Open Access Article
Open Access ArticleAdamantylglycine as a high-affinity peptide label for membrane transport monitoring and regulation†
Malavika
Pramod
a,
Mohammad A.
Alnajjar
a,
Sandra N.
Schöpper
a,
Thomas
Schwarzlose
b,
Werner M.
Nau
 *b and
Andreas
Hennig
*b and
Andreas
Hennig
 *a
*a
aCenter for Cellular Nanoanalytics (CellNanOs) and Department of Biology and Chemistry, Universität Osnabrück, Barbarastraße 7, Osnabrück 49069, Germany. E-mail: andreas.hennig@uni-osnabrueck.de
bSchool of Science, Constructor University, Campus Ring 1, Bremen 28759, Germany. E-mail: wnau@constructor.university
First published on 11th April 2024
Abstract
The non-canonical amino acid adamantylglycine (Ada) is introduced into peptides to allow high-affinity binding to cucurbit[7]uril (CB7). Introduction of Ada into a cell-penetrating peptide (CPP) sequence had minimal influence on the membrane transport, yet enabled up- and down-regulation of the membrane transport activity.
The molecular recognition of biomacromolecules such as peptides, proteins, nucleic acids, and other biologically active molecules by synthetic receptors is of significant interest for life science applications, for example, in proteomics, imaging, diagnostics, and drug delivery.1,2 Various classes of synthetic host molecules have been identified in this regard, among which cucurbit[n]urils (CBs) stand out due to their very high binding affinities with well-designed guest molecules.3,4 Ultra-high host–guest binding affinities in water are key for biological applications, for example in pull-down assays, stabilization of biopharmaceuticals, and bioimaging.3,5 Within recent years, attempts have been made to identify peptide binding epitopes with a strong affinity to CBs, in particular to the highly water-soluble homologue cucurbit[7]uril (CB7), which included sequences of naturally occurring, proteinogenic amino acids,6 as well as artificial binding epitopes7 and noncanonical amino acids.2,8–10
Stable CB7 complexes with noncanonical amino acids were first explored by Urbach and co-workers and included the p-tert-butyl and p-aminomethyl derivatives of phenylalanine (tBuPhe and AMPhe, Fig. 1).8 Compared to phenylalanine (Phe), the dissociation constants, Kd, were remarkably enhanced and peptides with an N-terminal AMPhe showed an exceedingly high affinity of Kd = 0.95 nM. Genetic encoding of these unnatural amino acids was subsequently demonstrated by Liu and co-workers and proved to be more efficient for tBuPhe than for AMPhe.2 This enabled the regulation of protein functions by host–guest complexation of the protein-incorporated tBuPhe. Recently, AMPhe was engineered into the N-terminus of the B-chain of the diabetes hormone insulin providing a semisynthetic insulin variant that can be bound by CB7 with a Kd value of 99 nM.10
 | ||
| Fig. 1 Structures of the proteinogenic amino acid phenylalanine (Phe) and the noncanonical amino acids tBuPhe, AMPhe, and Ada. | ||
As a useful complement, we report herein (S)-adamantylglycine (Ada) as a high-affinity CB7 binding motif in peptides. The unmodified amino acid Ada has a picomolar affinity to CB7,11 which is retained when it is appended to heptaarginine (R7). R7 is a well-known cell-penetrating peptide (CPP) and was previously investigated by us as a model CPP for counterion-activated membrane transport in large unilamellar phospholipid vesicles (LUVs).12,13 We compare herein R7, Phe-R7, and Ada-R7 in fluorescence-based membrane transport assays and show that Ada can serve as a high-affinity peptide label with minimal effects on the peptide translocation efficacy and kinetics. The picomolar affinity, in conjunction with the convenient introduction into peptides by solid-phase peptide synthesis, establishes Ada as a highly useful peptide label, for example, in peptide-based pull-down assays with CB7-functionalized beads14 or for intracellular peptide localization experiments with CB7-based fluorescent probes.15
Complexation of Ada-R7 (see ESI† for synthesis) by CB7 was first confirmed by 1H NMR spectroscopy (Fig. 2). The spectrum of Ada-R7 shows multiplets from the α-CH protons in the range from ca. 4.1–4.5 ppm and the δ-CH2 protons of the Arg side chain at 3.25 ppm, which overlap with the α-CH proton of the Ada amino acid.11 The methylene proton signals of Ada overlap with the β- and γ-CH2 protons of the Arg residues in the range from 1.55–1.95 ppm, whereas the methine (–CH) group of the unbound adamantyl residue shows up at 2.03 ppm as a clearly separated broad singlet.
In the presence of CB7, the 1H NMR spectra showed clear indications of inclusion of the adamantyl residue in the CB7 cavity. New signals appeared in the range from 0.9–1.4 ppm, which are assigned to the upfield-shifted CB7-bound adamantyl residue.11 Cavity binding of the Ada residue was also apparent from the appearance of a new singlet at 3.65 ppm, which is assigned to the α-CH proton of the bound Ada residue in accordance with previous observation of the adamantane amino acid.11 The position and shape of the peaks assigned to the arginine residues remained largely unaffected by the presence of CB7, whereas a new shoulder appeared at the position of the δ-CH2 protons of the Arg side chain suggesting a loose association of the guanidinium side chain with the remaining free portal of the CB7 cavity.16
The binding constant of Ada-R7 was determined next, by NMR competition using p-xylylenediamine (PXD) as a reference (Fig. S9, see Chart S1 for a structure overview, ESI†).11,17 By comparing the integrated peak areas corresponding to the protons of free PXD and CB7-bound PXD as well as free and bound α-CH and alkyl-protons of the Ada residue, the binding constant of Ada-R7 to CB7 was determined as (4.8 ± 0.8) × 1011 M−1. Complete equilibration of the mixture was ensured by comparing a spectrum, in which Ada-R7 was first mixed with CB7 before addition of PXD, with a second spectrum, in which Ada-R7 was added to the preformed CB7/PXD complex. Identical spectra of both NMR samples indicated that equilibrium had been reached. The binding constant was further confirmed by competitive ITC titrations with PXD (Fig. S10, ESI†), which gave an affinity of 7.0 × 1011 M−1, in good agreement with the NMR value. Competitive fluorescence titrations with the host-dye reporter pair composed of CB7 and berberine (BE) afforded a linear fluorescence decrease indicative of quantitative displacement of BE with increasing peptide concentrations, as expected for the very high binding affinity of the Ada residue to CB7.11
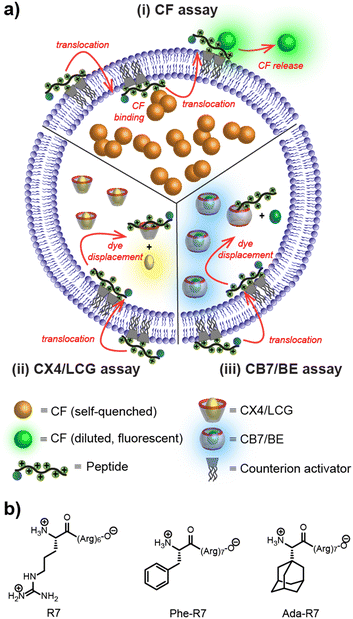
The next goal was to clarify to which extent peptide translocation across the lipid bilayer membrane of LUVs is affected by introducing the adamantylglycine amino acid into a known peptide sequence. We have therefore selected heptaarginine (R7) as a well-established CPP, which crosses liposome membranes after counterion activation.12,13 The membrane transport activity was investigated on one hand with the well-established efflux assay based on carboxyfluorescein (CF) and, on the other hand, with more recently reported supramolecular tandem assays that use liposome-encapsulated host-dye reporter pairs, either CX4/LCG or CB7/BE (see Fig. 3, Chart S1 and Fig. S17–S26, ESI†).18 The first two assays show a “switch-on” fluorescence response upon CPP entry, while the CB7/BE assay is of the “switch-off” type.
First, benchmark values were established with R7 and Phe-R7 (Table 1). These hydrophilic, polycationic peptides require an amphiphilic counterion activator, e.g. amphiphilic calixarenes,12,13,19 to translocate across the hydrophobic membrane of liposomes. We have selected here the lower-rim substituted pentyl ether derivative of p-sulfonatocalix[4]arene (CX4-C5) with a reported activator efficiency in the nanomolar range,12 and measured the effective concentrations (EC50) to afford 50% peptide-mediated dye efflux in the CF assay or 50% dye displacement in the CX4/LCG and CB7/BE assay. In excellent agreement with previous results (Table 1),12,13 this revealed EC50 values in the range of 0.1 to 1 μM for the membrane activity of R7 and Phe-R7 with CX4-C5 counterion activation in all three assays. The only exception was R7 with the CB7/BE assay, which showed a very high EC50 (>10 μM) due to the lack of a distinctive CB7 binding motif in the R7 peptide. The latter result is also relevant with respect to the mechanism of membrane-active peptides such as CPPs and antimicrobial peptides (AMPs),13,20,21 since it confirms that R7/CX4-C5 peptide-counterion complexes shuttle across the lipid membrane without causing any membrane perturbations through which hydrophilic molecules can escape from the liposomes.13 Dye efflux, as in the case of the CF assay, most likely proceeds via ternary activator/peptide/CF complexes that can shuttle back from the vesicle lumen to the liposome exterior as previously suggested (Fig. 3a).21 We therefore conclude that counterion activation with CX4-C5 affords peptide translocation without membrane rupture.12,13
| Assay | R7 | Phe-R7 | Ada-R7 | |||
|---|---|---|---|---|---|---|
| EC50 (μM) | K a (M−1) | EC50 (μM) | K a (M−1) | EC50 (μM) | K a (M−1) | |
a All transport experiments were conducted with POPC/POPS (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) LUVs (20 μM total phospholipid concentration) in 10 mM NaH2PO4, pH 7.2. Binding constants were determined by competitive fluorescence titration in the same buffer, unless otherwise noted.
b With 12 μM POPC LUVs in 10 mM Hepes, pH 7.0; see ref. 13.
c With 12 μM POPC/POPS LUVs in 10 mM (NH4)2HPO4, pH 7.0; see ref. 13.
d
K
a of Ada-R7 to CB7 was determined by competitive ITC in H2O, pH 7.0 and by 1H NMR in D2O, pD 7.4 (in square brackets); error ca. 10%. 1) LUVs (20 μM total phospholipid concentration) in 10 mM NaH2PO4, pH 7.2. Binding constants were determined by competitive fluorescence titration in the same buffer, unless otherwise noted.
b With 12 μM POPC LUVs in 10 mM Hepes, pH 7.0; see ref. 13.
c With 12 μM POPC/POPS LUVs in 10 mM (NH4)2HPO4, pH 7.0; see ref. 13.
d
K
a of Ada-R7 to CB7 was determined by competitive ITC in H2O, pH 7.0 and by 1H NMR in D2O, pD 7.4 (in square brackets); error ca. 10%.
|
||||||
| CF | 0.33 ± 0.03 | n.a. | 0.15 ± 0.01 | n.a. | 0.16 ± 0.02 | n.a. |
| CX4/LCG | 1.08 ± 0.06 | (7.2 ± 0.5) × 107 | 0.84 ± 0.03 | (3.3 ± 0.9) × 108 | 1.01 ± 0.06 | (1.2 ± 0.7) × 109 |
| [0.93 ± 0.06]b | [0.59 ± 0.05]b | |||||
| CB7/BE | >10 | (1.7 ± 0.1) × 105 | 0.15 ± 0.02 | (2.9 ± 0.5) × 107 | 0.15 ± 0.02 | 7.0 × 1011d |
| [0.05 ± 0.03]c | [4.8 × 1011]d | |||||
Subsequently, the membrane activity was investigated with the three assays for Ada-R7. Notably, the attachment of the adamantylglycine residue to the N-terminus of the R7 peptide had only a negligible influence on the measured membrane activity as obtained by the three assays (Table 1). When comparing the results for the same heptaarginine peptide (either with an N-terminal phenylalanyl or adamantylglycinyl residue or without N-terminal amino acid residue), the EC50 values showed very good consistency. This clearly demonstrates that adamantylglycine, unlike other amino acids such as tryptophan,22 has a negligible influence on membrane activity, classifying adamantylglycine as a minimally invasive peptide label for transport studies.
Lastly, we were interested whether binding of externally added CB7 had an influence on the membrane transport activity with the adamantylglycine peptide. Addition of CB7 had indeed a small, but sizable influence, whereas the transport activity systematically increased with increasing CB7 concentration (Fig. 4). This was surprising since an increased size should commonly lead to reduced membrane permeation on account of hindered diffusion. A control experiment in presence of aminomethyladamantane (AMADA) confirmed that CB7 binding to Ada-R7 is indeed responsible for the increased activity. AMADA has a very high affinity to CB7 and very slow dissociation kinetics,11 which irreversibly blocks the CB7 cavity during the transport experiment. This led to the restoration of the much lower transport activity (red trace in Fig. 4), suggesting that the transport activity of the Ada-R7 peptide can be up- and down-regulated by CB7 binding.
In conclusion, we have introduced herein adamantylglycine (Ada) as a high-affinity amino acid to facilitate peptide-CB7 binding. The small size of Ada renders it minimally invasive in peptide transport experiments. This will be useful for peptide binding to CB7 in competitive environments, e.g., in biological fluids or inside cells. Furthermore, the very high affinity will enable a biorthogonal up- and down-regulation of membrane transport activity of cell-penetrating peptides.
The work was funded by the Deutsche Forschungsgemeinschaft (DFG HE 5967/6-1 and NA 686/15-1).
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. Yin, Q. Cheng, D. Bardelang and R. Wang, JACS Au, 2023, 3, 2356–2377 CrossRef CAS PubMed; Q. Wu, Q. Lei, H.-C. Zhong, T.-B. Ren, Y. Sun, X.-B. Zhang and L. Yuan, Chem. Commun., 2023, 59, 3024–3039 RSC; D. V. D. W. Kankanamalage, J. H. T. Tran, N. Beltrami, K. Meng, X. Zhou, P. Pathak, L. Isaacs, A. L. Burin, M. F. Ali and J. Jayawickramarajah, J. Am. Chem. Soc., 2022, 144, 16502–16511 CrossRef PubMed; J. Chen, R. J. Hooley and W. Zhong, Bioconjugate Chem., 2022, 33, 2245–2253 CrossRef PubMed; N. Barooah, J. Mohanty and A. C. Bhasikuttan, Langmuir, 2022, 38, 6249–6264 CrossRef PubMed; A. Som, M. Pahwa, S. Bawari, N. D. Saha, R. Sasmal, M. S. Bosco, J. Mondal and S. S. Agasti, Chem. Sci., 2021, 12, 5484–5494 RSC; C. J. Addonizio, B. D. Gates and M. J. Webber, Bioconjugate Chem., 2021, 32, 1935–1946 CrossRef PubMed; Y.-H. Liu, Y.-M. Zhang, H.-J. Yu and Y. Liu, Angew. Chem., Int. Ed., 2021, 60, 3870–3880 CrossRef PubMed; A. Kataki-Anastasakou, S. Hernandez and E. M. Sletten, ACS Chem. Biol., 2021, 16, 2124–2129 CrossRef PubMed; W. Xue, P. Y. Zavalij and L. Isaacs, Angew. Chem., Int. Ed., 2020, 59, 13313–13319 CrossRef PubMed; L. Liu, Y. You, K. Zhou, B. Guo, Z. Cao, Y. Zhao and H.-C. Wu, Angew. Chem., Int. Ed., 2019, 58, 14929–14934 CrossRef PubMed; C. L. Schreiber and B. D. Smith, Nat. Rev. Chem., 2019, 3, 393–400 CrossRef PubMed; R. Sasmal, N. Das Saha, M. Pahwa, S. Rao, D. Joshi, M. S. Inamdar, V. Sheeba and S. S. Agasti, Anal. Chem., 2018, 90, 11305–11314 CrossRef PubMed; S. van Dun, C. Ottmann, L.-G. Milroy and L. Brunsveld, J. Am. Chem. Soc., 2017, 139, 13960–13968 CrossRef PubMed; J. Murray, K. Kim, T. Ogoshi, W. Yao and B. C. Gibb, Chem. Soc. Rev., 2017, 46, 2479–2496 RSC.
- W. Cao, X. Qin, Y. Wang, Z. Dai, X. Dai, H. Wang, W. Xuan, Y. Zhang, Y. Liu and T. Liu, Angew. Chem., Int. Ed., 2021, 60, 11196–11200 CrossRef CAS PubMed.
- J. An, S. Kim, A. Shrinidhi, J. Kim, H. Banna, G. Sung, K. M. Park and K. Kim, Nat. Biomed. Eng., 2020, 4, 1044–1052 CrossRef CAS PubMed; D. Shetty, J. K. Khedkar, K. M. Park and K. Kim, Chem. Soc. Rev., 2015, 44, 8747–8761 RSC.
- W. Liu, S. K. Samanta, B. D. Smith and L. Isaacs, Chem. Soc. Rev., 2017, 46, 2391–2403 RSC; L. Cao, M. Šekutor, P. Y. Zavalij, K. Mlinarić-Majerski, R. Glaser and L. Isaacs, Angew. Chem., Int. Ed., 2014, 53, 988–993 CrossRef CAS PubMed; S. Moghaddam, C. Yang, M. Rekharsky, Y. H. Ko, K. Kim, Y. Inoue and M. K. Gilson, J. Am. Chem. Soc., 2011, 133, 3570–3581 CrossRef PubMed.
- M. J. Webber, E. A. Appel, B. Vinciguerra, A. B. Cortinas, L. S. Thapa, S. Jhunjhunwala, L. Isaacs, R. Langer and D. G. Anderson, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 14189–14194 CrossRef CAS PubMed; D.-W. Lee, K. M. Park, M. Banerjee, S. H. Ha, T. Lee, K. Suh, S. Paul, H. Jung, J. Kim, N. Selvapalam, S. H. Ryu and K. Kim, Nat. Chem., 2011, 3, 154–159 CrossRef PubMed; K. L. Kim, G. Sung, J. Sim, J. Murray, M. Li, A. Lee, A. Shrinidhi, K. M. Park and K. Kim, Nat. Commun., 2018, 9, 1712 CrossRef PubMed.
- L. C. Smith, D. G. Leach, B. E. Blaylock, O. A. Ali and A. R. Urbach, J. Am. Chem. Soc., 2015, 137, 3663–3669 CrossRef CAS PubMed; J. W. Lee, H. H. L. Lee, Y. H. Ko, K. Kim and H. I. Kim, J. Phys. Chem. B, 2015, 119, 4628–4636 CrossRef PubMed; S. Sonzini, A. Marcozzi, R. J. Gubeli, C. F. van der Walle, P. Ravn, A. Herrmann and O. A. Scherman, Angew. Chem., Int. Ed., 2016, 55, 14000–14004 CrossRef PubMed; F. Ma, X. Zheng and Z. Li, Phys. Chem. Chem. Phys., 2021, 23, 13724–13733 RSC; F. Ma, X. Zheng, L. Xie and Z. Li, J. Mol. Liq., 2021, 328, 115479 CrossRef; Y. Zhao, F. Li, F. Ma, J. Zhi, G. Wu and X. Zheng, Phys. Chem. Chem. Phys., 2023, 25, 7893–7900 RSC.
- R. Meudom, N. Zheng, S. Zhu, M. T. Jacobsen, L. Cao and D. H.-C. Chou, Curr. Res. Chem. Biol., 2022, 2, 100013 CrossRef CAS.
- L. A. Logsdon, C. L. Schardon, V. Ramalingam, S. K. Kwee and A. R. Urbach, J. Am. Chem. Soc., 2011, 133, 17087–17092 CrossRef CAS PubMed.
- X. Chen, Z. Huang, R. L. Sala, A. M. McLean, G. Wu, K. Sokołowski, K. King, J. A. McCune and O. A. Scherman, J. Am. Chem. Soc., 2022, 144, 8474–8479 CrossRef CAS PubMed.
- H. R. Anderson, W. L. Reeves, A. T. Bockus, P. Suating, A. G. Grice, M. Gallagher and A. R. Urbach, Bioconjugate Chem., 2023, 34, 212–217 CrossRef CAS PubMed.
- M. A. Alnajjar, W. M. Nau and A. Hennig, Org. Biomol. Chem., 2021, 19, 8521–8529 RSC.
- S. Peng, A. Barba-Bon, Y.-C. Pan, W. M. Nau, D.-S. Guo and A. Hennig, Angew. Chem., Int. Ed., 2017, 56, 15742–15745 CrossRef CAS PubMed.
- A. Barba-Bon, Y.-C. Pan, F. Biedermann, D.-S. Guo, W. M. Nau and A. Hennig, J. Am. Chem. Soc., 2019, 141, 20137–20145 CrossRef CAS PubMed.
- J. Murray, J. Sim, K. Oh, G. Sung, A. Lee, A. Shrinidhi, A. Thirunarayanan, D. Shetty and K. Kim, Angew. Chem., Int. Ed., 2017, 56, 2395–2398 CrossRef CAS PubMed.
- A. T. Bockus, L. C. Smith, A. G. Grice, O. A. Ali, C. C. Young, W. Mobley, A. Leek, J. L. Roberts, B. Vinciguerra, L. Isaacs and A. R. Urbach, J. Am. Chem. Soc., 2016, 138, 16549–16552 CrossRef CAS PubMed; A. Norouzy, Z. Azizi and W. M. Nau, Angew. Chem., Int. Ed., 2015, 54, 792–795 CrossRef PubMed; Y. Liu, C. Hu, J. A. Serna, F. Biedermann and P. A. Levkin, RSC Chem. Biol., 2023, 4, 760–764 RSC.
- A. Hennig and W. M. Nau, Front. Chem., 2020, 8 Search PubMed; J. Hostaš, D. Sigwalt, M. Šekutor, H. Ajani, M. Dubecký, J. Řezáč, P. Y. Zavalij, L. Cao, C. Wohlschlager, K. Mlinarić-Majerski, L. Isaacs, R. Glaser and P. Hobza, Chem. – Eur. J., 2016, 22, 17226–17238 CrossRef PubMed.
- L. Cao and L. Isaacs, Supramol. Chem., 2014, 26, 251–258 CrossRef CAS.
- A. Hennig, Org. Mater., 2022, 4, 53–60 CrossRef CAS.
- Y.-C. Pan, A. Barba-Bon, H.-W. Tian, F. Ding, A. Hennig, W. M. Nau and D.-S. Guo, Angew. Chem., Int. Ed., 2021, 60, 1875–1882 CrossRef CAS PubMed; J. N. Martins, B. Raimundo, A. Rioboo, Y. Folgar-Cameán, J. Montenegro and N. Basílio, J. Am. Chem. Soc., 2023, 145, 13126–13133 CrossRef PubMed; F. Perret, M. Nishihara, T. Takeuchi, S. Futaki, A. N. Lazar, A. W. Coleman, N. Sakai and S. Matile, J. Am. Chem. Soc., 2005, 127, 1114–1115 CrossRef PubMed.
- J. C. Espeche, R. Varas, P. Maturana, A. C. Cutro, P. C. Maffía and A. Hollmann, Peptide Sci., 2023, e24305 Search PubMed; H. T. Kratochvil, Robert W. Newberry, B. Mensa, M. Mravic and W. F. DeGrado, Faraday Discuss., 2021, 232, 9–48 RSC; S. Guha, J. Ghimire, E. Wu and W. C. Wimley, Chem. Rev., 2019, 119, 6040–6085 CrossRef CAS PubMed; M. Vazdar, J. Heyda, P. E. Mason, G. Tesei, C. Allolio, M. Lund and P. Jungwirth, Acc. Chem. Res., 2018, 51, 1455–1464 CrossRef PubMed; M. Gestin, M. Dowaidar and Ü. Langel, in Peptides and Peptide-based Biomaterials and their Biomedical Applications, ed. A. Sunna, A. Care and P. L. Bergquist, Springer International Publishing, Cham, 2017, pp. 255–264 CrossRef PubMed; S. Futaki and I. Nakase, Acc. Chem. Res., 2017, 50, 2449–2456 CrossRef PubMed; N. Chuard, K. Fujisawa, P. Morelli, J. Saarbach, N. Winssinger, P. Metrangolo, G. Resnati, N. Sakai and S. Matile, J. Am. Chem. Soc., 2016, 138, 11264–11271 CrossRef PubMed; W. B. Kauffman, T. Fuselier, J. He and W. C. Wimley, Trends Biochem. Sci., 2015, 40, 749–764 CrossRef PubMed; R. Brock, Bioconjugate Chem., 2014, 25, 863–868 CrossRef PubMed; E. G. Stanzl, B. M. Trantow, J. R. Vargas and P. A. Wender, Acc. Chem. Res., 2013, 46, 2944–2954 CrossRef PubMed; C. Ciobanasu, J. P. Siebrasse and U. Kubitscheck, Biophys. J., 2010, 99, 153–162 CrossRef PubMed.
- T. Miyatake, M. Nishihara and S. Matile, J. Am. Chem. Soc., 2006, 128, 12420–12421 CrossRef CAS PubMed.
- S. Khemaissa, A. Walrant and S. Sagan, Q. Rev. Biophys., 2022, 55, e10 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, supporting results, and details on assay procedures. See DOI: https://doi.org/10.1039/d4cc00602j |
| This journal is © The Royal Society of Chemistry 2024 |