Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel radiolabelled salmochelin derivative for bacteria-specific PET imaging: synthesis, radiolabelling and evaluation†
Renato
Margeta
a,
Sonja
Schelhaas
a,
Sven
Hermann
a,
Michael
Schäfers
a,
Silke
Niemann‡
b and
Andreas
Faust‡
 *a
*a
aEuropean Institute for Molecular Imaging (EIMI), University Münster, Röntgenstraße 16, 48149, Münster, Germany
bInstitute of Medical Microbiology, University Hospital Münster, Domagkstraße 10, 48149, Münster, Germany
First published on 15th February 2024
Abstract
For specific imaging of bacterial infections we aimed at targeting the exclusive bacterial iron transport system via siderophore-based radiotracers. De novo synthesis and radiolabeling yielded the salmochelin-based PET radiotracer [68Ga]Ga-RMA693, which showed a favourable biodistribution and a bacteria-specific uptake in an animal model of Escherichia coli infection.
Bacterial infections such as vascular graft infections, endocarditis, or sepsis remain a leading cause of morbidity and mortality worldwide despite tremendous progress in both diagnosis and treatment. Infections can be difficult to localize and are often associated with nonspecific clinical presentation such as fever and pain. Further, diagnostic blood cultures may be false-negative under or after antibacterial therapy or in the case of bacterial strains that do not grow in blood cultures. These diagnostic challenges have triggered the use of imaging techniques to localize infections. To date, [18F]FDG-positron emission tomography (PET) is available in clinical molecular imaging of infections which relies on the uptake of radiolabelled glucose in activated immune cells given their increased glucose metabolism.1–3 Thus, this imaging technique does not target bacteria but rather the immune response to bacteria, and thus it is not possible, e.g., to distinguish between sterile inflammation and infection. Besides using bacterial cell wall components like D-amino acids or the bacteria specific uptake of complex carbohydrates PET radiotracers targeting the bacteria-specific iron transport system are a possible way.4
An ideal bacteria-specific PET tracer should exclusively be taken up by bacteria and not by host cells. In order to achieve high sensitivity, a molecular signal amplification strategy would be desirable, e.g. through transporter-driven cellular uptake and accumulation in the bacterium. In addition, the tracer should be highly stable in blood and serum so that it is not degraded by, e.g. enzymes during transport through the blood with only a small amount of the tracer actually reaching the site of infection.5
Iron is essential for almost all organisms. To capture enough iron during infections, bacteria have developed specific strategies, such as the production of siderophores, low molecular weight (500–1500 Da) iron chelators with a high affinity for Fe(III). These molecules bind Fe(III) with a higher affinity than eukaryotic transferrin or lactoferrin and can thereby capture Fe(III). Gram-negative bacteria, such as Escherichia coli or Klebsiella pneumoniae, are surrounded by two membranes, the cytoplasmic cell membrane and the outer membrane. Dedicated bacterial outer membrane transporters have a high affinity for iron-siderophore complexes (e.g. FepA for Fe-enterobactin and IroN for Fe-salmochelin) and thereby scavenge these complexes from the environment and transfer them into the periplasmic space. There they are associated to binding proteins and translocated to the cytoplasmic membrane and, via specific transporters, to the cytoplasm.6 Thus, efforts have been made to couple antibiotics to siderophores in order to efficiently deliver them into pathogens via the siderophore-mediated iron transport systems in a Trojan horse strategy.8 For PET a few siderophore-based radiopharmaceuticals have been developed. Desferrioxamine-derivatives labelled with gallium-68 ([68Ga]Ga-DFO-B) showed promising results in Pseudomonas aeruginosa and Staphylococcus aureus infected mice combined with a fast renal clearance.9 Several attempts were made to introduce artificial analogues of enterobactin as drug conjugates or imaging tools.10 In one study a related 68Ga-labelled enterobactin analogue showed limited in vivo performance.11
Enterobactin as a common siderophore is secreted by Enterobacteriaceae such as E. coli or K. pneumoniae. The host counteracts this iron uptake mechanism of the bacteria by producing lipocalin-2. This protein binds to the iron-laden siderophore, preventing its uptake by the bacteria.12 However, bacteria such as Salmonella species, some Klebsiella strains and (uro)pathogenic and avian pathogenic E. coli strains can produce salmochelin S4, a di-C-glucosylated enterobactin (Fig. 1). This so-called stealth siderophore is not bound by lipocalin-2 and thereby allows bacteria to acquire iron even in the presence lipocalin-2.13
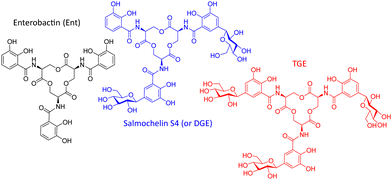 | ||
| Fig. 1 Structures of selected salmochelin derivatives: enterobactin (Ent), di-(DGE or salmochelin S4), and tri-C-glucosylated Ent (RMA693 or TGE7). | ||
In a former study salmochelin S4 was used as Trojan horse conjugated with ciprofloxacin.14 This conjugate as tetradentate ligand showed limited bacterial uptake in comparison to a hexadentate enterobactin-conjugate15 which underline the importance of the molecular shape for recognition. In our study, we use the gallium-68 (replacing iron) carrying salmochelin-derivative RMA693 as hexadentate complex to target E. coli (Fig. 1). It enables fast and near-quantitative radiolabelling with gallium-68 at room temperature and near-neutral pH.16 Additionally, the symmetric tri-C-glucosylated enterobactin offers a scalable synthesis and complex isolation can be avoided. After radiolabelling with gallium-68 in vitro experiments to characterize bacterial uptake and first in vivo experiments in mice were performed with successful PET-based imaging of bacterial infections.
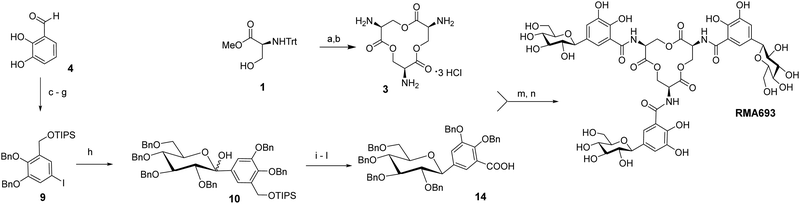
Scheme 1 summarizes the total synthesis of RMA693 as precursor for radiolabelling. Most steps are related to literature-known methods but were optimized to enable large-scale synthesis and purification via precipitation or crystallization instead of column chromatography. Starting with the commercially available protected L-serine macrolactonization and subsequent deprotection yielded the trilactone ring 3 in large scale (82%, two steps).17 To get the iodinated catechol-derivative 6 we selectively mono benzylated18 the 2,3-dihydroxy benzaldehyde 4 followed by iodination19 in para-position to the phenol for later C-glycosylation. Further benzylation, reduction of the benzylaldehyde 7 and silyl protection of the resulting alcohol 8 yielded the key intermediate 9. Accordingly, we did not perform C-glycosylations via metal-catalysed cross coupling reactions like Negishi- or Stille-coupling.20 We lithiated 9 and with glucono-1,5-lactone we built up selectively the β-C-glycoside 10 as hemiketal. After reduction of the lactol with triethylsilane in the presence of BF3·OEt221 the silylated benzyl alcohol was deprotected and oxidized in a two-step procedure using Dess Martin periodinane and Lindgren-oxidation yielding the carboxylic acid 14.22 Again, no column chromatography was necessary, and all steps could be performed on a multigram scale (see ESI† for details). The free amino groups from lactone 3 were combined with carboxylic acid 14via peptide coupling activated by PyAOP and the resulting perbenzylated RMA693 was deprotected quantitatively under standard conditions yielding the precursor RMA693 for complexation either with iron or gallium. It was observed that RMA693 is very sensitive to nucleophiles and oxidizing agents which also others reported during their work on enterobactin.22 This sensitivity was attributed to the macrolactone ring, which is identical for RMA693. Coordination of RMA693 with tris(2,2,6,6-tetramethyl-3,5-heptanedionato)iron(III) and -gallium(III) yielded nearly quantitatively the corresponding complexes as purple (Fe) and white (Ga) solids (Scheme 2). For manual radiolabelling generator-eluted gallium-68 (350–600 MBq) was neutralized and diluted with PBS-buffer (pH 7.4). After 10 min at 50 °C the precursor was totally consumed and the radiochemical purity of the resulting radiotracer [68Ga]Ga-RMA693 was higher than 99% (TLC and HPLC). It is stable for at least 120 minutes in the blood serum of mice and humans (Fig. S2, ESI†) and in used media for in vitro evaluation (Fig. S4, ESI†).
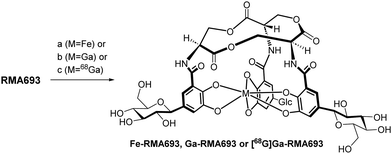 | ||
Scheme 2 (a) Fe(TMHD)3, MeOH/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, rt, 97%; (b) Ga(TMHD)3, MeOH/EtOAc 1 1, rt, 97%; (b) Ga(TMHD)3, MeOH/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, rt, 95%; (c) [68Ga][GaCl4]− (350–600 MBq), PBS-buffer, NaHCO3(aq), 50 °C, 10 min. 1, rt, 95%; (c) [68Ga][GaCl4]− (350–600 MBq), PBS-buffer, NaHCO3(aq), 50 °C, 10 min. | ||
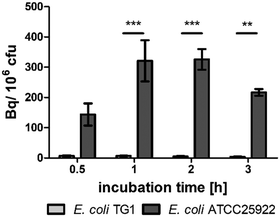
In vitro, E. coli ATCC25922 showed uptake of [68Ga]Ga-RMA693 in contrast to E. coli TG1 and also S. aureus LS1 (Fig. 2 and Fig. S3, ESI†). While the strain-dependent [68Ga]Ga-RMA693 accumulation could be detected with as little as 0.1 MBq ml−1 (Fig. S3a, ESI†) the uptake of the radiotracer was also shown to be correlated with the amount applied, with 1 MBq ml−1 giving a tenfold higher signal (Fig. S3b, ESI†). The tracer was not taken up by S. aureus LS1 or by E. coli TG1 which demonstrates the bacterial specificity of [68Ga]Ga-RMA693, since salmochelin uptake and thus uptake of the newly developed tracer depends on the presence of the specific transporter system. Gram-positive staphylococci such as S. aureus as well as E. coli TG1, an E. coli K-12 derivative, lack such transporters.23
When incubated with 0.1 MBq ml−1[68Ga]Ga-RMA693 in iron-depleted cation-adjusted medium (ID-CAMHB), we observed a 30-fold increase in the accumulation of the tracer in E. coli ATCC25922 compared to incubation in iron-containing medium (Fig. S3c, ESI†). Even under these conditions, E. coli TG1 showed no uptake of [68Ga]Ga-RMA693 (Fig. S3c, ESI†). When 1 MBq ml−1 tracer was applied, a nearly 10-fold accumulation was achieved, except for E. coli TG1. Furthermore, the highest uptake was reached already after one hour of incubation (Fig. 2). In Enterobacteriaceae, the uptake of iron by the bacterial cell is strictly regulated and the transport systems are only induced when iron levels are low. As a result, the expression of the salmochelin receptor (IroN) in the outer membrane of the bacteria is increased.24 This explains the increased and faster uptake of [68Ga]Ga-RMA693 in iron-depleted medium compared to uptake in iron-containing environment. During infection, the host decreases the accessibility of iron to the bacteria, e.g., by increasing the iron level in macrophages and decreasing it in blood plasma.25 Due to this nutritional immunity the in vitro results imply that the tracer should be sufficiently taken up by bacteria also in vivo.
To characterize the tracer pharmacokinetics in vivo, a biodistribution study in adult CD-1 mice was performed after intravenous injection of ∼10 MBq of [68Ga]Ga-RMA693. PET imaging demonstrated fast and notable elimination of [68Ga]Ga-RMA693 from the blood via the kidneys into the urinary bladder (Fig. S5a and b, ESI†). Besides this renal route of tracer elimination we did not observe any accumulation of [68Ga]Ga-RMA693 in other tissues or organs. Quantitative analysis visualized rapid clearance of the tracer from the blood and renal excretion of >90% of the injected dose at the end of the study (90 min post injection, (Fig. S5c, ESI†)). Finally, in vivo data were confirmed by ex vivo gamma counting of harvested organs and tissues (Fig. S5d, ESI†).
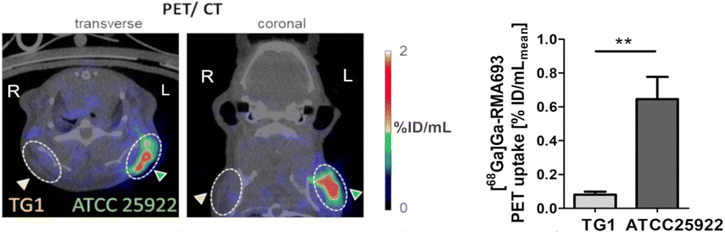
Next, we tested [68Ga]Ga-RMA693 in a mouse model of bacterial infection. Here, a infection was induced by subcutaneous inoculation of E. coli ATCC25922 in the left shoulder region. The E. coli TG1 was used as a negative control and was injected in the right shoulder region. 3 h after infection, [68Ga]Ga-RMA693 was injected intravenously and PET/CT imaging was performed. Images show accumulation of [68Ga]Ga-RMA693 restricted to the area of E. coli ATCC 25922 infection in the left shoulder, in contrast to the E. coli TG1 infection on the contralateral side (Fig. 3). Finally, in vivo data were confirmed by ex vivo gamma counting of harvested tissues (Fig. S6a, ESI†). The bacterial load of the tissue was determined. From both shoulders we recovered living bacteria (in the mouse presented in Fig. 3: left shoulder: 1.51 × 106 colony forming units (cfu) mL−1E. coli ATCC25922; right shoulder: 7.2 × 106E. coli TG1 cfu mL−1). To determine whether the [68Ga]Ga-RMA693 uptake semiquantitatively reflects the number of bacteria at the infection site, we analyzed the correlation between the mean [68Ga]Ga-RMA693 PET uptake and the number of viable bacteria in the tissue. Indeed, for E. coli ATCC 25922 tracer uptake was linearly correlated with the bacterial load (r = 0.886, p < 0.05). In contrast, there was no such correlation for E. coli TG1 (r = 0.486, p = 0.356) (Fig. S6b and c, ESI†). This result demonstrates that [68Ga]Ga-RMA693 can be used to selectively detect pathogenic E. coli, which are often capable of producing and utilizing salmochelin.
In conclusion, aiming to visualize bacterial infections in vivo in a non-invasive and bacteria-specific manner, we have developed a radiotracer for PET imaging targeting the bacterial siderophore-mediated iron transport system. Its scaffold is salmochelin-based, which delivers radioactive gallium instead of iron into the bacterial cell in a Trojan horse strategy. The synthetic development allows large-scale synthesis of tri-glucosylated enterobactin as well as easy and fast 68Ga-radiolabeling with high radiochemical yield and radiochemical purity without further time-consuming purification. We show the strain-dependent uptake of the radiotracer in vitro and demonstrate in a first in vivo study the potential to specifically detect bacterial infections by PET imaging using our new radiotracer. Further studies are currently ongoing to investigate the sensitivity of the tracer, also in different infectious mouse models.
This work was supported by the Interdisciplinary Centre for Clinical Research (IZKF, University Hospital of Münster, Germany, Fau2-014-17, CoreUnit PIX) and the Collaborative Research Centre (CRC) 1450–431460824, project A04, University of Münster, Germany.
Conflicts of interest
There are no conflicts to declare.Notes and references
- E. I. Reinders Folmer, G. C. I. von Meijenfeldt, M. J. van der Laan, A. W. J. M. Glaudemans, R. H. J. A. Slart, B. R. Saleem and C. J. Zeebregts, Eur. J. Vasc. Endovasc. Surg., 2018, 56, 719 CrossRef PubMed.
- V. Hoerr, M. Franz, M. W. Pletz, M. Diab, S. Niemann, C. Faber, T. Doenst, P. C. Schulze, S. Deinhardt-Emmer and B. Löffler, Int. J. Med. Microbiol., 2018, 308, 640 CrossRef CAS PubMed.
- E. H. Dibble, D. C. Yoo, G. L. Baird and R. B. Noto, AJR Am. J. Roentgenol., 2019, 213, 1358 CrossRef PubMed.
- S. Alberto, A. A. Ordonez, C. Arjun, G. K. Aulakh, N. Beziere, E. Dadachova, T. Ebenhan, U. Granados, A. Korde, A. Jalilian, W. Lestari, A. Mukherjee, M. Petrik, T. Sakr, C. L. S. Cuevas, M. M. Welling, J. R. Zeevaart, S. K. Jain and D. M. Wilson, J. Nucl. Med., 2023, 64, 1676 CrossRef CAS PubMed; W. Roll, A. Faust, S. Hermann and M. Schäfers, J. Nucl. Med., 2023, 64, 59S CrossRef PubMed.
- A. Axer, S. Hermann, G. Kehr, D. Clases, U. Karst, L. Fischer-Riepe, J. Roth, M. Fobker, M. Schäfers, R. Gilmour and A. Faust, ChemMedChem, 2018, 13, 241 CrossRef CAS PubMed.
- R. C. Hider and X. Kong, Nat. Prod. Rep., 2010, 27, 637 RSC.
- X. Yu, Y. Dai, T. Yang, M. R. Gagné and H. Gong, Tetrahedron, 2011, 67, 144–151 CrossRef CAS.
- M. G. P. Page, Clin. Infect. Diseases, 2019, 69, S529 CrossRef CAS PubMed.
- M. Petrik, E. Umlaufova, V. Raclavsky, A. Palyzova, V. Havlicek, J. Pfister, C. Mair, Z. Novy, M. Popper, M. Haiduch and C. Decristoforo, Eur. J. Nucl. Med. Mol. Imaging, 2021, 48, 372 CrossRef CAS PubMed.
- K. Ferreira, H.-Y. Hu, V. Fetz, H. Prochnow, B. Rais, P. P. Müller and M. Brönstrup, Angew. Chem., Int. Ed., 2017, 56, 8272 CrossRef CAS PubMed; P. Klahn, R. Zscherp and C. C. Jimidar, Synthesis, 2022, 3499 CrossRef; R. Zscherp, J. Coetzee, J. Vornweg, J. Grundenberg, J. Herrmann, R. Müller and P. Klahn, Chem. Sci., 2021, 12, 10179 RSC.
- C. Peukert, L. N. B. Langer, S. M. Wegener, A. Tutov, J. P. Bankstahl, B. Karge, F. M. Bengel, T. L. Ross and M. Brönstrup, J. Med. Chem., 2021, 64, 12359 CrossRef CAS PubMed.
- T. Flo, K. Smith, S. Sato, D. J. Rodriguez, M. A. Holmes, R. K. Strong, S. Akira and A. Aderem, Nature, 2004, 432, 917 CrossRef CAS PubMed.
- B. Bister, D. Bischoff, G. J. Nicholson, M. Valdebenito, K. Schneider, G. Winklemann, K. Hantke and R. D. Süssmuth, Biometals, 2004, 17, 471 CrossRef CAS PubMed.
- M. A. Joaqui-Joaqui, M. K. Pandey, A. Bansal, M. V. Ramakrishnam Raju, F. Armstrong-Pavlik, A. Dundar, H. L. Wong, T. R. DeGrado and V. C. Pierre, Inorg. Chem., 2020, 59, 12025 CrossRef CAS PubMed.
- T. J. Sanderson, C. M. Black, J. W. Southwell, E. J. Wilde, A. Pandey, R. Herman, G. H. Thomas, E. Boros, A.-K. Duhme-Klair and A. Routledge, ACS Infect. Dis., 2020, 6, 2532 CrossRef CAS PubMed.
- W. Neumann, M. Sassone-Corsi, M. Raffatellu and E. M. Nolan, J. Am. Chem. Soc., 2018, 140, 5193 CrossRef CAS PubMed.
- R. J. A. Ramirez, L. Karamanukyan, S. Ortiz and C. G. Gutierrez, Tetrahedron Lett., 1997, 38, 749–752 CrossRef CAS.
- I. E. Wrona, A. E. Gabarda, G. Evano and J. S. Panek, J. Am. Chem. Soc., 2005, 127, 15026–15027 CrossRef CAS PubMed.
- A. V. Joshua, S. K. Sharma and D. N. Abrams, Synth. Commun., 2008, 38, 434–440 CrossRef CAS.
- H. Gong and M. R. Gagné, J. Am. Chem. Soc., 2008, 130, 12177–12183 CrossRef CAS PubMed; F. Zhu, J. Rodriguez, T. Yang, I. Kevlishvili, E. Miller, D. Yi, S. O’Neill, M. J. Rourke, P. Liu and M. A. Walczak, J. Am. Chem. Soc., 2017, 139, 17908–17922 CrossRef PubMed; E. J. Wilde, E. V. Blagova, T. S. Sanderson, D. J. Raines, R. P. Thomas, A. Routledge, A. K. Duhme-Klair and K. S. Wilson, J. Inorg. Biochem., 2019, 190, 75–84 CrossRef PubMed.
- T. C. Ho, H. Kamimura, K. Ohmori and K. Suzuki, Org. Lett., 2016, 18, 4488–4490 CrossRef CAS PubMed.
- T. Zheng, J. L. Bullock and E. M. Nolan, J. Am. Chem. Soc., 2012, 134, 18388–18400 CrossRef CAS PubMed.
- M. Valdebenito, A. L. Crumbliss, G. Winkelmann and K. Hantke, Int. J. Med. Microbiol., 2006, 296, 513–520 CrossRef CAS PubMed; K. Hantke, G. Nicholson, W. Rabsch and G. Winkelmann, Proc. Natl. Acad. Sci., 2003, 100, 3677–3682 CrossRef PubMed; J. E. Cassat and E. P. Skaar, Semin. Immunopathol., 2012, 34, 215–235 CrossRef PubMed.
- A. R. Mey, C. Gómez-Garzón and S. M. Payne, EcoSal Plus, 2021, 9, eESP00342020 CrossRef PubMed.
- R. Golonka, B. S. Yeoh and M. Vijay-Kumar, J. Innate Immun., 2019, 11, 249–262 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Chemical and radiochemical synthesis and all analytical data, in vivo stability and biodistribution of the radiotracer, further in vitro assays, etc. See DOI: https://doi.org/10.1039/d4cc00255e |
| ‡ SN and AF contributed equally and share the senior authorship. |
| This journal is © The Royal Society of Chemistry 2024 |