., 2019, 58, 9439 CrossRef CAS PubMed; (d) Y. Ni, F. Gordillo-Gámez, M. P. Alvarez, Z. Nan, Z. Li, S. Wu, Y. Han, J. Casado and J. Wu, J. Am. Chem. Soc., 2020, 142, 12730 CrossRef CAS PubMed; (e) J. Zhu, Y. Han, Y. Ni, S. Wu, Q. Zhang, T. Jiao, Z. Li and J. Wu, J. Am. Chem. Soc., 2021, 143, 14314 CrossRef CAS PubMed; (f) A. Basavarajappa, M. D. Ambhorea and V. G. Anand, Chem. Commun., 2021, 57, 4299 RSC.
- (a) L. S. Penrose and R. Penrose, Br. J. Psychol., 1958, 49, 31 CrossRef CAS PubMed; (b) W. Nakanishi, T. Matsuno, J. Ichikawa and H. Isobe, Angew. Chem., Int. Ed., 2011, 50, 6048 CrossRef CAS PubMed; (c) Z. He, E. Wang, J. W. Y. Lam, Y. Li, Z. Lin and B. Z. Tang, ChemPlusChem, 2015, 80, 1245 CrossRef CAS PubMed.
- H. Nie, Q.-H. Li, S. Zhang, C.-M. Wang, W.-H. Lin, K. Deng, L.-J. Shu, Q.-D. Zeng and J.-H. Wan, Org. Chem. Front., 2021, 8, 6806 RSC.
- (a) H. Tanaka, Y. Inoue and T. Mori, ChemPhotoChem, 2018, 2, 386 CrossRef CAS . Review: ; (b) J.-L. Ma, Q. Peng and C.-H. Zhao, Chem. – Eur. J., 2019, 25, 15441 CrossRef CAS PubMed.
- (a) H. Abe, T. Yoneda, Y. Ohishi and M. Inouye, Chem. – Eur. J., 2016, 22, 18944 CrossRef CAS PubMed; (b) Y. Xu, S. Gsanger, M. B. Minameyer, I. Imaz, D. Maspoch, O. Shyshov, F. Schwer, X. Ribas, T. Drewello, B. Meyer and M. von Delius, J. Am. Chem. Soc., 2019, 141, 18500 CrossRef CAS PubMed; (c) J. Wang, Y.-Y. Ju, K.-H. Low, Y.-Z. Tan and J. Liu, Angew. Chem., Int. Ed., 2021, 60, 11814 CrossRef CAS PubMed.
- Reviews: (a) P. von Ragué Schleyer, Chem. Rev., 2001, 101, 1115 CrossRef PubMed; (b) T. M. Krygowski and M. K. Cyrański, Chem. Rev., 2001, 101, 1385 CrossRef CAS PubMed; (c) N. Martín and L. T. Scott, Chem. Soc. Rev., 2015, 44, 6397 RSC ; Book: ; (d) M. Solà, A. I. Boldyrev, M. K. Cyrański, T. M. Krygowski and G. Merino, Aromaticity and Antiaromaticity: Basics and Applications, John Wiley & Sons Ltd, 2023 Search PubMed.
- (a) L. T. Scott, G. J. DeCicco, J. L. Hyun and G. Reinhardt, J. Am. Chem. Soc., 1983, 105, 7760 CrossRef CAS; (b) L. T. Scott, M. J. Cooney, D. W. Rogers and K. Dejroongruang, J. Am. Chem. Soc., 1988, 110, 7244 CrossRef CAS; (c) C. Lepetit, B. Silvi and R. Chauvin, J. Phys. Chem. A, 2003, 107, 464 CrossRef CAS; (d) Y. Miyazawa, Z. Wang, M. Matsumoto, S. Hatano, I. Antol, E. Kayahara, S. Yamago and M. Abe, J. Am. Chem. Soc., 2021, 143, 7426 CrossRef CAS PubMed.
- (a) M. Simonetta and S. Winstein, J. Am. Chem. Soc., 1954, 76, 18 CrossRef CAS; (b) L. T. Scott, Pure Appl. Chem., 1986, 58, 105 CrossRef CAS Review: ; (c) R. V. Williams, Chem. Rev., 2001, 101, 1185 CrossRef CAS PubMed.
- J. Liu, H. Zhang, L. Hu, J. Wang, J. W. Y. Lam, L. Blancafort and B. Z. Tang, J. Am. Chem. Soc., 2022, 144, 7901 CrossRef CAS PubMed.
- (a) S. Clementi, V. Mancini and G. Marino, J. Chem. Soc. D, 1970, 1457 RSC; (b) J. O. Barcina, M. d R. C. Heras, M. Mba, R. G. Aspe and N. Herrero-García, J. Org. Chem., 2009, 74, 7148 CrossRef PubMed; (c) H. Zhang, X. Zheng, N. Xie, Z. He, J. Liu, N. L. C. Leung, Y. Niu, X. Huang, K. S. Wong, R. T. K. Kwok, H. H. Y. Sung, I. D. Williams, A. Qin, J. W. Y. Lam and B. Z. Tang, J. Am. Chem. Soc., 2017, 139, 16264 CrossRef CAS PubMed.
- Z. Jiang, K. Sekine and Y. Kuninobu, Chem. Commun., 2022, 58, 843 RSC.
- S. Toyota, Chem. Rev., 2010, 110, 5398 CrossRef CAS PubMed.
- M. Söderström, C. Matt and L. R. Odell, ACS Omega, 2023, 8, 4320 CrossRef PubMed.
- L. C. Cross and W. Klyne, Pure Appl. Chem., 1976, 45, 11 CrossRef.
- E. L. Eliel, S. H. Wilen and M. P. Doyle, Basic Organic Stereochemistry, Wiley, New York, 2001 Search PubMed.
- Chiral-stationary phase HPLC for the racemic resolution of product 3 has been tried. However, the two enantiomers of product 3 could not be isolated successfully. IA-3, IB N-3, IC-3, ID-3, IE-3, IF-3, IG-3, IH-3 and IJ-3 columns with various kinds of eluents (tetrahydrofuran, n-hexane, ethyl acetate, dichloromethane, chloroform, diethylamine and their combination) have been tried.
- We measured the circular dichroism (CD) spectra of 10−4 M DCM solution of the mixtures of 3 and (+)-10-camphorsulfonic acid ((+)-CSA) or (−)-CSA with different ratio. Comparing with spectra of (+)-CSA and (−)-CSA solution, red shift and enhancement of the ellipticity were observed (Fig. S21, ESI†). These results indicated that the addition of the chiral additive induced the chirality of 3.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data, and 1H, 13C NMR charts. CCDC 2335048 (3). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc00890a |
| This journal is © The Royal Society of Chemistry 2024 |