Open Access Article
Open Access ArticleThe size-dependent valence and conduction band-edge energies of Cu quantum dots†
Takahiro
Matsui
a,
Hiroto
Watanabe
 *a,
Shoichi
Somekawa
b,
Sayaka
Yanagida
b,
Yuya
Oaki
*a,
Shoichi
Somekawa
b,
Sayaka
Yanagida
b,
Yuya
Oaki
 a and
Hiroaki
Imai
a and
Hiroaki
Imai
 *a
*a
aDepartment of Applied Chemistry, Faculty of Science and Technology, Keio University, 3-14-1 Hiyoshi, Kohoku-ku, Yokohama 223-8522, Japan. E-mail: hiroaki@applc.keio.ac.jp
bTokyo Metropolitan Industrial Technology Research Institute, 2-4-10 Aomi. Koto-ku, Tokyo 135-0064, Japan
First published on 13th March 2024
Abstract
Ultra-small metal particles having band gaps are regarded as a new class of functional materials. We investigated the size dependencies of the band-edge energies on Cu quantum-dots in the size range of 0.7–2.1 nm. The extremely high conduction band-edge energies owing to the strong quantum-size effects were observed for sizes below 1 nm.
A shiny luster, which originates from the light reflection by their free electrons, is one of the most common characteristics of metals.1 The continuous energy band of metals around their Fermi level allows the presence of free electrons. When the particle sizes are reduced to a few tens of nanometers, the collective oscillation of the free electrons resonates with visible light and displays brilliant colors, which is called localized surface plasmon resonance (LSPR) (Fig. 1a).2 Upon further reduction of the particle size to near their Fermi wavelength (∼0.5 nm for Au), their optical and electronic properties change dramatically.3 In this size region, the electronic structure changes from a metallic to a molecule-like state (Fig. 1c). LSPR absorption is no longer observable due to the absence of free electrons and new absorption edges that originate from the electronic transition across the energy-gap.4 In the earlier studies, these tiny particles, mostly called “metal clusters,” were only formed as a cluster ion beam in a vacuum chamber.4b Since these metal clusters are formed and exist only for a short time in the chamber, their research has been limited to the field of fundamental physical chemistry.4b,5 Recently, metal clusters were regarded as practical materials owing to the development of large-scale synthetic routes via solution processes.6 Thus, these tiny metal clusters are now gathering interest in the field of materials chemistry as a new class of fluorophores and photocatalysts. For example, thiol-protected Au clusters are one of the most studied species.6a,b They show size-dependent photoluminescence in the UV and near-infrared regions. For Au, the transition from the metallic to the molecule-like state occurs at a size of ∼1.6–1.8 nm (Au144–Au187).7 Thus, the appearance of energy-gap states is not only the case for 2-dimensional clusters having a few-atoms, but also occurs for much larger 3-dimensional particles having a few tens to a few hundreds of atoms. These particles were recognized as metal quantum-dots (QDs) (Fig. 1b).3a
 | ||
| Fig. 1 Schematic illustration of Cu nanoparticles (Cu NPs), Cu quantum dots (Cu QDs) and Cu clusters. | ||
From the viewpoint of solid-state physics, the formation of electronic gap states with the size reduction of the metal particles is ascribed to quantum size effects, which have been well studied in the field of semiconductors.8 When the sizes of the semiconductor particles are similar to their exciton Bohr radius, their band gap energies (Egs) increase with the size reduction. Therefore, the Eg of QDs is controlled by changing their particle sizes. Semiconductor QDs show size-dependent functionalities, such as color-tunable bright luminescence.9–12
In the field of photocatalysis, valence and conduction band-edge energies (Evb and Ecb) are important because the photocatalytic activity strongly depends on the alignment of their energies and the redox potential of the target reactions.8b,13 Therefore, the photocatalytic reactivities are controllable by changing the sizes of the QDs. For example, the Evb and Ecb of WO3 QDs were optimized by controlling their sizes below 1 nm to improve the photocatalytic hydroxylation of benzene.14 The size dependency of Evb and Ecb owing to the quantum size effect is theoretically calculated using an effective mass approximation (EMA) method and shows good agreement with experimental values.15
The aim of the present study is to determine the size dependency of the Evb and Ecb of Cu QDs by using spectroscopic approaches. Different from Au and Pt, Cu has a relatively high Fermi level in its bulk state.16 Thus, Cu QDs smaller than 1 nm are excellent candidates for photocatalysts having a high Ecb level. Although the size dependency of the Evb and Ecb of Cu QDs has been theoretically investigated,17 experimental work was lacking because of the difficulty of the size-selective synthesis. Previously, our research group found a large expansion of Eg for CuO QDs with sizes below 1 nm, which were synthesized by using pores of porous silicas (PSs) as templates.18 In the present study, we synthesized Cu QDs through the reduction of CuO QDs and then unveiled the size dependency of the Evb, and Ecb of the Cu QDs (Fig. 1). In particular, for the 1 nm Cu QDs, an extremely high Ecb was observed, which corresponded to the high photocatalytic reduction ability of the Cu QDs expected from the theoretical study.19 Furthermore, we found that the band expansion of the metal QDs was explainable via the EMA method by taking into account the physical quantities obtained from a recent spectroscopic study of Cu.20
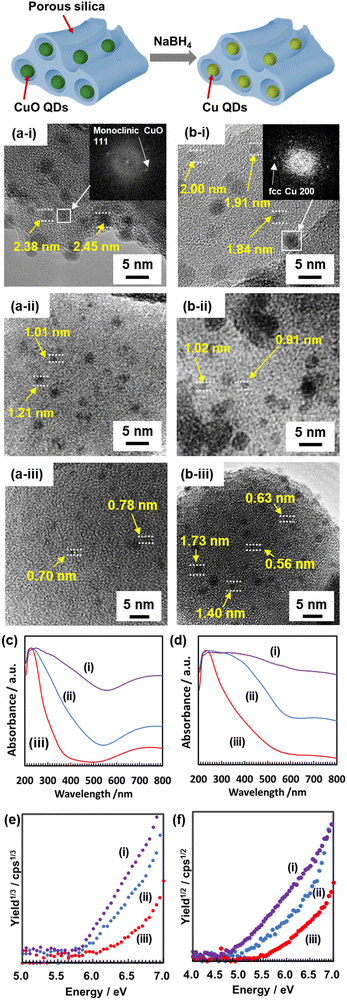
Here, four kinds of PSs with different pore sizes were used as templates for the production of CuO QDs (see Section S2 in the ESI† for the synthesis and analyses of the PSs).21 CuO QDs were prepared in the PSs using a simple impregnation method (see Section S3 in the ESI† for the experimental details). As shown in the typical transmission electron microscopy (TEM) images (Fig. 2a, and Fig. S3 in the ESI†), fine particles were prepared in the pores of PSs, and the particle size was well controlled in a range from 0.6 to 2.1 nm. The presence of CuO was confirmed by observing a lattice fringe image and a fast Fourier transform (FFT) pattern corresponding to the (111) plane of monoclinic CuO (inset in Fig. 2a-i). From UV-visible (UV-vis) absorption spectra, we observed a broad absorption of Cu2+d–d transition around 800 nm and size-dependent absorption edges around 300 to 500 nm for CuO QDs (see Fig. 2c, Fig. S8 and S9 in the ESI†). As described in our previous study,18 the band gap energy of the CuO QDs increased remarkably owing to the strong quantum size effect expressed below 2 nm. The detailed band structure analyses of the CuO QDs are described in Section 6 in the ESI.†
For the Cu QD preparation, a reductant solution of NaBH4 was injected into the pores of PSs containing CuO QDs. Fig. 2b and Fig. S4 in the ESI† show typical TEM images of Cu QDs. In particular, for Cu QDs, the aggregation and growth of the particles occurred during the high magnitude TEM observations (Fig. S5 in the ESI†). We analyzed the particle size distribution profiles by deconvoluting the primal and large particles formed by electron beam exposure to determine the average size and dispersity of the primal particles from TEM images. The primal particle size of the Cu QDs was well controlled in the range from 0.7 to 2.1 nm. As shown in the inset of Fig. 2b-i, we observed a lattice fringe image and an FFT pattern corresponding to the (200) planes of fcc Cu for the sample after the reduction. This clearly indicates the successful formation of Cu QDs. The presence of Cu QDs was also confirmed by the X-ray absorption near-edge structure (XANES) spectra of the samples before and after the reduction (Fig. S7 in the ESI†). The present Cu QDs show no fluorescence due to the absence of surface passivation agents.
The UV-vis spectra indicate that the absorption energy shifts due to the quantum size effect (Fig. 2d and Fig. S10a in the ESI†). After the reduction, the absorption of the Cu2+d–d transition disappeared, and the absorption edges shifted to a lower-energy side. These changes in the UV-vis spectra also indicate the reduction of CuO QDs to Cu QDs. The size dependency of the band gap energies of the Cu QDs was calculated using Tauc plots22 (Fig. S10b in the ESI†) as shown in Fig. 3a. The dotted curve in Fig. 3a represents the theoretical fitting of the present experimental data using an EMA method (see Section S7 in the ESI† for detailed analyses using the EMA method). Adopting a value of μ = 1.15 m0 as a reduced mass of Cu, the present experimental data were well fitted using the EMA method. For the 2.1 nm Cu particles, the absorption peak assigned to the LSPR was observed at 580 nm (Fig. S11 in the ESI†).23 Based on this result, the transition from the metallic state (= LSPR active) to the semiconductor-like state occurs at around 2 nm. This threshold diameter is slightly larger than that of the thiolate-protected Au QDs (1.6–1.8 nm).7
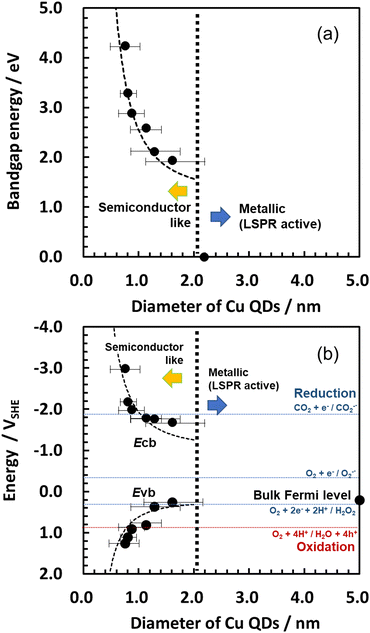 | ||
| Fig. 3 Size dependency of (a) Eg, (b) Evb, and Ecb for Cu QDs. The dotted curves represent calculation data using an EMA method. Several redox potentials of photocatalytic reactions are represented with horizontal lines. The error bars represent the FWHM of the particle size distribution profiles shown in Fig. S4 (ESI†). | ||
For the band-structure analysis of the Cu QDs, their Evb was measured using photoemission yield spectroscopy in air (PYSA) (Fig. 2f and Fig. S13 in the ESI†) (Fig. 2e and Fig. S12 in the ESI† show the PYSA spectra for CuO QDs).24 PYSA is a powerful tool for investigating the Evb for semiconductors and the Fermi level for metals. The PYSA stimulation with very weak UV light (10–100 nW, 4.0–7.0 eV) under ambient conditions is a suitable method to determine the Evb of QDs, which is sensitive to the measurement conditions.24b By combining the Eg obtained from UV-vis spectra and the Evb obtained from PYSA, the size dependency of the Evb and Ecb of Cu QDs was obtained and is summarized in Fig. 3b (see Section S6 in the ESI† for detailed analyses). Both the Evb and Ecb show dramatic shifts below 1 nm. The energy shift of the Ecb is larger than that of the Evb. This is in contrast to the case of CuO QDs (Fig. S14 in the ESI†). The present experimental values were fitted via EMA separately for the Evb and Ecb. Since the electronic configuration of a Cu atom is described as 1s2 2s2 2p6 3s2 3p6 3d10 4s1 4p0, the partially filled 4s orbitals mainly contribute to the occupied energy state around the Fermi level.17b When the quantum size effect became significant, this orbital shifts to the lower-energy side and forms a valence band. On the other hand, unoccupied energy states, which are mainly formed by 4p orbitals, exist above the Fermi level. Recently, Sonoda found that the lowest unoccupied bulk electronic state of Cu lies 1.24 eV above the Fermi level (structure D in the article) by using angle-resolved two-photon photoemission spectroscopy.20 This energy state shifts to the higher-energy side and constructs the conduction-band edge owing to the quantum size effect. The effective mass of the electron in this state was estimated to be 1.67 m0. Therefore, we used the following physical quantities for the EMA fitting: Ecb(bulk) = −1.03 VSHE, Evb(bulk) = +0.21 VSHE, me = 1.67 m0, μ = 1.15 m0, and mh = 3.69 m0 (see Section S7 in the ESI† for details of the EMA method). Using these values, the present experimental data were well fitted with EMA (Fig. 3b). Therefore, similar to the semiconductor QDs, the formation of the band gap states and their expansion due to the size reduction of metal QDs are explainable with a theory based on the quantum size effects of nanoparticles. The present results show a similar tendency with previous studies.4b,19b
Here, the Ecb for 1 nm QDs exceeds −2.0 VSHE and shifts to −3.0 VSHE for 0.7 nm Cu QDs. These Ecb values are higher than those of the single-electron reduction potential of O2 (−0.35 VSHE)25 and CO2 (−1.90 VSHE).26 Thus, as described in the previous molecular orbital calculations, Cu QDs are efficient photocatalysts, especially for the reduction reaction. Cysteine-capped Cu10 was reported to have high photocatalytic activity for the degradation of an organic dye.19b This is probably due to the high Ecb, which produces highly active superoxide radicals. The Cu10 also has high stability against aerobic oxidation owing to its low HOMO level expected in the previous study.19b In the present study, we experimentally observed the low Evb levels (+ 1.26 VSHE), which were located below the reduction potential of molecular oxygen for 0.7 nm Cu QDs.25 Taking the relationship between the Evb and the particle sizes into account, oxidative stability is expected for Cu QDs smaller than 2 nm. However, the Evb levels of the Cu QDs were only slightly lower than the oxidation potential of water.25 Thus, relatively low efficiency is expected for the photocatalytic water oxidation. The construction of a Z-scheme hetero-junction27 with oxide semiconductors is required for the best photocatalytic performance of the Cu QDs.
In summary, we produced Cu QDs of various sizes, ranging from 0.7 to 2.1 nm, by reducing CuO QDs synthesized in the pores of PSs. The size dependency of their Evb and Ecb was investigated experimentally using UV-vis and PYSA techniques. We observed a significant shift in the Evb and Ecb, which was due to strong quantum size effects when the particle sizes were below 1 nm. Since the Ecb for 0.7 nm Cu QDs exceeds the single-electron reduction potentials of O2 and CO2, Cu QDs would be potential candidates for efficient photocatalysts for the reduction reactions.
We thank Dr Y. Nakajima (RIKEN KEIKI Co., Ltd.) for helpful discussions and kind assistance with the PYSA experiments. We thank Dr T. Kaya (Keio University) for the TEM experiments. This work was partially supported by JSPS KAKENHI grant number JP21H01627.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. A. White, Physical Properties of Materials, CRC Press, Boca Raton, 2018, pp. 47 Search PubMed.
- K. M. Mayer and J. H. Hafner, Chem. Rev., 2011, 111, 3828 CrossRef CAS PubMed.
- (a) J. Zheng, P. R. Nicovich and R. M. Dickson, Annu. Rev. Phys. Chem., 2007, 58, 409 CrossRef CAS PubMed; (b) Y. Lu and W. Chen, Chem. Soc. Rev., 2012, 41, 3594 RSC.
- (a) W. A. de Heer, Rev. Mod. Phys., 1993, 65, 611 CAS; (b) K. J. Taylor, C. L. Pettiette-Hall, O. Cheshnovsky and R. E. Smalley, J. Chem. Phys., 1992, 96, 3319 CrossRef CAS.
- (a) A. Sebetci and Z. B. Güvenç, Surf. Sci., 2003, 525, 66 CrossRef CAS; (b) M. A. Tafoughalt and M. Samah, Comput. Theor. Chem., 2014, 1033, 23 CAS; (c) T. Tsuneda, J. Comput. Chem., 2019, 40, 206 CAS.
- (a) J. Zheng, C. Zhang and R. M. Dickson, Phys. Chem. Lett., 2004, 93, 077402-1 Search PubMed; (b) K. G. Stamplecoskie and P. V. Kamat, J. Am. Chem. Soc., 2014, 136, 11093 CrossRef CAS PubMed; (c) I. Chakraborty, R. G. Bhuin, S. Bhat and T. Pradeep, Nanoscale, 2014, 6, 8561 RSC; C. Vázquez-Vázquez, M. Bañobre-López, A. Mitra, M. A. López-Quintela and J. Rivas, Langmuir, 2009, 25, 8208 Search PubMed; (d) T. Imaoka and K. Yamamoto, Bull. Chem. Soc. Jpn., 2019, 92, 941 CrossRef CAS.
- Y. Negishi, T. Nakazaki, S. Malola, S. Takano, Y. Niihori, W. Kurashige, S. Yamazoe, T. Tsukuda and H. Hakkinen, J. Am. Chem. Soc., 2015, 137, 1206 CrossRef CAS PubMed.
- (a) F. P. García de Arquer, D. V. Talapin, V. I. Klimov, Y. Arakawa, M. Bayer and E. H. Sargent, Science, 2021, 373, eaaz8541 CrossRef PubMed; (b) D. Kandi, S. Martha and K. M. Parida, Int. J. Hydrogen Energy, 2017, 42, 9467 CrossRef CAS.
- M. L. Landry, T. E. Morrell, T. K. Karagounis, C. H. Hsia and C. Y. Wang, J. Chem. Educ., 2014, 91, 274 CrossRef CAS.
- M. Shen, W. Jia, Y. You, F. Li, S. Tian, J. Li, Y. Jin and D. Han, Nanoscale Res. Lett., 2013, 8, 253 CrossRef PubMed.
- H. R. You, J. Y. Park, D. H. Lee, Y. Kim and J. Choi, Appl. Sci., 2020, 10, 975 CrossRef CAS.
- T. Nakotte, H. Luo and J. Pietryga, Nanomaterials, 2020, 10, 172 CrossRef CAS PubMed.
- (a) T. Inoue, A. Fujishima, S. Konishi and K. Honda, Nature, 1979, 277, 637 CrossRef CAS; (b) T. Suzuki, H. Watanabe, Y. Oaki and H. Imai, Chem. Commun., 2016, 52, 6185 RSC.
- A. Ohno, H. Watanabe, T. Matsui, S. Somekawa, M. Tomisaki, Y. Einaga, Y. Oaki and H. Imai, Catal. Sci. Technol., 2021, 11, 6537 RSC.
- (a) L. Brus, J. Phys. Chem., 1986, 90, 2555 CrossRef CAS; (b) F. Rodríguez-Mas, J. C. Ferrer, J. L. Alonso, D. Valiente and S. Fernández de Ávila, Crystals, 2020, 10, 226 CrossRef.
- T. Tani, J. Soc. Photogr. Imaging Jpn., 2015, 78, 16 Search PubMed.
- (a) M. Kabir, A. Mookerjee and A. K. Bhattacharya, Eur. Phys. J. D, 2004, 31, 477 CrossRef CAS; (b) C. G. Li, Z. G. Shen, Y. F. Hu, Y. N. Tang, W. G. Chen and B. Z. Ren, Sci. Rep., 2017, 7, 1345 Search PubMed; (c) U. J. Rangel-Peña, R. L. Camacho-Mendoza, S. González-Montiel, L. Feria and J. Cruz-Borbolla, Clust. Sci., 2021, 32, 1155 Search PubMed.
- H. Tamaki, H. Watanabe, S. Kamiyama, Y. Oaki and H. Imai, Angew. Chem., Int. Ed., 2014, 53, 10706 CAS.
- (a) S. Huseyinova, J. Blanco, F. G. Requejo, J. M. Ramallo-López, M. C. Blanco, D. Buceta and M. A. López-Quintela, J. Phys. Chem. C, 2016, 120, 15902 CrossRef CAS; (b) S. Huseyinova, J. M. B. Trillo, J. M. Ramallo-López, F. G. Requejo, D. Buceta and M. A. López-Quintela, Phys. Chem. Chem. Phys., 2023, 25, 6025 CAS.
- Y. Sonoda, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 245410 Search PubMed.
- H. Watanabe, K. Fujikata, Y. Oaki and H. Imai, Microporous Mesoporous Mater., 2015, 214, 41 CrossRef CAS.
- J. Tauc, Mater. Res. Bull., 1970, 5, 721 CrossRef CAS.
- P. Liu, H. Wang, X. Li, M. Rui and H. Zeng, RSC Adv., 2015, 5, 79738 RSC.
- (a) D. Yamashita, Y. Nakajima, A. Ishizaki and M. Uda, J. Surf. Anal., 2008, 14, 433 CAS; (b) J. Jasieniak, M. Califano and S. E. Watkins, ACS Nano, 2011, 5, 5888 CrossRef CAS PubMed; (c) E. O. Kane, Phys. Rev., 1962, 127, 131 CrossRef CAS.
- W. H. Koppenol, D. M. Stanbury and P. L. Bounds, Free Radical Biol. Med., 2010, 49, 317 CrossRef CAS PubMed.
- S. Lu, F. Lou and Z. Yu, Catalysts, 2022, 12, 228 CrossRef CAS.
- B.-J. Ng, L. K. Putri, X. Y. Kong, Y. W. The, P. Pasbakhsh and S.-P. Chai, Adv. Sci., 2020, 7, 1903171 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: (1) Materials. (2) Preparation and characterization of porous silicas. (3) Preparation and characterization of the CuO QDs and Cu QDs. (4) Particle size analysis of the CuO QDs and Cu QDs. (5) Determination of the valence states of the CuO QDs and Cu QDs by X-ray absorption near-edge structure measurements. (6) Determination of the band structures of the CuO QDs and Cu QDs. (7) Curve fitting with effective mass approximation (EMA) for the CuO QDs and Cu QDs. See DOI: https://doi.org/10.1039/d4cc00260a |
| This journal is © The Royal Society of Chemistry 2024 |