Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Porous protein crystals: synthesis and applications
Alec Arthur
Jones
 a and
Christopher D.
Snow
a and
Christopher D.
Snow
 *ab
*ab
aSchool of Biomedical Engineering, Colorado State University, Fort Collins, CO 80523-1301, USA. E-mail: christopher.snow@colostate.edu
bDepartment of Chemical and Biological Engineering, Colorado State University, Fort Collins, CO 80523-1301, USA
First published on 30th April 2024
Abstract
Large-pore protein crystals (LPCs) are an emerging class of biomaterials. The inherent diversity of proteins translates to a diversity of crystal lattice structures, many of which display large pores and solvent channels. These pores can, in turn, be functionalized via directed evolution and rational redesign based on the known crystal structures. LPCs possess extremely high solvent content, as well as extremely high surface area to volume ratios. Because of these characteristics, LPCs continue to be explored in diverse applications including catalysis, targeted therapeutic delivery, templating of nanostructures, structural biology. This Feature review article will describe several of the existing platforms in detail, with particular focus on LPC synthesis approaches and reported applications.
1. Introduction
Proteins are well-suited as a substrate for material applications, due to their biocompatibility,1 and structural diversity.2 A variety of mesostructures emerge as a property of their constitutive nanostructures; these include protein nanoparticles,3 nanocages,4,5 hydrogels,6 plate-like crystals and sheets,7,8 and porous scaffolds.9–11Porous materials, with their ability to host and transport guest molecules within their frameworks, have been explored in recent years for diverse scientific and industrial applications. Among these, large-pore protein crystals (LPCs) represent a distinctive class of crystalline materials characterized by a three-dimensional network of interconnected voids and channels. Large-pore protein crystals exhibit remarkable structural diversity compared with traditional nanomaterials, owing to the diversity of protein sequences found in nature. A typical bacterial protein sequence of 300 amino acids,12 could have 20300 distinct amino acid sequences; assuming ∼8 conformational degrees of freedom per residue,13,14 the number of possible conformations extends to ∼1.72 × 10661 theoretical conformations, effectively an infinite landscape of structural diversity. While most of these hypothetical structures would be unstable and any plausible structure would be restricted to narrowly defined minima within this energy landscape, the number of stable structures is nevertheless vast. The ability of proteins to undergo genetic selection, along with the wholesale shuffling and recombination of domains15 enables a realistic sampling of this landscape. The enormous diversity of protein structures (and functions) has sparked a growing interest in their potential applications as biomimetic scaffolds.
The unique architecture of protein crystals arises from the self-assembly of protein molecules into ordered, crystalline structures. Protein crystals typically contain a high surface area to volume ratio16 with a well-defined lattice of interconnected pores. Pore diameters typically range from tens of Angstroms to greater than 10 nm (Fig. 1) with corresponding wide-ranging solvent content between 27% and 65%.11,17–21 These solvent channels enable the accommodation of a wide array of guest molecules, from small ions to large biomolecules such as peptides, nucleic acids, and other proteins. Possessing solvent channels of sufficient size for guest macromolecule transport would be one characteristic of the LPC subset of protein crystals. The Matthews coefficient (VM), defined as the fraction of crystal volume per unit of protein molecular weight (Å3 Da−1), is a useful parameter for specifying solvent content. More recent estimates on the distribution of VM, based on reported crystallographic data, suggest that VM of reported protein crystals is quite broad, from extremely high density (just over 1 Å3 Da−1, approximating tightly-packed spheres) to extremely low density in the case of LPCs (just below 10 Å3 Da−1).22,23 Due to their high solvent content and void space, LPCs are also uniquely sensitive to dehydration, which typically results in contraction of the overall crystal structure. Dehydration can be achieved in a controlled manner by altering the relative humidity or osmolarity of the surrounding environment of the crystal.24 While deformation of the nanostructure is often undesirable for material applications of LPCs, for structural investigations, crystals may be deliberately dehydrated to improve their diffraction resolution, although cracks or other broad morphological defects can potentially negate the improved diffraction limit.25
Understanding the formation mechanisms of LPCs is pivotal for their controlled synthesis. Crystallization of biomolecules is notably distinct from crystallizing small molecules, owing primarily to their increased molecular weight and complexity. The crystallization process involves an interplay of many factors, including protein concentration, pH, temperature, ionic strength, and the presence of precipitants and nucleating agents.26 Proteins have additional considerations such as susceptibility to misfolding and ease of expression and purification. Recent advances in protein engineering and biophysical techniques have facilitated the rational design and optimization of LPCs.
Notably, while LPC growth is similar to the growth of other less porous protein crystals, a key distinguishing characteristic is that sufficiently large pores could allow building blocks throughout the crystal to exchange with solution. A candidate quantitative threshold for LPCs, therefore, would be any protein crystal with solvent channels of sufficient diameter to allow transport of the constituent crystal building blocks – in other words, LPCs may be defined as protein crystals whose pore radius is at least twice the hydrodynamic radius of the constituent monomer. Precise nanopore sizes within a crystal lead to a sharp cutoff on transport depending on guest size. Accordingly, biomolecular crystals have been explored as molecular sieves for selective adsorption and separation of biomolecules.27 The confined environment within LPCs also provides a unique platform for enzyme immobilization and colocalization, enhancing catalytic efficiency and stability.28–30 Specific applications for LPCs that have been investigated include catalysis, templating of nanostructures, and targeted therapeutic delivery of bioactive compounds, all of which will be described in this review. This Feature Article will provide a broad overview of the current state of LPCs, with particular focus on their methods of synthesis and specific applications.
2. Synthesis methods
2.1. Control of size and morphology
Historically, protein crystallization has been most often used for the specific purpose of generating large, uniform crystals that are ideal for X-ray diffraction.31 Due to their irregular shape and complex interactions with each other and the surrounding solvent, many proteins have a tendency to crystallize into structures which feature sizable solvent-filled cavities and pores. Multiple synthesis routes have been described for generating nanoporous scaffolds from proteins. The most commonly reported approach involves first growing the crystal using conventional methods, such as sitting- or hanging-drop vapor diffusion or batch crystallization. Vapor diffusion methods generally result in larger, fewer crystals, and are more suited towards generating crystals for X-ray diffraction. One common approach involves mixing concentrated protein stock with a high-ionic strength precipitant buffer (e.g. a buffered high-salt solution, such as sodium chloride or ammonium sulphate). The wells also contain a reservoir containing the undiluted precipitant solution. The wells are subsequently sealed, and water molecules diffuse through the vapor phase between the well solution and the droplet containing protein. Due to the lower salt concentration within the protein droplet, equilibrium between the droplet and the well results in a gradual net decrease in volume in the protein droplet. The resulting gradual increase in protein and salt concentration occurs slowly enough that nucleation proceeds very slowly, and metastable growth of crystals can begin immediately after nucleation (Fig. 2). | ||
| Fig. 2 Phase diagram showing the trajectory, over time, of protein and precipitant concentration for vapour diffusion (blue arrow) and batch crystallization (red arrow). | ||
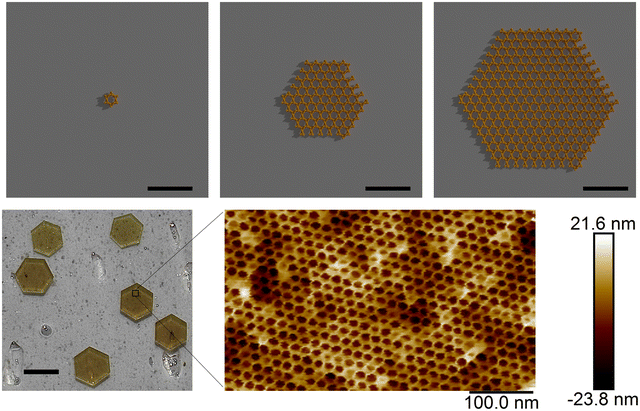
Batch crystallization is distinct from vapor diffusion in that both the protein and precipitant are mixed in a single volume, without any vapor-phase equilibration. At sufficiently high protein and precipitant concentrations, nucleation proceeds very rapidly, resulting in many smaller crystals. This approach is well-suited for generating a large number of small crystals, although it requires optimization to avoid issues such as excess protein aggregation. The “CJ” crystals used by our group, from a putative polyisoprenoid-binding protein from Campylobacter jejuni have been shown to possess very large solvent channels (>13 nm diameter), via X-ray diffraction9 and atomic force microscopy.32 We have frequently relied on batch crystallization to generate large quantities of small porous microcrystals for a variety of applications.
Methods for generating porous protein structures include growth of complete structures from constituent monomers (“bottom-up”), or crystal growth followed by disassembly into sub-structures, such as protein nanotubes or cages. While most of the systems described in this review conform to a bottom-up approach, a number of approaches that instead use the crystal form as a template have been described. For instance, Ueno and coworkers constructed a supramolecular nanotube assembly, by mutating a key interface residue (I149C) in RuBisCO from Thermococcus kodakaraensis.33 After growth, the crystals were crosslinked in an oxidizing buffer for 24 hours; following cross-linking, the crystals were dissolved into their constituent nanotubes by soaking in an alkaline solution, and subsequently characterized via transmission electron microscopy.33 Proposed applications of such tubular assemblies include templating of nanomaterials, as confinement within the tube enables size-restricted growth of inorganic materials like nanorods (a similar approach by the Ueno group is described in more detail in Section 3.3). Similar top-down strategies for synthesizing porous protein structures, specifically protein cages, have been employed by the Care group34 and Hilvert group.5
Porous proteinaceous materials are not restricted to large proteins alone. Heinz-Kunert et al. constructed a porous peptide framework from a synthetic peptide building block.35 The building block consisted of a simple α helix, containing rigid, planar, and highly aromatic bipyridyl residues extending from the N- and C-termini; the bipyridyl side chains can participate in π-stacking with neighbouring peptides, resulting in the formation of a porous lattice.35 While this system is restricted to small-length peptides, the resulting scaffold shares the characteristic tunability of LPCs via amino acid substitutions, as evidenced by changes in molar uptake of Nile red in response to substitutions at the pore-facing residues.35 Similarly, cyclic peptide nanotubes (cPNTs) have received increasing attention in recent years for a number of applications, particularly for therapeutic delivery,36,37 owing to their high biocompatibility and ease of synthesis. Similar to LPCs, cPNTs can be synthesized with a range of pore sizes,38,39 in some cases as high as 2 μm in diameter.40 Furthermore, a significant advantage of these systems is the ease with which they can be incorporated into the lipid bilayer of the plasma membrane.
Any discussion of LPC microcrystal synthesis would not be complete without consideration for production scale-up. Furthermore, synthesis of LPC microcrystals is unlike synthesis of conventional meso- and microporous scaffolds, insofar as it relies on a biologically derived building block, whereas more traditional systems, including mesoporous silica particles and other inorganic and polymer-based systems are traditionally composed of non-biologically derived substances. Scaled production of lysozyme nano- and microcrystals has been described using an oscillatory flow reactor,41,42 in which control of size and crystal abundance is achieved via increased turbulent mixing. More critically, however, the necessity to express and purify a recombinant protein from a host organism results in a significant increase in both time and expense, compared with other porous nano- and microparticle scaffolds. One potential method of overcoming these obstacles involves overexpressing and crystallizing the scaffold protein in vivo or in living cells. Abe et al. from the Ueno group have observed crystallization of polyhedrin in insect cells (S. frugiperda and B. mori),27 recapitulating earlier observations by Mori et al.43 Similar in vivo crystallization approaches have been used for generating Cry3 crystals.44 More recent work by the Ueno group describes a cell-free system for generating nanocrystals, by co-incubating template mRNA with wheat germ extract;45 although these crystallization reactions were only performed at millilitre scales, this approach, similar to in vivo crystallization, obviates the need for downstream purification of the protein.
For most of the downstream applications described in this article, it is favourable to generate monodisperse crystals of very small sizes (ideally less than 5 μm in diameter). For this reason, many methods have been explored for generating nano- and microcrystals in large batches. Crystal size, as well as crystallization efficiency (defined as the ratio of crystallized protein to total protein) is influenced most heavily by protein concentration, followed by precipitant concentration, temperature, pH, and the presence of nucleating agents.46
The canonical methods for growing protein crystals include batch crystallization, microdialysis, and sitting- and hanging-drop vapor diffusion. While vapor diffusion is a preferred method for growing large crystals, droplet handling is typically impractical for generating many small crystals. Nevertheless, it can be useful in the context of studying the behaviour of LPCs at a visible scale. Within our group, thanks to the conserved nanostructure of both large and small crystals, we routinely predict the behaviour of smaller porous microcrystals from X-ray diffraction and fluorescence microscopy of larger crystals, which are easier to manipulate and observe.11,47,48
2.2. Enhancing crystal stability
Most crystals derived from biomolecules are susceptible to dissolution when transferred outside of their mother liquor. Therefore, it is often necessary to chemically crosslink the crystals following growth and washing, to ensure that they retain their structural integrity following solvent exchange. Some rare protein crystals, such as crystals derived from the insecticidal, Bacillus thuringiensis-derived Cry toxins44,49 do not require additional downstream cross-linking. S-layer proteins, which confer structural rigidity to the outer membrane of certain prokaryotic species, have also been shown to form highly stable two-dimensional porous crystals on the surface of living cells.50–52Commonly reported methods for stabilizing protein crystals (Fig. 3) include chemical methods such as formaldehyde,53 glutaraldehyde,54,55 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide,56,57 and glyoxal.58,59 Oxidation of proximal cysteines to promote the formation of disulfide bonds within LPCs is another well-established strategy.60–62 While sortases63–65 and SpyCatcher/SpyTag66 have been well studied in the context of enzyme immobilization and installation of guests within or on scaffolds, these systems have been less characterized for their use in stabilizing the scaffold itself.
 | ||
| Fig. 3 An overview of the various reported methods for cross-linking PPCs, or stabilizing guest-crystal interactions, via covalent bonds. | ||
Solution exchange and downstream handling are potential challenges when cross-linking LPCs. In an ideal scenario, the crystals should be stabilized within their mother liquor immediately following or during growth, as downstream handling and washing steps and solution exchanges can result in mishandling and damaging of the crystals. Ultraviolet photocrosslinking is an alternative strategy for LPC stabilization that remains largely unexplored. UV excitation of tyrosine-rich proteins in the presence of strong oxidizers, such as Ru(II) complexes, results in the formation of reactive radical species which can subsequently form covalent bonds with neighbouring tyrosines.67 Similar chemistries are believed to contribute to the mechanical strength of insect wings68 and fibroin and keratin structures in Tussah silk.69 Dityrosine crosslinking has been employed for other structure determination-based methods such as mass spectrometry70,71 and NMR,72 and has seen some use as a method for stabilizing protein films and nanoparticles.73 At the same time, it should be noted that radical chemistry can result in the undesirable formation of free-radical species and unintended side reactions within the crystal; UV photocrosslinking is a common method for sterilization and inactivating enzymes in vitro.74 For biochemically inert scaffolds however, the reduced handling and transfer steps may make UV-induced crosslinking an attractive strategy for stabilizing LPCs.
2.3. Validation
While this article has described some of the methods for generating LPCs, as well as stabilizing them outside of their mother liquor, it is worth noting that the methods for characterizing LPCs themselves are similarly diverse, and these depend in large part on the nature of the crystals themselves. Properties of interest include crystal size, porosity, dimensionality, and tolerance to desiccation. Many of the methods for studying the morphology and behaviour of LPCs overlap significantly with established techniques used in materials engineering, and structural and cell biology.For small crystals, scanning electron microscopy (SEM) is often used to validate both the size and correct habit of porous microcrystals. For tiny crystals (<1 μm diameter), dynamic light scattering (DLS) is another technique used for size validation and has the additional benefit of being able to measure the surface zeta potential. The latter property is critical for ensuring that crystals are monodisperse in solution. DLS involves measuring the frequency in intensity of incident scattered light off of a sample over time, in order to build a correlation function that reveals the overall rate of diffusion of particles in a sample. Size determination, in turn, relies on the relationship between the diffusion coefficient (D) and the hydrodynamic radius (RH) of a given particle, as described by the Stokes–Einstein equation:75,76
Regarding kinetics, crystal formation is commonly hypothesized to proceed in a two-step fashion – nucleation, followed by metastable growth.77–79 Furthermore, the thermodynamics of crystal nucleation are believed to result in a two-step process – aggregation into protein-rich clusters, followed by phase transition into an ordered crystal structure (for this reason, nucleation is recognized as the rate-limiting step in microcrystal formation). The free energy associated with homogeneous nucleation (characteristic of batch crystallization) is dependent on the volume (bulk) free energy, solvent-accessible (interfacial) free energy, and the surface tension between the nucleus and surrounding solution.80
Because crystal nucleation is preceded by the formation of disordered liquid clusters, one of the challenges of using DLS to monitor crystal growth is its inability to distinguish between amorphous protein clusters and well-ordered nanocrystals. Fortunately, because crystalline materials demonstrate much higher form birefringence than amorphous aggregate, DLS can be used in combination with depolarized DLS (DDLS) in order to reliably distinguish nanocrystals from disordered aggregates, allowing for real-time monitoring of crystal formation.81 The Chan group has also routinely used SEM and DLS on Cry3Aa crystals to validate their morphology and size.10
For direct evaluation of crystal porosity, transmission electron microscopy (TEM) is one of the more commonly reported methods.82 Our group has used SEM to characterize the size and shape of LPC microcrystals, and atomic force microscopy (AFM) to characterize the pore surface of macroscopic crystals Fig. 4, bottom). Some advantages of AFM over TEM include its non-destructive nature, ease of sample preparation, and the ability to monitor guest–crystal interactions in situ.32,83 We have also recently employed tuneable resistive pulse sensing (TRPS) for microcrystal batches to obtain size distributions and concentrations of microcrystal batches. This platform is increasingly used for quantifying extracellular vesicles and nanoparticles.84
3. Functionalization and applications
3.1. Enzyme scaffolds
It has been hypothesized that many enzymes and enzyme complexes have naturally evolved their scaffolding and active sites to reduce the distance that substrates need to diffuse through a pathway from one enzyme's active site to another – putative examples from nature include a host of dehydrogenases85–87 as well as more extreme examples like tryptophan synthase.88–90 It has been previously argued that for efficient channelling to occur, the processing rate (that is, the rate at which the enzyme processes a given substrate within a confined geometry, defined as kcatN/V, where kcat is the catalytic constant of an enzyme, and N is the number of enzyme molecules confined to a given volume, V) must exceed the escape rate (i.e., the frequency at which a substrate diffuses away from the confined enzyme, or D/r2) for a particular enzyme; this is ultimately influenced by the catalytic efficiency of the enzyme, as well as the degree of confinement.91,92 The controlled confinement within crystal pores offers a unique platform for enzyme immobilization and biocatalysis. By harnessing the ordered structure and tailored pore sizes of LPCs, enzymes can be selectively encapsulated, resulting in enhanced stability and catalytic efficiency.93,94 The integration of enzymes within LPCs not only expands the scope of biocatalytic processes but also may enable sustainable industrial applications due to increased reusability.Kowalski et al. from our group previously characterized enzymatic activity in CJ crystals.47 CJ crystallizes to form P622 crystals with large pores (∼13 nm) along their hexagonal face, along with smaller flanking pores (∼3 nm). Because CJ crystals typically have a hexahistidine tag at the C-terminus, the resulting tags project into the major pore. As a result, guest proteins which also have a polyhistidine tag are able to interact with the histidine tag inside of the crystal via shared metal affinity,95 resulting in non-covalent “tethering” of the protein guest. In this study, CJ crystals were grown to large sizes (>0.1 mm) using sitting-drop vapor diffusion, and subsequently cross-linked using glyoxal. The resulting scaffolds remained resistant to dissolution after transferring outside their mother liquor. After cross-linking, the crystals were soaked in a basic buffer containing nickel, in order to saturate the histidine tags in the crystal with nickel ions. Separately, bovine serum albumin (BSA), horseradish peroxidase (HRP), and glucose oxidase (GOX) were fluorescently labelled. Uptake of HRP was observed via confocal fluorescence microscopy, and crystal saturation was achieved after 16 hours. Co-loading of both GOX and HRP within the same crystal resulted in visible HRP activity throughout the crystal over a 2-minute period, after adding glucose and a fluorescent substrate. It was additionally observed that the crystal could be reused up to at least 5 recycles. These results demonstrated the feasibility of using protein crystals as a multi-enzyme scaffold, although this approach remained limited insofar as guest loading was non-covalent, and pH and metal dependent as a result of histidine tag tethering.
Recyclability of the scaffold in an industrial context is additionally a vital consideration. Heater et al. from the Chan group previously characterized the use of Cry3Aa crystals as a catalytic scaffold for the production of biodiesel from waste cooking oil.96 Following their investigations of Cry3Aa structures, which revealed the presence of large pores (∼50 Å × 50 Å), Heater et al. managed to confer lipase activity on crystals comprised of Cry3Aa-lipase fusion protein; the resulting crystals contained a lipase enzyme fused to the C-terminus of Cry3Aa, projecting into the pore space of conventional Cry3Aa crystals.96 The Cry3Aa-lipase fusion was subsequently screened (while the crystals were still encapsulated within cells) for improved activity via directed evolution. Optimized crystals displayed slightly higher Km values, and greatly reduced kcat values. When the crystals were purified in vitro, they demonstrated fatty acid transesterification to fatty acid methyl esters (FAME) in a variety of harsh solvent conditions and following prolonged exposure to increased temperatures. The same crystals were capable of an impressive 15 reuse cycles despite having been centrifuged, washed twice with hexane, and vacuum-dried between each cycle, with relative percent conversion remaining greater than 77%. The greatly reduced turnover may be explained as a result of suboptimal orientation of the immobilized enzyme. Similarly reduced catalytic efficiency was observed by Kowalski et al. for HRP immobilized within CJ crystal pores. It is probable that turnover and substrate binding are heavily influenced by a variety of factors that are difficult to control, such as the orientation of the enzyme active site relative to the crystal pore, as well as possible off-target binding between the guest enzyme and the pore wall.
An additional factor to consider with any reusable scaffold is modularity. It is self-evident that modification of a single platform to be able to accommodate multiple alternative guests is economically advantageous. Covalent installation of guest enzymes within a LPC is likewise desirable, as the resulting irreversible bond is neither pH or redox dependent, allowing the scaffold to tolerate a wider range of chemistries and solution conditions.97 Following on their work with Cry3Aa-lipase crystals, Sun et al. attempted to introduce greater modularity in the Cry3Aa scaffold by fusing SpyCatcher at the C-terminus.98 “SpyCatcher” is a 12.3 kDa protein, redesigned from the immunoglobulin-like CnaB2 domain from Streptococcus pyogenes, which is capable of reacting with a 13 amino acid peptide (“SpyTag”) to form an intermolecular, isopeptide amide bond;99 second and third generation variants which exhibit improved second-order rate constants have since been developed via directed evolution and rational design.100 This platform has been used in a wide variety of applications, including enzyme immobilization,101–103 recombinant protein purification,104 and cyclization of protein termini to improve their stability.105,106 After growing microcrystals from the fusion protein, guest loading was accomplished by sequentially soaking in three guest SpyTag002 fusion enzymes involved in the menaquinone synthesis pathway (MenF, MenD, and MenH) (Fig. 5), with the lowest molecular weight guests soaked into the crystal last.98 Relative activity of the guest enzymes within the crystals was retained at higher temperatures, contrasting with the enzymes in free solution.98 By introducing SpyCatcher at the Cry3Aa terminus within the crystals, the Cry3Aa scaffolds were able to accommodate multiple different guest enzymes in a modular fashion, thereby enabling the confinement of an entire metabolic pathway inside a single crystal scaffold.98 The main limitation of this system is the narrowness of the crystal pores necessitates sequential guest loading on the basis of molecular weight/size. Crystals with larger diameter pores could allow larger domain guests to load without steric occlusion of the solvent channels.
3.2. Therapeutic delivery
Previously well-characterized platforms for therapeutic delivery of DNA, RNA, proteins, and small molecules include gold nanoparticles,107–109 mesoporous silica particles,110–112 liposomes,113,114 and polymer-based scaffolds such as chitosan115 and agarose-based hydrogels.116 While each of these platforms are traditionally treated as independent frontiers in medical research, and the methods of synthesis can vary substantially, there is a considerable degree of overlap in terms of their experimental and analytical methods. Alongside these existing platforms, LPCs represent an emerging frontier.Porous micro- and nanocrystals are particularly amenable to delivery applications, as larger protein crystals localized to the bloodstream could result in capillary occlusion.117 Additionally, morphology and aspect ratio of particles, which can be difficult to control for certain protein crystals, has been shown to greatly influence circulation time and organ and tissue localization.118 As a result, there have been few reported accounts testing delivery in vivo using LPCs. The Chan lab tested in vivo delivery of antimicrobial peptides housed in 1 μm-diameter Cry3Aa crystals in BALB/c mice via intravenous injection, and observed consistent localization of Cry3Aa crystals to the liver, lungs, and kidneys, up to 24 hours post-injection.10 A more recent study by Yang et al. from the same group demonstrated delivery of p53 protein to mammalian cells in vitro using a modified version of Cry3Aa (dubbed Pos3Aa), which contained multiple lysine substitutions to increase the surface charge of the crystals. The Cry protein used to grow these crystals was fused to either mCherry fluorescent protein, or the p53 tumour-suppressing protein.119 An additional justification for these mutations was based on ample evidence that uptake and endosomal escape are heavily dependent on the presence of numerous positively charged residues, such as lysine and arginine.120–125 Independent staining of endosomes and crystals revealed that while cellular uptake occurred for both versions of Cry3Aa, endosomal escape into the cytoplasm was only observed for Pos3Aa crystals. Using different endocytic inhibitors, it was further observed that uptake was not receptor mediated, but instead occurred via macropinocytosis.119 Crystals comprised of Pos3Aa-p53 fusion protein were able to sensitize mice to chemotherapy treatments, consistent with release and activity of p53 within the tumour cells. Cells exposed to crystals containing the mCherry fusion displayed diffuse fluorescence throughout the cytoplasm, which the authors had initially hypothesized may be due to dissolution of the crystals. Unexpectedly however, when they attempted to dissolve the crystals in PBS at physiological temperature and pH, they observed an independent band corresponding to the molecular weight of mCherry alone, rather than the fusion protein, thereby suggesting cleavage and release of the guest fusion protein; subsequent experiments indicated that sustained release of mCherry was observed over a period of three days.119 Although the authors did not speculate as to the precise mechanism of cleavage, the results of this study demonstrate that LPCs can be engineered towards stable delivery of exogenous therapeutic proteins.
Outstanding questions related to the use of new LPCs in living systems include their potential cytotoxicity, immunogenicity, and their compatibility in vasculature, as previously mentioned. The question of cytotoxicity arises from both the potential toxicity of the scaffold protein, as well as the use of any reactive cross-linkers for stabilizing the crystals following synthesis. While our group has previously shown that large crystals grown from CJ do not induce significant cytotoxicity,56 and Cry protein crystals do not appear to be cytotoxic or haemolytic,10 parasporins (also derived from Bacillus thuringiensis) display clear cytotoxicity against HeLa and HT-29 cancer cells.126 This example emphasizes the importance of careful evaluation of the potential in vivo effect of each scaffold protein, as well as consideration of possible carryover of undesirable bioactive substances (e.g. endotoxins) from the host organism used to grow the crystals. The second question as to what extent protein crystals can negatively impact circulation is implicitly addressed in the Chan group studies; intravenous injection of mice with ∼1 μm diameter crystals did not result in any apparent ill-effects on the mice.10,119 In addition to safety, additional work remains related to specific cell targeting and tissue localization. In most of the described platforms, the crystals have remained largely unmodified, although it is conceivable that the protein sequence could be modified to display specific cell-binding motifs. Previous work with liposomes, dendrimers, and protein nanoparticles has demonstrated that binding and uptake by specific cell types can be achieved via decoration of the particle surface with nanobodies which recognize an epitope unique to a particular cell surface;127 it is reasonable to believe that a similar strategy could be employed for targeting protein microcrystals.
In addition to delivery of antimicrobials and therapeutic proteins, cross-linked LPC microcrystals have also been explored as delivery vehicles for antigens. Previous work by St. Clair et al. compared the antigen response in rats between soluble human serum albumin (HSA), and glutaraldehyde-cross-linked HSA microcrystals (1–5 μm in diameter), and observed up to 30-fold increase in antibody titres for lightly-cross-linked HSA crystals.128 Similar strategies have more recently shifted towards the use of protein nanoparticles3,129 or peptide-based assemblies,130 due to their relative ease of synthesis.
3.3. Templated fabrication of nanostructures
LPCs can also serve as a valuable template for the fabrication of inorganic nanoparticles, nanorods, dendrimers, and nanowires. Such nanostructures have a potentially broad range of applications, particularly in biosensing,131–135 MRI contrast agents,136 and targeted therapeutic delivery systems.137,138 Traditional methods for synthesizing nanoparticles, nanorods, and nanowires occurs in bulk solution, where it can be difficult to control the size, shape, and stoichiometry of the produced structures. Through biomimetic mineralization, the ordered pore structure of the crystal provides a scaffold for the controlled nucleation and growth of inorganic materials. Depending on the side chains lining the crystal pores, concentration of metal ions can occur within the pore space or along the walls of the pores, supporting subsequent nucleation. For instance, nucleation and growth of magnetite nanoparticles within bacterial magnetosomes is believed to be dependent on the presence of multiple acidic residues in magnetosome-associating proteins.139 Magnetite nucleation is achieved through a combination of iron binding within a confined region, and localized iron supersaturation.140 Following nucleation within the crystal pores, metastable growth of larger nanoparticles and nanorods can be initiated by adjusting metal ion concentration, pH, or other solution conditions. Templating enables control over the size and composition of the resulting nanoparticles and facilitates the development of functional nanomaterials, in particular nanoparticles, nanorods, and nanowires. Within our own group, Kowalski et al. demonstrated sequestration of gold nanoparticles to the interior of CJ crystals141 and subsequent nanorod growth (not published).Previous methods involving the use of nanoporous protein scaffolds as templates for nanorod and nanoparticle production have relied on a diverse range of protein-based scaffolds, including hen egg white lysozyme (HEWL) crystals,140,142,143 bacterial polyisoprenoid-binding proteins,141 modified ferritin cages,144,145 S-layer protein arrays146 and BSA adsorbed to titanium oxide.147 Ferritin cages, in particular, continue to be heavily investigated due to well-established protocols for heterologous expression, purification, and in vitro assembly.148–152 While unmodified ferritin cages do not contain open-ended pores, they do possess an interior cavity on a comparable size scale to the porous systems described in this article (between 7 and 8 nm in diameter). While ferritin cages have been investigated in the context of delivery,153–155 they have also been used to generate CdS,156,157 ZnSe,158 Pd,159 and magnetite160 nanoparticles of well-defined shapes and sizes, for potential use as MRI contrast agents or therapeutic delivery vectors.
Two-dimensional, porous arrangements of S-layer proteins, which are canonically involved in maintaining cell wall integrity in bacteria and archaea,161 have received particularly strong focus in recent decades, following on the early work of Sleytr et al.162 While S-layer proteins have been shown to self-assemble,163,164 a more commonly reported method of synthesis involves cell lysis and isolation of cell wall components via ultracentrifugation.164 Cytosolic expression and assembly of prokaryotic S-layer protein – GFP fusion protein has been achieved in yeast;165 instead of forming sheets, the resulting crystals were rod-like (consistent with prior observations of in vitro self-assembly S-layer crystals),166,167 although the porosity of the material was nevertheless confirmed by TEM. S-layer platforms have been used for deposition and nucleation of FePt nanoparticles from the gas phase,168 as well as for controlled placement of ferromagnetic nanoparticles from solution.169 In addition to nanoparticles, metal nanorods and nanowires170,171 have also been constructed using similar strategies, typically in combination with chemical vapor deposition.
Similar templating strategies have also been used for generating biologically-derived nanostructures. A recent approach by Abe et al. from the Ueno group involves the use of protein nanotubes formed from Trypanosoma brucei cysteine protease cathepsin B (TbCatB), a protein which has been shown to crystallize in vivo in insect cells which have been baculovirus-infected.172 The described approach displays many similarities with their previous work on RuBisCO nanotubes from T. kodakaraensis,33 insofar as it utilizes a tubular protein assembly with a single channel, rather than a larger crystal containing a large number of pores. One-dimensional assembly ensures a confined space for templating of thin cylindrical materials, such as nanorods, nanowires, or filaments. In this particular context, the authors used the resulting tubular structures (which resist dissolving above pH 4) to generate proteinaceous filaments, stabilized by disulfide bonds between neighbouring cysteine residues.172 This strategy could be extended towards the synthesis of proteinaceous nanorods and nanoparticles, towards potential applications in therapeutic delivery and catalysis.
3.4. Structure determination
Beyond the functional applications discussed above, LPCs have been explored as scaffolds for third-party proteins or other biomolecules which may otherwise be difficult or impossible to crystallize. Traditional X-ray diffraction methods are dependent on the molecular uniformity of the target within a crystal, yet certain molecules remain difficult to crystallize due to difficulties with expression in prokaryotic hosts, their susceptibility to detergents,173 and for proteins with hydrophobic patches, there is the additional concern of poor protein solubility during crystallization screens.174Because of these technical challenges, scaffold-assisted structure determination (SASD) has emerged as a potential strategy for generating a three-dimensional lattice for arbitrary proteins or biomolecules, by installing the target molecule upon a pre-existing crystal scaffold. Sufficiently high occupancy of the guest molecule throughout the crystal scaffold may result in resolvability of the guest, assuming a uniform orientation and structure of the guest molecule across every unit cell of the scaffold. This approach was initially proposed by Nadrian Seeman in the 1980s as a DNA-based scaffold which could accommodate guest proteins.175 Huber et al. from our group recently described a similar approach for resolving a small-molecule guest within a protein crystal. The scaffold protein was mutated to include a pore-facing cysteine, which was then conjugated to a small molecule guest.11 Similar guest structure determination strategies have been recently reviewed.176
A recent platform in the spirit of this approach was recently reported by Orun et al. from our group in 2023.177 In this platform, the porous crystal is a hybrid material consisting of replication factor E54 (RepE54)178 and its 21-bp cognate DNA sequence, and contains protein complexes at the cell vertices. The resulting crystal scaffold was dubbed co-crystal 1 (CC1) (Fig. 6). Expansion of CC1 crystals from a single unit cell proceeds in a “Lincoln log” fashion, wherein two distinct protein–protein interfaces stabilize the crystal along the z-axis, while the crystal is further expanded in the xy-plane via the lengths of DNA running between the vertices; the resulting scaffolds were stabilized by chemical ligation of the 5′ and 3′ termini of the aligned DNA segments.177 Critically, this topology theoretically allows the modular insertion of nearly any DNA sequence to support subsequent guest capture.
It should be noted that there are two potential limitations with this type of hybrid scaffold. The first is the observed tendency of the crystals to adopt an interpenetrating lattice which results in a steep reduction in overall porosity, restricting the number of possible guest molecules. The second limitation is a consequence of using DNA as a co-crystallizing element. To stabilize the co-crystals outside of their mother liquor, it is necessary to ligate the termini of DNA oligomers between the neighbouring unit cells; because of this, the 5′ and 3′ ends of the DNA molecules must be properly aligned. Due to the biophysical constraints of a stable right-handed DNA helix, this means that the length of DNA between the stacked protein vertices must be a multiple of around 10–11 base pairs. Successfully growing well-diffracting crystal scaffolds becomes more challenging as greater flexibility in the scaffold is introduced, which can limit diffraction resolution for large DNA expansion co-crystals. Notably, protein–DNA co-crystals have many other potential applications beyond structure determination; for instance, an increase in flexibility and pore size, combined with their inherent biocompatibility, may be useful for a wide range of biomedical and biomaterials applications such as hybrid biomaterials, biosensing, and therapeutic delivery.179–184
3.5. Emerging and future applications
In addition to the aforementioned applications, LPC scaffolds have been utilized for a variety of niche applications, the majority of which are dependent on the unique properties of the protein itself. For instance, the CJ crystals that our lab routinely works with are able to rapidly adsorb nucleic acids to their interior,32,185 and this property is currently being exploited by our group across multiple projects.There is a precedent for the use of recombinant proteins in textile applications. Spider silk,186,187 elastin,188 resilin,189,190 collagen,191 fibroin,192 and keratin193 are all well-characterized structural proteins, and have been utilized in textiles in recent years, most commonly via electrospinning of individual fibres, or casting and self-assembly in foams and hydrogels. However, precisely controlled porosity is difficult in such systems. In pursuit of multifunctional textiles involving attachment of protein crystal depots, Hartje et al. from our group recently demonstrated integration of individual lysozyme and CJ crystals on bulk cotton, via chemical cross-linking with dihydrazides or dialdehydes, respectively.194 The resulting crystals were able to adsorb guest sulforhodamine-labeled cytochrome P450 protein, although the activity of guest P450 enzyme was not tested. Despite repeated laundering of the functionalized fabrics, at least 20% of the attached crystals were retained when chemically cross-linked to fabric.194 The authors suggest that crystal retention might be improved via more robust chemical crosslinking;194 additional strategies could involve in situ crystal growth on the surface of the fabric, which could reduce downstream handling and transfer of crystals. This approach may enable additional functionalization of cloth surfaces, particularly when combined with biosensing crystals or scaffolds which interact with specific pathogens.
Yet another novel application of LPCs is as a marker for mark-release-recapture (MRR) studies. It was previously demonstrated that CJ crystals were able to adsorb DNA to the crystal interior,185 so Stuart et al. from our group synthesized CJ microcrystals loaded with a synthetic DNA marker; after saturating these microcrystals with DNA, they were deposited in tubs of stagnant water and allowed to incubate for several days alongside mosquito larvae.185 Emerging adult mosquitoes were subsequently captured and reared in an insectary, whereupon they were sacrificed, and the original barcode sequence was recovered from the adult mosquitoes via qPCR-based detection and next-generation sequencing.185 This study demonstrates a novel application of LPCs for marking and detection of mosquitoes, which have traditionally relied on fluorescent powders,195 paints/dyes, or radioisotopes.196
4. Conclusions
This review has described both the synthesis and applications of several existing LPC platforms, although these represent a small fraction of the opportunities for this material family. Due to the inherent structural and functional diversity of proteins, the number of possible lattice structures and functionality is limited only by human imagination. The past year alone has seen extremely rapid advances in machine learning-based methods,197–199 and it is increasingly possible to identify sequences that conform to any desired tertiary structure. In 2023, the Baker group reported designed 3D protein crystals capable of rapid in vitro self-assembly.200 Additional machine learning tools, such as AlphaFold, have been used to predict allosteric and protein–ligand interactions,201 and may be used to alter the structure and binding affinities of LPCs. With the advent of reliable protein crystal design, we anticipate rapid growth in the number of engineered crystals with functional applications. This emerging family of high-precision biomaterials represents a novel frontier for catalysis, therapeutic delivery, and a host of unexplored applications.Author contributions
The manuscript was written by A. A. J., and edited by C. D. S.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. A. J. was supported during this work with Anschutz Family Foundation funding. Figures were prepared in PyMOL 2.3.1, ChemDraw Professional 22.2.0.3300, and Blender 4.0.Notes and references
- M. B. Stie, K. Kalouta, V. Vetri and V. Foderà, J. Controlled Release, 2022, 344, 12–25 CrossRef CAS PubMed.
- N. C. Abascal and L. Regan, Open Biol., 2018, 8, 180113 CrossRef CAS PubMed.
- B. Zhang, C. W. Chao, Y. Tsybovsky, O. M. Abiona, G. B. Hutchinson, J. I. Moliva, A. S. Olia, A. Pegu, E. Phung, G. B. E. Stewart-Jones, R. Verardi, L. Wang, S. Wang, A. Werner, E. S. Yang, C. Yap, T. Zhou, J. R. Mascola, N. J. Sullivan, B. S. Graham, K. S. Corbett and P. D. Kwong, Sci. Rep., 2020, 10, 18149 CrossRef CAS PubMed.
- X. Tan, H. Chen, C. Gu, J. Zang, T. Zhang, H. Wang and G. Zhao, Commun. Chem., 2020, 3, 151 CrossRef CAS PubMed.
- E. Sasaki, D. Böhringer, M. van de Waterbeemd, M. Leibundgut, R. Zschoche, A. J. R. Heck, N. Ban and D. Hilvert, Nat. Commun., 2017, 8, 14663 CrossRef PubMed.
- C. Huerta-López and J. Alegre-Cebollada, Nanomaterials, 2021, 11(7), 1656 CrossRef PubMed.
- M. Charrier, D. Li, V. R. Mann, L. Yun, S. Jani, B. Rad, B. E. Cohen, P. D. Ashby, K. R. Ryan and C. M. Ajo-Franklin, ACS Synth. Biol., 2019, 8, 181–190 CrossRef CAS PubMed.
- Y.-M. Tu, W. Song, T. Ren, Y. Shen, R. Chowdhury, P. Rajapaksha, T. E. Culp, L. Samineni, C. Lang, A. Thokkadam, D. Carson, Y. Dai, A. Mukthar, M. Zhang, A. Parshin, J. N. Sloand, S. H. Medina, M. Grzelakowski, D. Bhattacharya, W. A. Phillip, E. D. Gomez, R. J. Hickey, Y. Wei and M. Kumar, Nat. Mater., 2020, 19, 347–354 CrossRef CAS PubMed.
- T. R. Huber, L. F. Hartje, E. C. McPherson, A. E. Kowalski and C. D. Snow, Small, 2017, 13, 1602703 CrossRef PubMed.
- Z. Yang, J. Zheng, C.-F. Chan, I. L. K. Wong, B. S. Heater, L. M. C. Chow, M. M. M. Lee and M. K. Chan, Biomaterials, 2019, 217, 119286 CrossRef CAS PubMed.
- T. R. Huber, E. C. McPherson, C. E. Keating and C. D. Snow, Bioconjugate Chem., 2018, 29, 17–22 CrossRef CAS PubMed.
- A. Tiessen, P. Pérez-Rodríguez and L. J. Delaye-Arredondo, BMC Res. Notes, 2012, 5, 85 CrossRef CAS PubMed.
- P. L. Privalov, in Advances in Protein Chemistry, ed. C. B. Anfinsen, J. T. Edsall and F. M. Richards, Academic Press, 1979, vol. 33, pp. 167–241 Search PubMed.
- Y. Sawada and S. Honda, Biophys. J., 2006, 91, 1213–1223 CrossRef CAS PubMed.
- S. Nepomnyachiy, N. Ben-Tal and R. Kolodny, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 11703–11708 CrossRef CAS PubMed.
- O. Svensson, M. Gilski, D. Nurizzo and M. W. Bowler, IUCrJ, 2019, 6, 822–831 CrossRef CAS PubMed.
- L. Xu, S. D. Benson and R. M. Burnett, J. Struct. Biol., 2007, 157, 424–431 CrossRef CAS PubMed.
- K. O. Ramberg, S. Engilberge, T. Skorek and P. B. Crowley, J. Am. Chem. Soc., 2021, 143, 1896–1907 CrossRef CAS PubMed.
- Y.-T. Lai, E. Reading, G. L. Hura, K.-L. Tsai, A. Laganowsky, F. J. Asturias, J. A. Tainer, C. V. Robinson and T. O. Yeates, Nat. Chem., 2014, 6, 1065–1071 CrossRef CAS PubMed.
- J. Li, J. Carroll and D. J. Ellar, Nature, 1991, 353, 815–821 CrossRef CAS PubMed.
- J. L. Miller, J. L. Coq, A. Hodes, R. Barbalat, J. F. Miller and P. Ghosh, PLoS Biol., 2008, 6, e131 CrossRef PubMed.
- K. A. Kantardjieff and B. Rupp, Protein Sci., 2003, 12, 1865–1871 CrossRef CAS PubMed.
- C. X. Weichenberger and B. Rupp, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2014, 70, 1579–1588 CrossRef CAS PubMed.
- C. M. C. Lobley, J. Sandy, J. Sanchez-Weatherby, M. Mazzorana, T. Krojer, R. P. Nowak and T. L. Sorensen, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2016, 72, 629–640 CrossRef CAS PubMed.
- J. Sanchez-Weatherby and I. Moraes, Adv. Exp. Med. Biol., 2016, 922, 73–89 CrossRef CAS PubMed.
- A. McPherson and J. A. Gavira, Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun., 2013, 70, 2–20 CrossRef PubMed.
- S. Abe, H. Tabe, H. Ijiri, K. Yamashita, K. Hirata, K. Atsumi, T. Shimoi, M. Akai, H. Mori, S. Kitagawa and T. Ueno, ACS Nano, 2017, 11, 2410–2419 CrossRef CAS PubMed.
- T. Ueno, Chem. - Eur. J., 2013, 19, 9096–9102 CrossRef CAS PubMed.
- M. Kojima, S. Abe and T. Ueno, Biomater. Sci., 2022, 10, 354–367 RSC.
- H. Tabe, H. Takahashi, T. Shimoi, S. Abe, T. Ueno and Y. Yamada, Appl. Catal., B, 2018, 237, 1124–1129 CrossRef CAS.
- J. M. Thomas, Nature, 2012, 491, 186–187 CrossRef CAS PubMed.
- D. Wang, J. D. Stuart, A. A. Jones, C. D. Snow and M. J. Kipper, Nanoscale, 2021, 13, 10871–10881 RSC.
- T. K. Nguyen, H. Negishi, S. Abe and T. Ueno, Chem. Sci., 2019, 10, 1046–1051 RSC.
- I. Boyton, S. C. Goodchild, D. Diaz, A. Elbourne, L. E. Collins-Praino and A. Care, ACS Omega, 2022, 7, 823–836 CrossRef CAS PubMed.
- S. L. Heinz-Kunert, A. Pandya, V. T. Dang, P. N. Tran, S. Ghosh, D. McElheny, B. D. Santarsiero, Z. Ren and A. I. Nguyen, J. Am. Chem. Soc., 2022, 144, 7001–7009 CrossRef CAS PubMed.
- J. Ke, J. Zhang, J. Li, J. Liu and S. Guan, Int. J. Mol. Sci., 2022, 23, 12071 CrossRef CAS PubMed.
- C. Wu and H. Wang, ChemBioChem, 2023, 24, e202300018 CrossRef CAS PubMed.
- R. Moral and S. Paul, Langmuir, 2024, 40, 882–895 CrossRef CAS PubMed.
- A. Méndez-Ardoy, I. Insua, J. R. Granja and J. Montenegro, Methods Mol. Biol., 2022, 2371, 449–466 CrossRef PubMed.
- D. J. Rubin, S. Amini, F. Zhou, H. Su, A. Miserez and N. S. Joshi, ACS Nano, 2015, 9, 3360–3368 CrossRef CAS PubMed.
- F. Castro, A. Ferreira, J. A. Teixeira and F. Rocha, Cryst. Growth Des., 2016, 16, 3748–3755 CrossRef CAS.
- F. Castro, I. Cunha, A. Ferreira, J. A. Teixeira and F. Rocha, Chem. Eng. Res. Des., 2022, 178, 575–582 CrossRef CAS.
- H. Mori, R. Ito, H. Nakazawa, M. Sumida, F. Matsubara and Y. Minobe, J. Gen. Virol., 1993, 74, 99–102 CrossRef CAS PubMed.
- M. S. Nair, M. M. Lee, A. Bonnegarde-Bernard, J. A. Wallace, D. H. Dean, M. C. Ostrowski, R. W. Burry, P. N. Boyaka and M. K. Chan, PLoS One, 2015, 10, e0127669 CrossRef PubMed.
- S. Abe, J. Tanaka, M. Kojima, S. Kanamaru, K. Hirata, K. Yamashita, A. Kobayashi and T. Ueno, Sci. Rep., 2022, 12, 16031 CrossRef CAS PubMed.
- J. C. Falkner, A. M. Al-Somali, J. A. Jamison, J. Zhang, S. L. Adrianse, R. L. Simpson, M. K. Calabretta, W. Radding, G. N. Phillips and V. L. Colvin, Chem. Mater., 2005, 17, 2679–2686 CrossRef CAS.
- A. E. Kowalski, L. B. Johnson, H. K. Dierl, S. Park, T. R. Huber and C. D. Snow, Biomater. Sci., 2019, 7, 1898–1904 RSC.
- K. Han, Z. Zhang and F. A. Tezcan, J. Am. Chem. Soc., 2023, 145, 19932–19944 CrossRef CAS PubMed.
- R. Adalat, F. Saleem, N. Crickmore, S. Naz and A. R. Shakoori, Toxins, 2017, 9, 80 CrossRef PubMed.
- J. Herrmann, F. Jabbarpour, P. G. Bargar, J. F. Nomellini, P.-N. Li, T. J. Lane, T. M. Weiss, J. Smit, L. Shapiro and S. Wakatsuki, Biophys. J., 2017, 112, 1841–1851 CrossRef CAS PubMed.
- C. J. Comerci, J. Herrmann, J. Yoon, F. Jabbarpour, X. Zhou, J. F. Nomellini, J. Smit, L. Shapiro, S. Wakatsuki and W. E. Moerner, Nat. Commun., 2019, 10, 2731 CrossRef PubMed.
- V. R. Mann, F. Manea, N. J. Borys, C. M. Ajo-Franklin and B. E. Cohen, Biochemistry, 2021, 60, 1063–1074 CrossRef CAS PubMed.
- K. Lu, W. Ye, L. Zhou, L. B. Collins, X. Chen, A. Gold, L. M. Ball and J. A. Swenberg, J. Am. Chem. Soc., 2010, 132, 3388–3399 CrossRef CAS PubMed.
- E.-K. Yan, H.-L. Cao, C.-Y. Zhang, Q.-Q. Lu, Y.-J. Ye, J. He, L.-J. Huang and D.-C. Yin, RSC Adv., 2015, 5, 26163–26174 RSC.
- F. A. Quiocho and F. M. Richards, Proc. Natl. Acad. Sci. U. S. A., 1964, 52, 833–839 CrossRef CAS PubMed.
- L. F. Hartje, H. T. Bui, D. A. Andales, S. P. James, T. R. Huber and C. D. Snow, ACS Biomater. Sci. Eng., 2018, 4, 826–831 CrossRef CAS PubMed.
- A. R. Orun, S. Dmytriw, A. Vajapayajula and C. D. Snow, Crystals, 2022, 12, 49 CrossRef.
- Q. Zhang, E. Crosland and D. Fabris, Anal. Chim. Acta, 2008, 627, 117–128 CrossRef CAS PubMed.
- C. J. Lusty, J. Appl. Cryst., 1999, 32, 106–112 CrossRef CAS.
- T. Hiromoto, T. Ikura, E. Honjo, M. Blaber, R. Kuroki and T. Tamada, Front. Mol. Biosci., 2022, 9, 908394 CrossRef CAS PubMed.
- M. Pu, Z. Xu, Y. Peng, Y. Hou, D. Liu, Y. Wang, H. Liu, G. Song and Z.-J. Liu, Protein Cell, 2018, 9, 659–663 CrossRef CAS PubMed.
- D.-A. Silva, L. Stewart, K.-H. Lam, R. Jin and D. Baker, FEBS J., 2018, 285, 1783–1785 CrossRef CAS PubMed.
- J. Fauser, S. Savitskiy, M. Fottner, V. Trauschke and B. Gulen, Bioconjugate Chem., 2020, 31, 1883–1892 CrossRef CAS PubMed.
- A. Susmitha, J. S. Arya, L. Sundar, K. K. Maiti and K. M. Nampoothiri, J. Biotechnol., 2023, 367, 11–19 CrossRef CAS PubMed.
- H. H. Wang, B. Altun, K. Nwe and A. Tsourkas, Angew. Chem. Int. Ed., 2017, 56, 5349–5352 CrossRef CAS PubMed.
- L.-X. Cai, Y.-Q. Lin, Y.-M. Chu, X.-P. Chen, L.-X. Liu, M. Zhang and G.-Y. Zhang, Bio. Protoc., 2022, 12, e4282 CAS.
- C. Liu, J. Hua, P. F. Ng and B. Fei, J. Mater. Sci. Technol., 2021, 63, 182–191 CrossRef CAS.
- J. Lee, M. Ju, O. H. Cho, Y. Kim and K. T. Nam, Adv. Sci., 2019, 6, 1801255 CrossRef PubMed.
- D. J. Raven, C. Earland and M. Little, Biochim. Biophys. Acta, Protein Struct., 1971, 251, 96–99 CrossRef CAS PubMed.
- F. Müller, A. Graziadei and J. Rappsilber, Anal. Chem., 2019, 91, 9041–9048 CrossRef PubMed.
- K. Stahl, A. Graziadei, T. Dau, O. Brock and J. Rappsilber, Nat. Biotechnol., 2023, 1–10 Search PubMed.
- U. Schweizer, T. Hey, G. Lipps and G. Krauss, Nucleic Acids Res., 1999, 27, 3183–3189 CrossRef CAS PubMed.
- S. Zhang, D. H. Adamson, R. K. Prud’homme and A. J. Link, Polym. Chem., 2011, 2, 665–671 RSC.
- R. A. Luse and A. D. McLaren, Photochem. Photobiol., 1963, 2, 343–360 CrossRef CAS.
- W. Sutherland, London, Edinburgh Dublin Philos. Mag. J. Sci., 1905, 9(54), 781–785 CrossRef CAS.
- A. Einstein, Ann. Phys., 1905, 17, 549–560 CrossRef CAS.
- A. Sauter, F. Roosen-Runge, F. Zhang, G. Lotze, R. M. J. Jacobs and F. Schreiber, J. Am. Chem. Soc., 2015, 137, 1485–1491 CrossRef CAS PubMed.
- R. Maier, G. Zocher, A. Sauter, S. Da Vela, O. Matsarskaia, R. Schweins, M. Sztucki, F. Zhang, T. Stehle and F. Schreiber, Cryst. Growth Des., 2020, 20, 7951–7962 CrossRef CAS.
- W. Tian, W. Li and H. Yang, Cryst. Growth Des., 2023, 23, 5181–5193 CrossRef CAS PubMed.
- D. W. Oxtoby, J. Phys.: Condens. Matter, 1992, 4, 7627 CrossRef.
- R. Schubert, A. Meyer, K. Dierks, S. Kapis, R. Reimer, H. Einspahr, M. Perbandt and C. Betzel, J. Appl. Cryst., 2015, 48, 1476–1484 CrossRef CAS.
- Y. Zhou, Z. Dong, O. Terasaki and Y. Ma, Acc. Mater. Res., 2022, 3, 110–121 CrossRef CAS.
- D. Wang, M. Hedayati, J. D. Stuart, L. Y. C. Madruga, K. C. Popat, C. D. Snow and M. J. Kipper, Mater. Today Nano, 2023, 24, 100432 CrossRef CAS PubMed.
- G. R. Willmott, Anal. Chem., 2018, 90, 2987–2995 CrossRef CAS PubMed.
- S. Kumar, S. Sega, J. K. Lynn-Barbe, D. L. Harris, J. T. Koehn, D. C. Crans and D. C. Crick, Pathogens, 2023, 12, 1171 CrossRef CAS PubMed.
- J. Škerlová, J. Berndtsson, H. Nolte, M. Ott and P. Stenmark, Nat. Commun., 2021, 12, 5277 CrossRef PubMed.
- Ž. M. Svedružić, I. Odorčić, C. H. Chang and D. Svedružić, Sci. Rep., 2020, 10, 10404 CrossRef PubMed.
- P. Mehrabi, S. Sung, D. von Stetten, A. Prester, C. E. Hatton, S. Kleine-Döpke, A. Berkes, G. Gore, J.-P. Leimkohl, H. Schikora, M. Kollewe, H. Rohde, M. Wilmanns, F. Tellkamp and E. C. Schulz, Nat. Commun., 2023, 14(1), 2365 CrossRef CAS PubMed.
- M. F. Dunn, Arch. Biochem. Biophys., 2012, 519, 154–166 CrossRef CAS PubMed.
- M. F. Dunn, D. Niks, H. Ngo, T. R. M. Barends and I. Schlichting, Trends Biochem. Sci., 2008, 33, 254–264 CrossRef CAS PubMed.
- M. Castellana, M. Z. Wilson, Y. Xu, P. Joshi, I. M. Cristea, J. D. Rabinowitz, Z. Gitai and N. S. Wingreen, Nat. Biotechnol., 2014, 32, 1011–1018 CrossRef CAS PubMed.
- J. P. Dexter, P. S. Ward, T. Dasgupta, A. M. Hosios, J. Gunawardena and M. G. Vander Heiden, J. Biol. Chem., 2018, 293, 20051–20061 CrossRef CAS PubMed.
- K. Xu, X. Chen, R. Zheng and Y. Zheng, Front. Bioeng. Biotechnol., 2020, 8, 660 CrossRef PubMed.
- T. K. Nguyen, S. Abe, M. Kasamatsu, B. Maity, K. Yamashita, K. Hirata, M. Kojima and T. Ueno, ACS Appl. Nano Mater., 2021, 4, 1672–1681 CrossRef CAS.
- R. D. Healey, L. Couillaud, F. Hoh, A. Mouhand, A. Fouillen, P. Couvineau, S. Granier and C. Leyrat, Commun. Chem., 2023, 6, 1–13 CrossRef PubMed.
- B. S. Heater, W. S. Chan, M. M. Lee and M. K. Chan, Biotechnol. Biofuels, 2019, 12, 165 CrossRef CAS PubMed.
- S. Gad and S. Ayakar, Biotechnol. Rep., 2021, 32, e00670 CrossRef CAS PubMed.
- Q. Sun, B. S. Heater, T. L. Li, W. Ye, Z. Guo and M. K. Chan, Bioconjugate Chem., 2022, 33, 386–396 CrossRef CAS PubMed.
- B. Zakeri, J. O. Fierer, E. Celik, E. C. Chittock, U. Schwarz-Linek, V. T. Moy and M. Howarth, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, E690–E697 CrossRef CAS PubMed.
- A. H. Keeble, P. Turkki, S. Stokes, I. N. A. Khairil Anuar, R. Rahikainen, V. P. Hytönen and M. Howarth, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 26523–26533 CrossRef CAS PubMed.
- J. Tian, R. Jia, D. Wenge, H. Sun, Y. Wang, Y. Chang and H. Luo, Biotechnol. Lett., 2021, 43, 1075–1087 CrossRef CAS PubMed.
- G. P. Anderson, J. L. Liu, L. C. Shriver-Lake, D. Zabetakis, V. A. Sugiharto, H.-W. Chen, C.-R. Lee, G. N. Defang, S.-J. L. Wu, N. Venkateswaran and E. R. Goldman, Anal. Chem., 2019, 91, 9424–9429 CrossRef CAS PubMed.
- M. Liu, Y. Song, Y.-H. P. J. Zhang and C. You, ChemSusChem, 2023, 16, e202202153 CrossRef CAS PubMed.
- I. N. A. Khairil Anuar, A. Banerjee, A. H. Keeble, A. Carella, G. I. Nikov and M. Howarth, Nat. Commun., 2019, 10, 1734 CrossRef PubMed.
- C. Schoene, J. O. Fierer, S. P. Bennett and M. Howarth, Angew. Chem. Int. Ed., 2014, 53, 6101–6104 Search PubMed.
- S. Zhang, Q. Ren, S. J. Novick, T. S. Strutzenberg, P. R. Griffin and H. Bao, Nat. Commun., 2021, 12, 5451 CrossRef CAS PubMed.
- S. Guo, Y. Huang, Q. Jiang, Y. Sun, L. Deng, Z. Liang, Q. Du, J. Xing, Y. Zhao, P. C. Wang, A. Dong and X.-J. Liang, ACS Nano, 2010, 4, 5505–5511 CrossRef CAS PubMed.
- J. Guo, M. J. Armstrong, C. M. O’Driscoll, J. D. Holmes and K. Rahme, RSC Adv., 2015, 5, 17862–17871 RSC.
- T. Ruks, K. Loza, M. Heggen, C. Ottmann, P. Bayer, C. Beuck and M. Epple, ChemBioChem, 2021, 22, 1456–1463 CrossRef CAS PubMed.
- C. Bharti, U. Nagaich, A. K. Pal and N. Gulati, Int. J. Pharm. Investig., 2015, 5, 124–133 CrossRef CAS PubMed.
- F. Rizzi, R. Castaldo, T. Latronico, P. Lasala, G. Gentile, M. Lavorgna, M. Striccoli, A. Agostiano, R. Comparelli, N. Depalo, M. L. Curri and E. Fanizza, Molecules, 2021, 26(14), 4247 CrossRef CAS PubMed.
- M. Vallet-Regí, F. Schüth, D. Lozano, M. Colilla and M. Manzano, Chem. Soc. Rev., 2022, 51, 5365–5451 RSC.
- H. Elsana, T. O. B. Olusanya, J. Carr-Wilkinson, S. Darby, A. Faheem and A. A. Elkordy, Sci. Rep., 2019, 9, 15120 CrossRef PubMed.
- M. A. Obeid, A. Elburi, L. C. Young, A. B. Mullen, R. J. Tate and V. A. Ferro, Mol. Pharmaceutics, 2017, 14, 2450–2458 CrossRef CAS PubMed.
- B. R. Lee, K. T. Oh, H. J. Baik, Y. S. Youn and E. S. Lee, Int. J. Pharm., 2010, 392, 78–82 CrossRef CAS PubMed.
- N. Ninan, A. Forget, V. P. Shastri, N. H. Voelcker and A. Blencowe, ACS Appl. Mater. Interfaces, 2016, 8, 28511–28521 CrossRef CAS PubMed.
- M. Cooley, A. Sarode, M. Hoore, D. A. Fedosov, S. Mitragotri and A. S. Gupta, Nanoscale, 2018, 10, 15350–15364 RSC.
- P. Decuzzi, B. Godin, T. Tanaka, S. Y. Lee, C. Chiappini, X. Liu and M. Ferrari, J. Controlled Release, 2010, 141, 320–327 CrossRef CAS PubMed.
- Z. Yang, M. M. M. Lee and M. K. Chan, Biomaterials, 2021, 271, 120759 CrossRef CAS PubMed.
- I. Nakase, K. Noguchi, A. Aoki, T. Takatani-Nakase, I. Fujii and S. Futaki, Sci. Rep., 2017, 7, 1991 CrossRef PubMed.
- S. Silva, A. J. Almeida and N. Vale, Biomolecules, 2019, 9, 22 CrossRef PubMed.
- I. M. S. Degors, C. Wang, Z. U. Rehman and I. S. Zuhorn, Acc. Chem. Res., 2019, 52, 1750–1760 CrossRef CAS PubMed.
- Z. Sun, J. Huang, Z. Fishelson, C. Wang and S. Zhang, Biomedicines, 2023, 11, 1971 CrossRef CAS PubMed.
- B. R. McNaughton, J. J. Cronican, D. B. Thompson and D. R. Liu, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 6111–6116 CrossRef CAS PubMed.
- J. Yin, Q. Wang, S. Hou, L. Bao, W. Yao and X. Gao, J. Am. Chem. Soc., 2018, 140, 17234–17240 CrossRef CAS PubMed.
- M. A. M. Aboul-Soud, M. Z. Al-Amri, A. Kumar, Y. A. Al-Sheikh, A. E. Ashour and T. A. El-Kersh, Molecules, 2019, 24, 506 CrossRef PubMed.
- Y. Hu, C. Liu and S. Muyldermans, Front. Immunol., 2017, 8, 1442 CrossRef PubMed.
- N. S. Clair, B. Shenoy, L. D. Jacob and A. L. Margolin, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 9469–9474 CrossRef PubMed.
- T. K. Tan, P. Rijal, R. Rahikainen, A. H. Keeble, L. Schimanski, S. Hussain, R. Harvey, J. W. P. Hayes, J. C. Edwards, R. K. McLean, V. Martini, M. Pedrera, N. Thakur, C. Conceicao, I. Dietrich, H. Shelton, A. Ludi, G. Wilsden, C. Browning, A. K. Zagrajek, D. Bialy, S. Bhat, P. Stevenson-Leggett, P. Hollinghurst, M. Tully, K. Moffat, C. Chiu, R. Waters, A. Gray, M. Azhar, V. Mioulet, J. Newman, A. S. Asfor, A. Burman, S. Crossley, J. A. Hammond, E. Tchilian, B. Charleston, D. Bailey, T. J. Tuthill, S. P. Graham, H. M. E. Duyvesteyn, T. Malinauskas, J. Huo, J. A. Tree, K. R. Buttigieg, R. J. Owens, M. W. Carroll, R. S. Daniels, J. W. McCauley, D. I. Stuart, K.-Y. A. Huang, M. Howarth and A. R. Townsend, Nat. Commun., 2021, 12, 542 CrossRef CAS PubMed.
- R. F. Q. Grenfell, L. M. Shollenberger, E. F. Samli and D. A. Harn, Clin. Vaccine Immunol., 2015, 22, 336–343 CrossRef CAS PubMed.
- S. Malik, J. Singh, R. Goyat, Y. Saharan, V. Chaudhry, A. Umar, A. A. Ibrahim, S. Akbar, S. Ameen and S. Baskoutas, Heliyon, 2023, 9, e19929 CrossRef CAS PubMed.
- X. Liu, Y. Qiu, D. Jiang, F. Li, Y. Gan, Y. Zhu, Y. Pan, H. Wan and P. Wang, Microsyst. Nanoeng., 2022, 8, 1–15 CrossRef PubMed.
- C. Yu and J. Irudayaraj, Anal. Chem., 2007, 79, 572–579 CrossRef CAS PubMed.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078–1081 CrossRef CAS PubMed.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547–1562 CrossRef CAS PubMed.
- H. Wei, A. Wiśniowska, J. Fan, P. Harvey, Y. Li, V. Wu, E. C. Hansen, J. Zhang, M. G. Kaul, A. M. Frey, G. Adam, A. I. Frenkel, M. G. Bawendi and A. Jasanoff, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2102340118 CrossRef CAS PubMed.
- J. M. Rae and B. Jachimska, Nanoscale, 2021, 13, 2703–2713 RSC.
- T. Niidome, A. Shiotani, T. Mori and Y. Katayama, J. Controlled Release, 2010, 148, e65–6 CrossRef CAS PubMed.
- A. Yamagishi, K. Narumiya, M. Tanaka, T. Matsunaga and A. Arakaki, Sci. Rep., 2016, 6, 35670 CrossRef CAS PubMed.
- M. Savchenko, V. Sebastian, M. T. Lopez-Lopez, A. Rodriguez-Navarro, L. Alvarez De Cienfuegos, C. Jimenez-Lopez and J. A. Gavira, Cryst. Growth Des., 2023, 23, 4032–4040 CrossRef CAS PubMed.
- A. E. Kowalski, T. R. Huber, T. W. Ni, L. F. Hartje, K. L. Appel, J. W. Yost, C. J. Ackerson and C. D. Snow, Nanoscale, 2016, 8, 12693–12696 RSC.
- M. Guli, E. M. Lambert, M. Li and S. Mann, Angew. Chem., Int. Ed., 2010, 49, 520–523 CrossRef CAS PubMed.
- O. Deschaume, B. De Roo, M. J. Van Bael, J.-P. Locquet, C. Van Haesendonck and C. Bartic, Chem. Mater., 2014, 26, 5383–5393 CrossRef CAS.
- C. Lu, B. Maity, X. Peng, N. Ito, S. Abe, X. Sheng, T. Ueno and D. Lu, Commun. Chem., 2022, 5, 39 CrossRef CAS PubMed.
- B. Maity, Y. Hishikawa, D. Lu and T. Ueno, Polyhedron, 2019, 172, 104–111 CrossRef CAS.
- S. S. Mark, M. Bergkvist, P. Bhatnagar, C. Welch, A. L. Goodyear, X. Yang, E. R. Angert and C. A. Batt, Colloids Surf., B, 2007, 57, 161–173 CrossRef CAS PubMed.
- Z.-D. Gao, H.-F. Liu, C.-Y. Li and Y.-Y. Song, Chem. Commun., 2013, 49, 774–776 RSC.
- G. Nie, A. D. Sheftel, S. F. Kim and P. Ponka, Blood, 2005, 105, 2161–2167 CrossRef CAS PubMed.
- M. Hoppler, L. Meile and T. Walczyk, Anal. Bioanal. Chem., 2008, 390, 53–59 CrossRef CAS PubMed.
- H. Chen, S. Zhang, C. Xu and G. Zhao, Chem. Commun., 2016, 52, 7402–7405 RSC.
- S. Yin, B. Zhang, J. Lin, Y. Liu, Z. Su and J. Bi, Eng. Life Sci., 2021, 21, 630–642 CrossRef CAS PubMed.
- B. Maity and T. Ueno, in Protein Cages: Design, Structure, and Applications, ed. T. Ueno, S. Lim and K. Xia, Springer US, New York, NY, 2023, pp. 135–145 Search PubMed.
- M. Liang, K. Fan, M. Zhou, D. Duan, J. Zheng, D. Yang, J. Feng and X. Yan, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 14900–14905 CrossRef CAS PubMed.
- T. Tan, H. Wang, H. Cao, L. Zeng, Y. Wang, Z. Wang, J. Wang, J. Li, S. Wang, Z. Zhang and Y. Li, Adv. Sci., 2018, 5, 1801012 CrossRef PubMed.
- J. Zhang, D. Cheng, J. He, J. Hong, C. Yuan and M. Liang, Nat. Protoc., 2021, 16, 4878–4896 CrossRef CAS PubMed.
- K. K. W. Wong and S. Mann, Adv. Mater., 1996, 8, 928–932 CrossRef CAS.
- K. Iwahori and I. Yamashita, J. Phys.: Conf. Ser., 2007, 61, 492 CrossRef CAS.
- K. Iwahori, K. Yoshizawa, M. Muraoka and I. Yamashita, Inorg. Chem., 2005, 44, 6393–6400 CrossRef CAS PubMed.
- M. Peskova, L. Ilkovics, D. Hynek, S. Dostalova, E. M. Sanchez-Carnerero, M. Remes, Z. Heger and V. Pekarik, J. Colloid Interface Sci., 2019, 537, 20–27 CrossRef CAS PubMed.
- M. G. Walls, C. Cao, K. Yu-Zhang, J. Li, R. Che and Y. Pan, Microsc. Microanal., 2013, 19, 835–841 CrossRef CAS PubMed.
- U. B. Sleytr, B. Schuster, E.-M. Egelseer and D. Pum, FEMS Microbiol. Rev., 2014, 38, 823–864 CrossRef CAS PubMed.
- U. B. Sleytr, P. Messner, D. Pum and M. Sára, Angew. Chem., Int. Ed., 1999, 38, 1034–1054 CrossRef CAS PubMed.
- U. B. Sleytr, J. Ultrastruct. Res., 1976, 55, 360–377 CrossRef CAS PubMed.
- D. Pum, J. L. Toca-Herrera and U. B. Sleytr, Int. J. Mol. Sci., 2013, 14, 2484–2501 CrossRef CAS PubMed.
- A. Blecha, K. Zarschler, K. A. Sjollema, M. Veenhuis and G. Rödel, Microb. Cell Fact., 2005, 4, 28 CrossRef PubMed.
- P. Messner, D. Pum and U. B. Sleytr, J. Ultrastruct. Mol. Struct. Res., 1986, 97, 73–88 CrossRef CAS PubMed.
- M. Jarosch, E. M. Egelseer, C. Huber, D. Moll, D. Mattanovich, U. B. Sleytr and M. Sára, Microbiology, 2001, 147, 1353–1363 CrossRef CAS PubMed.
- U. Queitsch, C. Hamann, F. Schäffel, B. Rellinghaus, L. Schultz, A. Blüher and M. Mertig, J. Phys. Chem. C, 2009, 113, 10471–10476 CrossRef CAS.
- E. Valero, M. Martín, N. Gálvez, P. Sánchez, J. Raff, M. L. Merroun and J. M. Dominguez-Vera, Inorg. Chem., 2015, 54, 6758–6762 CrossRef CAS PubMed.
- C. B. Maliakkal, E. K. Mårtensson, M. U. Tornberg, D. Jacobsson, A. R. Persson, J. Johansson, L. R. Wallenberg and K. A. Dick, ACS Nano, 2020, 14, 3868–3875 CrossRef CAS PubMed.
- M. Abdolirad, R. Khalilzadeh and M. Alijanianzadeh, Superlattices Microstruct., 2018, 120, 370–376 CrossRef CAS.
- S. Abe, T. T. Pham, H. Negishi, K. Yamashita, K. Hirata and T. Ueno, Angew. Chem., 2021, 133, 12449–12453 CrossRef.
- A. A. Kermani, FEBS J., 2021, 288, 5788–5804 CrossRef CAS PubMed.
- Z. E. R. Newby, I. I. I. Joseph, D. O’Connel, F. Gruswitz, F. A. Hays, W. E. C. Harries, I. M. Harwood, J. D. Ho, J. K. Lee, D. F. Savage, L. J. W. Miercke and R. Stroud, Nat. Protoc., 2009, 4, 619 CrossRef CAS PubMed.
- N. C. Seeman, J. Theor. Biol., 1982, 99, 237–247 CrossRef CAS PubMed.
- A. R. Ward and C. D. Snow, Curr. Opin. Struct. Biol., 2020, 60, 85–92 CrossRef CAS PubMed.
- A. R. Orun, E. T. Shields, S. Dmytriw, A. Vajapayajula, C. K. Slaughter and C. D. Snow, ACS Nano, 2023, 17, 13110–13120 CrossRef CAS PubMed.
- H. Komori, F. Matsunaga, Y. Higuchi, M. Ishiai, C. Wada and K. Miki, EMBO J., 1999, 18, 4597–4607 CrossRef CAS PubMed.
- S. Karamikamkar, E. P. Yalcintas, R. Haghniaz, N. R. de Barros, M. Mecwan, R. Nasiri, E. Davoodi, F. Nasrollahi, A. Erdem, H. Kang, J. Lee, Y. Zhu, S. Ahadian, V. Jucaud, H. Maleki, M. R. Dokmeci, H.-J. Kim and A. Khademhosseini, Adv. Sci., 2023, 10, 2204681 CrossRef CAS PubMed.
- L. Wan, Q. Chen, J. Liu, X. Yang, J. Huang, L. Li, X. Guo, J. Zhang and K. Wang, Biomacromolecules, 2016, 17, 1543–1550 CrossRef CAS PubMed.
- J. Tang, X. Jia, Q. Li, Z. Cui, A. Liang, B. Ke, D. Yang and C. Yao, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2303822120 CrossRef CAS PubMed.
- Y. Yuan and N. Solin, ACS Appl. Bio Mater., 2022, 5, 3360–3370 CrossRef CAS PubMed.
- D. Lovskaya, A. Bezchasnyuk, M. Mochalova, P. Tsygankov, A. Lebedev, Y. Zorkina, E. Zubkov, A. Ochneva, O. Gurina, A. Silantyev, A. Majouga and N. Menshutina, Gels, 2022, 8, 765 CrossRef CAS PubMed.
- Y. A. Pardo, K. G. Yancey, D. S. Rosenwasser, D. M. Bassen, J. T. Butcher, J. E. Sabin, M. Ma, S. Hamada and D. Luo, Mater. Today, 2022, 53, 98–105 CrossRef CAS.
- J. D. Stuart, D. A. Hartman, L. I. Gray, A. A. Jones, N. R. Wickenkamp, C. Hirt, A. Safira, A. R. Regas, T. M. Kondash, M. L. Yates, S. Driga, C. D. Snow and R. C. Kading, PNAS Nexus, 2022, 1, pgac190 CrossRef PubMed.
- A. P. Kiseleva, P. V. Krivoshapkin and E. F. Krivoshapkina, Front. Chem., 2020, 8, 554 CrossRef CAS PubMed.
- T. B. Aigner, E. DeSimone and T. Scheibel, Adv. Mater., 2018, 30, 1704636 CrossRef PubMed.
- S. G. Wise, S. M. Mithieux and A. S. Weiss, Adv. Protein Chem. Struct. Biol., 2009, 78, 1–24 CAS.
- S. Zhao, X. Ye, X. Dai, X. Wang, S. Yu and B. Zhong, PLoS One, 2023, 18, e0282533 CrossRef CAS PubMed.
- R. Balu, N. K. Dutta, A. K. Dutta and N. R. Choudhury, Nat. Commun., 2021, 12, 149 CrossRef CAS PubMed.
- R. Zeng, K. Tang, H. Tian and Y. Pei, J. Polym. Sci., 2024, 62(6), 998 CrossRef CAS.
- H. A. Tran, T. T. Hoang, A. Maraldo, T. N. Do, D. L. Kaplan, K. S. Lim and J. Rnjak-Kovacina, Mater. Today, 2023, 65, 244–259 CrossRef CAS.
- W. Fang, R. Fan, A. S. Aranko, M. Hummel and H. Sixta, ACS Sustainable Chem. Eng., 2023, 11, 14807–14815 CrossRef CAS.
- L. F. Hartje, D. A. Andales, L. P. Gintner, L. B. Johnson, Y. V. Li and C. D. Snow, Crystals, 2023, 13(2), 352 CrossRef CAS.
- N. J. Culbert, M. Kaiser, N. Venter, M. J. B. Vreysen, J. R. L. Gilles and J. Bouyer, Parasites Vectors, 2020, 13, 192 CrossRef PubMed.
- J. B. Silver, Mosquito Ecology: Field Sampling Methods, Springer, Dordrecht, 3rd edn, 2007 Search PubMed.
- J. L. Watson, D. Juergens, N. R. Bennett, B. L. Trippe, J. Yim, H. E. Eisenach, W. Ahern, A. J. Borst, R. J. Ragotte, L. F. Milles, B. I. M. Wicky, N. Hanikel, S. J. Pellock, A. Courbet, W. Sheffler, J. Wang, P. Venkatesh, I. Sappington, S. V. Torres, A. Lauko, V. De Bortoli, E. Mathieu, S. Ovchinnikov, R. Barzilay, T. S. Jaakkola, F. DiMaio, M. Baek and D. Baker, Nature, 2023, 620, 1089–1100 CrossRef CAS PubMed.
- A. Madani, B. Krause, E. R. Greene, S. Subramanian, B. P. Mohr, J. M. Holton, J. L. Olmos, C. Xiong, Z. Z. Sun, R. Socher, J. S. Fraser and N. Naik, Nat. Biotechnol., 2023, 41, 1099–1106 CrossRef CAS PubMed.
- J. B. Ingraham, M. Baranov, Z. Costello, K. W. Barber, W. Wang, A. Ismail, V. Frappier, D. M. Lord, C. Ng-Thow-Hing, E. R. Van Vlack, S. Tie, V. Xue, S. C. Cowles, A. Leung, J. V. Rodrigues, C. L. Morales-Perez, A. M. Ayoub, R. Green, K. Puentes, F. Oplinger, N. V. Panwar, F. Obermeyer, A. R. Root, A. L. Beam, F. J. Poelwijk and G. Grigoryan, Nature, 2023, 623, 1070–1078 CrossRef CAS PubMed.
- Z. Li, S. Wang, U. Nattermann, A. K. Bera, A. J. Borst, M. Y. Yaman, M. J. Bick, E. C. Yang, W. Sheffler, B. Lee, S. Seifert, G. L. Hura, H. Nguyen, A. Kang, R. Dalal, J. M. Lubner, Y. Hsia, H. Haddox, A. Courbet, Q. Dowling, M. Miranda, A. Favor, A. Etemadi, N. I. Edman, W. Yang, C. Weidle, B. Sankaran, B. Negahdari, M. B. Ross, D. S. Ginger and D. Baker, Nat. Mater., 2023, 1–8 Search PubMed.
- A. Gazizov, A. Lian, C. Goverde, S. Ovchinnikov and N. F. Polizzi, 2023, 2023.10.15.562410.
| This journal is © The Royal Society of Chemistry 2024 |