Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of two nitrogen-containing polyaromatic compounds through gold catalysis/DBU-promoted cyclizations†
Vikas Ashokrao
Sadaphal
,
Tien-Lin
Wu
 and
Rai-Shung
Liu
and
Rai-Shung
Liu
 *
*
Department of Chemistry, National Tsing Hua University, Hsinchu, Taiwan, Republic of China. E-mail: rsliu@mx.nthu.edu.tw
First published on 25th March 2024
Abstract
This work reports an efficient synthesis of novel benzo[7,8]indolizino[2,3,4,5-ija]quinazoline derivatives between 2-(2-ethynylaryl)acetonitriles 1 and anthranils 2. The synthetic approach involves the initial formation of 7-formylindole intermediates that can be implemented by DBU to activate a novel indole–nitrile–aldehyde cyclization.
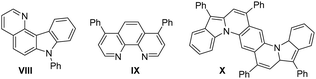
Nitrogen-doped polyarenes have attracted immense interest owing to their significance as electronic and pharmaceutical materials.1,2 Nevertheless, synthetic procedures to access such heteroaromatic compounds are long and tedious.1,2 Reported examples are largely limited to those polyarenes containing only one nitrogen atom. There is considerable interest in synthesizing polyarenes containing two or more nitrogen atoms, but little success is achieved toward material application.3Fig. 1 shows those polyarenes containing two nitrogen atoms, which have optoelectronic applications.4 New convenient syntheses of such N-doped polyarenes are highly desired to expand the present small scope.
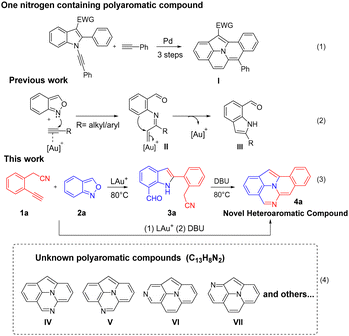
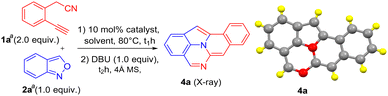
Scheme 1 (eqn (1)) shows a recent example of synthesizing fused four-membered benzenes such as benzo[7,8]indolizino[2,3,4,5-ija]quinoline (I) containing one nitrogen atom that is embedded in an indole moiety.5 We are aware that the two-nitrogen-containing analogues C13H8N2, such as species IV–VII, have not been reported in the literature (eqn (4)). Incorporation of one additional nitrogen in polyaromatic frameworks can alter the optoelectronic properties. The LUMO–HOMO energy levels will be significantly decreased if a pyridine ring replaces a benzene ring.4 Gold catalysis proves to be a powerful tool to access all-carbon fused benzene rings;6 however, their applications to the synthesis of nitrogen-containing fused benzenes are not well explored. Recently, Hashmi reported7 gold-catalyzed synthesis of new indole derivatives such as 2-amino-7-formylindole products (III) from the reactions of alkynes with anthranils (eqn (2)). So far, this catalytic reaction has no imminent impact on materials and medicinal chemistry. This work reports the new development of this catalytic reaction to access two-nitrogen containing fused benzenes such as benzo[7,8]indolizino[2,3,4,5-ija]quinazoline (4a), which is unprecedented in the literature (eqn (3)). This reaction sequence comprises two separate steps involving the initial treatment of 2-(2-ethynylaryl)acetonitriles 1a with anthranil 2a with a suitable gold catalyst in hot DCE, followed by a novel DBU-promoted8 indole–nitrile–aldehyde cyclization; intermediate 3a can be isolated and well characterized.
In material applications, 7-phenyl-7H-pyrido[3,2-c]carbazole (VIII)9a has applications in OLED devices (Fig. 1). 4,7-Diphenyl-1,10-phenanthroline (BPhen, IX)4 is a commonly used electron transport material (ETM) with good hole-blocking ability. Tetraphenylbis(indolo[1,2-a])quinoline (TPBIQ, X)9b has found application as an organic field transistor.
The reactions of 2-(2-ethynylaryl)acetonitriles 1a10 (2.0 equiv.) with anthranil 2a (1.0 equiv.) are optimized using various gold catalysts; the results are shown in Table 1. Alkyne substrate 1a was used in an excessive amount because protracted heating in this catalysis will cause slow alkyne decomposition. The reaction using IPrAuCl (10 mol%)/AgNTf2 (10 mol%) in hot DCE for a period of time (t1 = 20 h) ensured a nearly complete reaction; subsequently, DBU (1.0 equiv.) was added to the same solution with continuing heating (80 °C, t2 = 24 h). After workup, a new product 4a was isolated in 56% yield (entry 1). Other gold catalysts, LAuCl/AgNTf2 [L =PPh3 and P(OPh)3], gave the same product 4a in 45 and 37% yields, respectively (entries 2 and 3). Next, we altered the silver salts for IPrAuCl (AgX, X = SbF6 and OTf), further affording 4a in 50 and 33% yields, respectively (entries 4 and 5). Furthermore, silver-free IPrAuCl/NaBARF (BARF = B[3, 5-(CF3)2C6H3]4) was prepared, to yield our target 4a in 41% yield (entry 6). Notably, adding AgNTf2 (10 mol%) to this IPrAuCl/AgNTf2 (10 mol%) system increased the yield of 4a to 69% (entry 7). With this new IPrAuCl/AgNTf2 composition, the yields of compounds 4a in different solvents were as follows (entries 8–10): CF3C6H5 (38%), toluene (20%), and acetonitrile (0%). IPrAuCl without silver salts was inactive under the same reaction conditions (entry 11). AuCl3 was also less efficient, giving 4a in 39% yield (entry 12). AgNTf2 alone was catalytically inactive as well (entry 13). The molecular structure of compound 4a was characterized by X-ray diffraction;11 its ORTEP image is shown in Table 1. Our reaction sequence involves readily available reagents 1a and 2a, whereas the method in eqn (1) employs highly functionalized indoles as the starting reagent.
| Entry | Catalyst | Solvent | Time | Yieldb (%) | |
|---|---|---|---|---|---|
| t 1/t2 (h) | 4a | 2a | |||
| a Reaction conditions: 1a (1.6 mmol), 2a (0.8 mmol), gold catalyst (10 mol%), DBU (1.0 equiv.) in solvent (3.0 mL) under a N2 atmosphere. b Product yields are obtained after purification from a silica column. c Additional 10 mol% AgNTf2. DCE = 1,2 dichloroethane. ACN = acetonitrile. | |||||
| 1 | IPrAuCl/AgNTf2 | DCE | 20/24 | 56 | 8 |
| 2 | PPh3AuCl/AgNTf2 | DCE | 22/23 | 45 | 13 |
| 3 | (PhO)3PAuCl/AgNTf2 | DCE | 23/21 | 37 | 16 |
| 4 | IPrAuCl/AgSbF6 | DCE | 20/23 | 50 | 9 |
| 5 | IPrAuCl/AgOTf | DCE | 24/19 | 33 | 18 |
| 6 | IPrAuCl/NaBArF | DCE | 23/21 | 41 | 11 |
| 7c | IPrAuCl/AgNTf2 | DCE | 19/26 | 69 | — |
| 8c | IPrAuCl/AgNTf2 | Ph-CF3 | 24/21 | 38 | 16 |
| 9c | IPrAuCl/AgNTf2 | Toluene | 23/17 | 20 | 63 |
| 10c | IPrAuCl/AgNTf2 | ACN | 18/00 | — | 77 |
| 11 | IPrAuCl | DCE | 24/00 | — | 71 |
| 12 | AuCl3 | DCE | 24/20 | 39 | 15 |
| 13 | AgNTf2 | DCE | 16/00 | — | 83 |
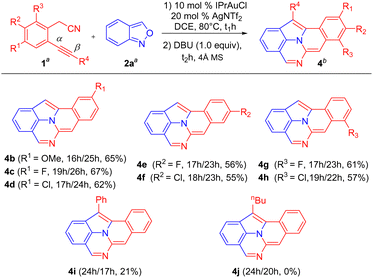
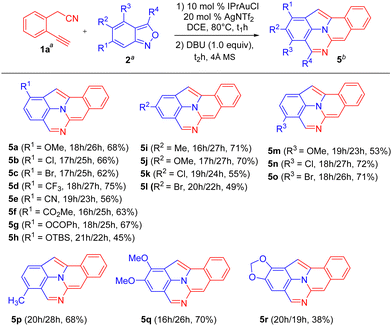
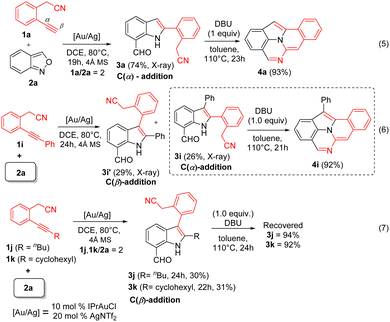
The generality of this new catalysis is assessed using various 2-(2-ethynylaryl)acetonitriles 1 and anthranil 2a; the results are summarized in Scheme 2. The operation used IPrAuCl/AgNTf2 in 10 mol% and 20 mol%, respectively, in the first step, and DBU (1.0 equiv.) in the second step. For substrates 1b–1d bearing various 5-phenyl substituents (R1 = OMe, F, and Cl), their standard operations yielded the desired products 4b–4d in 62–67% yields. We prepared additional substrates 1e and 1f containing 4-phenyl substituents (R2 = F and Cl), which delivered the desired products 4e and 4f in 56 and 55% yields, respectively. We also prepared substrates 1g and 1h bearing 3-phenyl substituents (R3 = F and Cl), and the corresponding products 4g and 4h were obtained in 61 and 57% yields, respectively. Substrate 1i, bearing a phenylethynyl group, afforded the desired product 4i, albeit with only 21% yield. Another internal alkyne substrate, 1j (R4 = n-butyl), failed to form the C(2)-substituted 7-formylindole intermediate through the C(α)-addition. The low efficiency of internal alkyne substrates is due to a distinct C(β)-regioselectivity for the anthranil attack on gold-π-alkyne, whereas our target 4 is produced from the alkynyl C(α)-regioselectivity. This assessment is further manifested by our control experiments (vide infra, eqn (5)–(7)).
Scheme 3 provides the outcome of the reaction of standard alkyne substrate 1a with various anthranils 2. A wide range of anthranils 2b–2h bearing various functional groups, including R1 = OMe, Cl, Br, CF3, CN, CO2Me, OCOPh, and OTBS, could produce the desired products 5a–5h in 45–75% yield; herein, only product 5h (R1 = OTBS) was obtained in low yield (45%). For anthranils 2i–2l containing R2 = Me, OMe, Cl, and Br, their reactions gave the desired products 5i–5l in 49–71% yields. Additional anthranils 2m–2o bearing R3 = OMe, Cl, and Br, delivered similar products 5m–5o in 53–72% yields. For anthranil 2p with R4 = Me, the corresponding product 5p was produced in 68% yield. For disubstituted anthranils 2q and 2r, bearing R1, R2 = OMe and OCH2O, this gold catalysis afforded the desired products 5q and 5r in 70 and 38% yields, respectively. In Scheme 3, only a few products such as 5h, 5l and 5r were produced in low yields (<50%).
Control experiments were conducted to study the effect of alkyne substrates. The gold-catalyzed reaction between 2-cyanomethyl-1-ethynylbenzene 1a (2.0 equiv.) and anthranil 2a yielded C(2)-substituted 7-formylindole 3a in 74% yield (Scheme 4, eqn (5)). The molecular structure of compound 3a was elucidated by X-ray diffraction.11 A further treatment of this species with DBU (1.0 equiv.) in hot toluene yielded the desired product 4a in 93% yield. For substrate 1i bearing an internal alkyne, two regioisomeric 7-formylindoles 3i′ and 3i, were obtained instead (eqn (6)). Their molecular structures were characterized by X-ray diffraction.11 Only the C(α)-addition product such as C(2)-substituted 7-formylindole 3i was active towards the DBU-promoted cyclization to yield our target 4i in 92% yield. Finally, we prepared alkyl-substituted alkynes 1j and 1k, herein the C(β)-addition products, i.e., C(3)-substituted 7-formylindole 3j and 3k, were obtained in low yields (eqn (7)). Importantly, these two species are chemically robust in the presence of DBU.
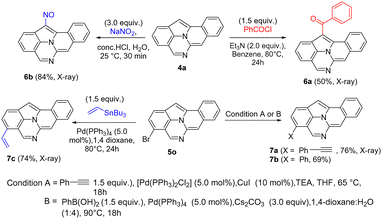
Scheme 5 depicts the chemical functionalization of compounds 4a and 5o. Treatment of species 4a with PhCOCl (1.5 equiv.) yielded a new acylation product 6a in 50% yield. Treatment of compound 4a with HCl/NaNO2, produced a nitrosyl-derived product 6b in 84% yield. Bromo-containing species 5o was active towards the Sonogashira reaction to yield the cross-coupling product 7a in 76% yield. The Suzuki–Miyaura cross-coupling of compound 5o with phenylboronic acid led to the formation of product 7b in 69% yield. Furthermore, the Stille-coupling reaction on species 5o with tributyl(vinyl)tin, delivered product 7c in 74% yield. The molecular structures of 6a, 6b, 7a and 7c were elucidated using X-ray diffraction.11
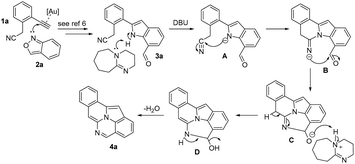
This gold catalysis and DBU-promoted cyclization involves an initial formation of 7-formylindole 3a (Scheme 6); its formation mechanism follows an early proposal in Hashmi's work6 (see eqn (2)). The second step comprises an intramolecular reaction among indole, nitrile and aldehyde; such a three-component cyclization is unprecedented in the literature. We postulate an initial deprotonation of the indole N–H group of intermediate 3a to form an amide anion A that attacks the nitrile group to generate an imino anion B; this step does not require a gold catalyst according to our control experiment in eqn (5). A final intramolecular cyclization is likely to occur between this imino anion B and the aldehyde to generate an oxy anion C, which upon protonation by the DBU/H+ complex will generate the last intermediate D. A final aromatization of this intermediate D delivers the observed product 4a.
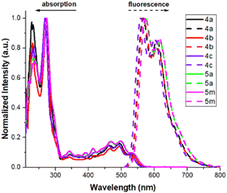
Photophysical properties of representatives 4a–4c, 5a and 5m were measured to examine their potential material applications. Fig. 2 shows the electronic absorption and emission spectra, and Table S1 (see ESI†) shows their corresponding wavelengths, absorption efficiencies and Stokes shifts. All these compounds have very similar values despite various substituents (Cl, F and OMe) on three different benzene rings. One strong absorption band is observed at 268–274 nm, corresponding to π–π* transition with large coefficients (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e = 4.47–4.91). The emission spectra are centered at 565–572 nm in the yellow–orange region. Notably, the Stokes shifts are quite large up to 291–299 nm−1, which reflects a fast relaxation process from the initial state to the emissive state.
e = 4.47–4.91). The emission spectra are centered at 565–572 nm in the yellow–orange region. Notably, the Stokes shifts are quite large up to 291–299 nm−1, which reflects a fast relaxation process from the initial state to the emissive state.
In summary, we have developed new gold catalysis between 2-cyanomethyl-1-ethynylbenzene with anthranils to construct benzo[7,8]indolizino[2,3,4,5-ija]quinazoline frameworks. In this reaction sequence, anthranils attack the gold-π-alkyne intermediate at the alkyne C(α)-regioselectivity forming 7-formylindole intermediates that can be isolated and characterized. Internal alkyne substrates are not efficient because the alkyne C(β)-regioselectivity occurs to yield 7-formylindole intermediates that are not active towards the DBU-activated cyclizations. The use of readily available substrates 1 and 2 to access two-nitrogen containing fused benzenes highlights the significance of this work.
The authors thank the National Science and Technology Council (NSTC 112-2113-M-007-013), Taiwan, for supporting this work.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644–4680 CrossRef CAS PubMed; (b) L. Xiao, H. Lan and J. Kido, Chem. Lett., 2007, 36, 802 CrossRef CAS; (c) U. H. F. Bunz, J. U. Engelhart, B. D. Lindner and M. Schaffroth, Angew. Chem., Int. Ed., 2013, 52, 3810–3821 CrossRef CAS PubMed; (d) X. F. Wu, H. Neumann and M. Beller, Chem. Rev., 2013, 113(1), 1–35 CrossRef CAS PubMed; (e) M. Stepien, E. Gonka, M. Zyla and N. Sprutta, Chem. Rev., 2017, 117, 3479–3716 CrossRef CAS PubMed; (f) S. Hiroto, Chem. – Asian J., 2019, 14(15), 2514–2523 CrossRef CAS PubMed; (g) Z. Zeng, H. Jin, K. Sekine, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2018, 57, 6935–6939 CrossRef CAS PubMed; (h) Z. Zeng, H. Jin, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2018, 57, 16549–16553 CrossRef CAS PubMed; (i) K. P. Kawahara, W. Matsuoka, H. Ito and K. Itami, Angew. Chem., Int. Ed., 2020, 59, 6383–6388 CrossRef CAS PubMed; (j) A. Borissov, Y. K. Maurya, L. Moshniaha, W.-S. Wong, M. Zyla-Karwowska and M. Stepien, Chem. Rev., 2022, 122, 565–788 CrossRef CAS PubMed.
- (a) M. Ishikura, T. Abe, T. Choshi and S. Hibino, Nat. Prod. Rep., 2013, 30, 694 RSC; (b) L. W. Ye, X. Q. Zhu, R. L. Sahani, Y. Xu, P. C. Qian and R.-S. Liu, Chem. Rev., 2021, 121, 9039–9112 CrossRef CAS PubMed; (c) Y. Li, J. Jin, W. Fan and D. Huang, Org. Lett., 2023, 25, 8284–8289 CrossRef CAS PubMed; (d) S. Nam and I. Kim, J. Org. Chem., 2023, 88, 745–754 CrossRef CAS PubMed.
- (a) M. Tasior, M. Chotkowski and D. T. Gryko, Org. Lett., 2015, 17(24), 6106–6109 CrossRef CAS PubMed; (b) B. Alcaide, P. Almendros, I. Fernandez, F. Herrera and A. Luna, Chem. – Eur. J., 2018, 24, 1448–1454 CrossRef CAS PubMed; (c) J. Qiao, X. Jia, P. Li, X. Liu, J. Zhao, Y. Zhou, J. Wang, H. Liu and F. Zhao, Adv. Synth. Catal., 2019, 361, 1419–1440 CrossRef CAS; (d) Y. Zhang, S. H. Pun and Q. Miao, Chem. Rev., 2022, 122, 14554–14593 CrossRef CAS PubMed.
- D. Chen, S.-J. Su and Y. Cao, J. Mater. Chem. C, 2014, 2, 9565 RSC.
- K. Alam, S. W. Hong, K. H. Oh and J. K. Park, Angew. Chem., Int. Ed., 2017, 56, 13387–13391 CrossRef CAS PubMed.
- (a) C. F. Shu, C.-B. Chen, W.-X. Chen and L.-W. Ye, Org. Lett., 2013, 15, 5542–5545 CrossRef CAS PubMed; (b) C. Zhang, K. Hong, C. Pei, S. Zhou, W. Hu, A. S. K. Hashmi and X. Xu, Nat. Commun., 2021, 12, 1182 CrossRef CAS PubMed.
- H. Jin, L. Huang, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 794–797 CrossRef CAS PubMed.
- (a) S. K. Guchhait, G. Priyadarshani and N. M. Gulghane, RSC Adv., 2016, 6, 56056 RSC; (b) R. R. Zalte, A. A. Festa, N. E. Golantsov, K. Subramani, V. B. Rybakov, A. V. Varlamov, R. Luque and L. G. Voskressensky, Chem. Commun., 2020, 56, 6527–6530 RSC.
- (a) C. J. Shin, D. M. Kang, M. S. Kang, N. H. Lee, H. G. Lee, H. K. Jung and M. Y. Chae, PCT Int. Appl., WO 2013089424 A1 20130620, 2013 Search PubMed; (b) L. Zhu, E.-G. Kim, Y. Yi, E. Ahmed, S. A. Jenekhe, V. Coropceanu and J.-L. Bredas, J. Phys. Chem. C, 2010, 114, 20401 CrossRef CAS.
- For gold-catalyzed reactions of alkyne substrate 1 with the pyridine-based oxide, see: S.-N. Karad and R.-S. Liu, Angew. Chem., Int. Ed., 2014, 53, 5444–5448 CrossRef CAS PubMed.
- Compound 3a (CCDC 2283774), 3i (CCDC 2292226), 3i′ (CCDC 2290023), 4a (CCDC 2283773), 6a (CCDC 2288008), 6b (2301731), 7a (CCDC 2301524) and 7c (CCDC 2301525) contain the supplementary crystallographic data for this paper†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2283773, 2283774, 2288008, 2290023, 2292226, 2301524, 2301525 and 2301731. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc00113c |
| This journal is © The Royal Society of Chemistry 2024 |