 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and investigation of a meta[6]cycloparaphenylene gold(I) N-heterocyclic carbene complex†
Felix
Bernt
 ab,
Christopher M.
Leonhardt
ab,
Christopher M.
Leonhardt
 ab,
Dominic
Schatz
ab,
Dominic
Schatz
 ab and
Hermann A.
Wegner
ab and
Hermann A.
Wegner
 *ab
*ab
aInstitute of Organic Chemistry, Justus Liebig University Giessen, Heinrich Buff Ring 17, Giessen 35392, Germany. E-mail: hermann.a.wegner@org.chemie.uni-giessen.de
bCentre for Materials Research (ZfM/LaMa), Justus Liebig University Giessen, Heinrich Buff Ring 16, Giessen 35392, Germany
First published on 8th February 2024
Abstract
Meta[n]cycloparaphenylenes (m[n]CPPs) as well as N-heterocyclic carbene (NHC) gold(I)-complexes are intriguing building blocks for material and life sciences due to their extraordinary structures resulting in unique photophysical properties. Herein, we report the combination of a m[6]CPP with a N-heterocyclic carbene serving as a ligand in a linear gold(I)-complex possessing the form [AuBr(NHC)]. Solid-state structures of both the precursor and the complex are presented and discussed. Moreover, we investigated the luminescence properties of both the imidazolium intermediate and the corresponding gold(I)-complex.
The development of novel concepts for fluorophores is driven by the constant quest for an improvement of the materials regarding their required properties in material and life sciences. In recent years, the combination of organic fluorophores with gold-NHC (N-heterocyclic carbene) complexes has become a common strategy to create hybrid materials that exhibit unique properties, not only useful in catalysis,1,2 or as precursors for gold nanomaterials,3–6 but for biological applications as well.7–13
The increased interest in NHCs can be attributed to the strong σ-donating capability of NHCs, which allows for the formation of coordination bonds with a wide range of transition metals throughout the periodic table. Moreover, NHCs demonstrate the unique ability to stabilize transition metals in both lower and higher oxidation states.14–16
A relatively new class of unique organic fluorophores are meta[n]cycloparaphenylenes (m[n]CPPs), published in 2019 by the group of Jasti.17 These m[n]CPPs exhibit a similar absorbance pattern to their all-para derivatives, but with a slight shift in wavelength from 340 nm to 327 nm.18 The absorption maxima at these wavelengths are attributed to the transition between the HOMO and the LUMO+1 or LUMO+2 states. Unlike their [n]CPP counterparts, the m[n]CPPs display a broken orbital centrosymmetry, resulting in the presence of a secondary absorption shoulder in their spectra. This unique feature is attributed to the visible HOMO to LUMO transition. Additionally, both the m[5]CCP and the m[6]CPP exhibit emission maxima that are size-dependent as well. This size-dependency in emission is a consequence of the partial planarization of the phenylene backbone. The fluorescence exhibited by these smaller m[n]CPPs makes them intriguing candidates for application in organic chemistry, materials science, and life sciences.19–23 In 2023, our group successfully developed a convenient synthesis strategy towards different substituted m[6]CPPs.24 Therein, we could demonstrate that the nitrile functionality can work as a convenient handle towards functional group conversion reactions without changing the absorption and emission maxima. We herein present the synthesis of a Au(I)–NHC methylene bridged m[6]CPP as a contribution to the ever-expanding areas of Au(I)–NHC chemistry and m[n]CPPs. Doing so, we planned to synthesize a m[6]CPP–NHC precursor, which should provide a platform to investigate the influence of the core structure on the m[6]CPP and vice versa. Moreover, this combination of m[6]CPP with a NHC-ligand will open new research areas for this new compound class, including materials and life sciences, as well as (organo)metal chemistry.
Initially, we focused on the synthesis of a Au(I)–NHC complex based on imidazolinium core motif 4 (Scheme 1, left). Therefore, we started with the conversion of nitrile-m[6]CPP 1 into its amino-methylene derivative 2 in a yield of 78%. The subsequent amide coupling reaction was carried out using a modified protocol based on Steglich esterification. After purification, we were able to successfully synthesize the desired m[6]CPP-oxalamide 3 in a yield of 63%. However, despite our best efforts we were not able to realize the subsequent reduction step to obtain the desired oxalic diamine required for the preparation of the desired carbene precursor 4.
In a second approach, we designed imidazolium precursor 7 substituted with m[6]CPP connected through a methylene-bridge to the imidazole core as well (Scheme 1, right). It is a common observation that such precursors tend to exhibit enhanced stability when compared to their saturated analogues. We decided to introduce a methyl group as a second wingtip on the envisioned Au(I)-complex 8 as this would enable us to use the commercially available 1-methyl imidazole and carboxy-m[6]CPP 5 as building blocks. Commencing from CN-m[6]CPP 1 once again, we proceeded with a synthetic protocol towards the carboxylic acid developed by our group.24 The yield of carboxy-m[6]CPP 1 was substantially improved to 99% by conducting the synthesis on a larger scale, which allowed us to just wash the product with cold ethanol and water for purification instead of applying purification via column chromatography. After obtaining carboxy-m[6]CPP 5, we carried out a two-step synthesis sequence using first, LiAlH4 and then PBr3 as the reagents. By following this approach, we successfully synthesised important intermediate (bromomethyl)-m[6]CPP 6 within two steps with a decent yield of 44%. Finally, we added N-methyl imidazole to (bromomethyl)-m[6]CPP 6, leading to the formation of imidazolium salt 7 with a very good yield of 81%. With imidazolium salt 7 in hand, we demonstrated its applicability as an [AuX(NHC)] ligand.
There are three main approaches for the synthesis of metal-NHC complexes, all of which share the utilization of an imidazolium salt or its derivatives.14,25–27 Among them, the so-called weak-base approach, independently developed by Nolan et al.25 and Gimeno et al.27 in 2013, seemed to be a promising strategy for our approach. It uses a weak, cost-effective base and solvents like NEt3, NaOAc, K2CO3, DCM, or acetone. This method is also compatible with ambient atmospheric conditions. Following their protocol, we stirred a solution of the imidazolium precursor 7 together with the Au(I) precursor [AuCl(SMe2)] and K2CO3 at 60 °C in acetone overnight. After evaporating the solvent and conducting further purification, we successfully isolated [AuBr(NHC)] 8 in 58% yield. Halide scrambling is a common observation, in the case of different counterions. However, analysis of compound 8 by NMR, IR spectroscopy, mass spectrometry and elemental analysis confirmed its homogeneity.
Single-crystals of imidazolium 7 suitable for X-ray diffraction measurements were obtained by vapour diffusion of n-pentane into a saturated solution of 7 dissolved in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of acetonitrile and dichloromethane (Fig. 1, left). Similarly, crystals of [AuBr(NHC)] 8 were obtained. In that case, the solid was dissolved in a 1
1 mixture of acetonitrile and dichloromethane (Fig. 1, left). Similarly, crystals of [AuBr(NHC)] 8 were obtained. In that case, the solid was dissolved in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1,2-dichlorobenzene and 1,2-dichloroethane and n-pentane was used for vapor diffusion (Fig. 1, right), resulting in minor solvate disorder, which has been addressed. Additionally, the structure has crystallographically-imposed mirror-symmetry.
1 mixture of 1,2-dichlorobenzene and 1,2-dichloroethane and n-pentane was used for vapor diffusion (Fig. 1, right), resulting in minor solvate disorder, which has been addressed. Additionally, the structure has crystallographically-imposed mirror-symmetry.
 | ||
| Fig. 1 Absorption (left) and emission (right) spectra of CN-m[6]CPP 1 (black), imidazolium bromide 7 (yellow), and the [AuBr(NHC)] complex 8 (red). | ||
Upon coordination and formation of the Au(I)-complex, the bond angle between N(1) and C(1) opened to 111.1°. Complex 8 shows an approximately linear two-coordinate geometry at the Au(I)-centre, as the C(23)–Au(1)–Br(1) angle is measured to be 179.7°. Moreover, the bond lengths within the imidazole core changed to 1.345 Å and 1.361 Å, respectively, while the bond length between C(23) and Au(1) was 1.975 Å.
Regarding the torsional angles, the m[6]CPP segment of 7 shows smaller values, ranging from 50° to 16°, compared to nitrile-substituted derivative 1 (56° to 23°).24 These torsional angles change upon coordination of the gold(I)-species ranging now from 58° to 17°.
Solution-state UV/Vis absorption and luminescence spectra were measured for both imidazolium-bromide 7 and complex 8 in chloroform. The absorption spectra of both substances show the characteristics of the m[6]CPP substituent, recognisable by the sharp absorption shoulder at around 326 nm and the broad shoulder at around 405 nm. While the main absorption is assigned to the HOMO−1 to LUMO and HOMO to LUMO+1 transitions, the second absorption shoulder is represented by the HOMO to LUMO transition.17 This was further confirmed by density functional theory (DFT) calculations at the PBE0/ZORA-def2-TZVPP level (for more details see ESI†).
The fluorescence properties of 7 and 8 have been investigated under ambient conditions in chloroform solutions. Both imidazolium 7 and Au(I)-complex 8 show two emission maxima at 493 nm and 519 nm, respectively, after irradiation with a wavelength of 326 nm. These maxima fit very well to the maxima of CN-m[6]CPP 1, which was measured under the same conditions.24 The fluorescence quantum yields of both substances have been determined to be 0.14 (7) and 0.22 (8) using CN-m[6]CPP 1 (Φ = 0.24)24 and CN-m[10]CPP (Φ = 0.71)24 as standards. The emission spectra are presented together with the experimental absorption spectra in Fig. 2.
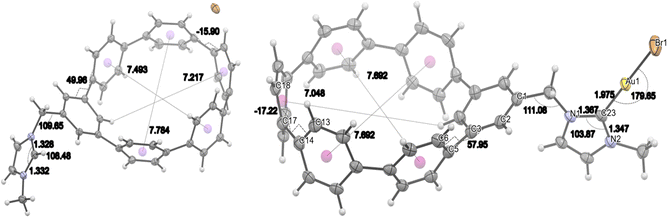 | ||
| Fig. 2 ORTEP drawings of imidazolium bromide 7 and [AuBr(NHC)] complex 8 at 50% probability. Additionally, important structural parameters are shown. | ||
In conclusion, we have successfully combined an intriguing chromophore (m[6]CPP) with the ligand NHC to design a novel Au-based complex. For the preparation, we have been able to utilize our post-functionalization protocol of CN-m[6]CPP 1 to access the asymmetric substituted m[6]CPP-oxalamide 3 and the imidazolium bromide 7. The latter has been subjected to the synthesis of [AuBr(NHC)] complex 8. The photophysical properties of both the precursor 7 and the final complex 8 are dominated by the m[6]CPP moiety. Although the quantum yield dropped significantly for the imidazolium precursor 7 compared to the unsubstituted m[6]CPP, this could be partially restored within gold(I)-complex 8. Moreover, theoretical calculations supported our experimental observations that the main radiative transitions just occur inside the m[6]CPP part, which is electronically decoupled from the other parts of the molecule. Additionally, investigations of the buried volume and steric properties are presented. With presenting the first example of a m[6]CPP gold(I)-complex, this novel compound combination will open new research fields including material and life sciences, as well as (organo)metal chemistry to the relatively young compound class of m[n]CPPs.
The data that support the findings of this study are available in the ESI† of this article. Deposition numbers 2303780 (for compound 7) and 2310446 (for compound 8) contain the supplementary crystallographic data for this paper.
F. B. and H. A. W. conceptualized the project. F. B. performed the organic synthesis and collected experimental data. C. M. L. performed the DFT calculations. Major contributions in solving and discussing the solid-state structures were made by D. S. All authors were involved in discussing the data and preparing the manuscript.
The authors greatly appreciate financial support provided by TCI and the European and Hessian Government (EFRE, 20005599). Moreover, the authors thank Lisa Marie Wagner for X-ray diffraction measurements. Dr Christian Würtele is gratefully acknowledged for his valuable advice during the process of solving the solid-state structures. We thank the JLU for funding the open access within the national German TIB Consortium with the Royal Society of Chemistry's Platinum Agreement. Finally, the authors thank Jannis Volkmann, Daniel Kohrs, and Jan H. Griwatz for helpful discussions during the laboratory work.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Pažický, A. Loos, M. J. Ferreira, D. Serra, N. Vinokurov, F. Rominger, C. Jäkel, A. S. K. Hashmi and M. Limbach, Organometallics, 2010, 29, 4448–4458 CrossRef.
- L. Rocchigiani and M. Bochmann, Chem. Rev., 2021, 121, 8364–8451 CrossRef CAS PubMed.
- K. Salorinne, R. W. Y. Man, P. A. Lummis, M. S. A. Hazer, S. Malola, J. C.-H. Yim, A. J. Veinot, W. Zhou, H. Häkkinen, M. Nambo and C. M. Crudden, Chem. Commun., 2020, 56, 6102–6105 RSC.
- A. I. Sullivan, J. F. DeJesus, S. Malola, S. Takano, T. Tsukuda, H. Häkkinen and C. M. Crudden, Chem. Mater., 2023, 35, 2790–2796 CrossRef CAS.
- M. R. Narouz, S. Takano, P. A. Lummis, T. I. Levchenko, A. Nazemi, S. Kaappa, S. Malola, G. Yousefalizadeh, L. A. Calhoun, K. G. Stamplecoskie, H. Häkkinen, T. Tsukuda and C. M. Crudden, J. Am. Chem. Soc., 2019, 141, 14997–15002 CrossRef CAS PubMed.
- R. W. Y. Man, H. Yi, S. Malola, S. Takano, T. Tsukuda, H. Häkkinen, M. Nambo and C. M. Crudden, J. Am. Chem. Soc., 2022, 144, 2056–2061 CrossRef CAS PubMed.
- M. Mora, M. C. Gimeno and R. Visbal, Chem. Soc. Rev., 2019, 48, 447–462 RSC.
- H. Ibrahim, C. Gibard, C. Hesling, R. Guillot, L. Morel, A. Gautier and F. Cisnetti, Dalton Trans., 2014, 43, 6981–6989 RSC.
- A. Citta, E. Schuh, F. Mohr, A. Folda, M. L. Massimino, A. Bindoli, A. Casini and M. P. Rigobello, Metallomics, 2013, 5, 1006–1015 CrossRef CAS PubMed.
- M. Kriechbaum, G. Winterleitner, A. Gerisch, M. List and U. Monkowius, Eur. J. Inorg. Chem., 2013, 5567–5575 CrossRef CAS.
- B. Bertrand, A. de Almeida, E. P. M. van der Burgt, M. Picquet, A. Citta, A. Folda, M. P. Rigobello, P. Le Gendre, E. Bodio and A. Casini, Eur. J. Inorg. Chem., 2014, 4532–4536 CrossRef CAS.
- R. Visbal, V. Fernández-Moreira, I. Marzo, A. Laguna and M. C. Gimeno, Dalton Trans., 2016, 45, 15026–15033 RSC.
- L. M. Groves, C. F. Williams, A. J. Hayes, B. D. Ward, M. D. Isaacs, N. O. Symonds, D. Lloyd, P. N. Horton, S. J. Coles and S. J. A. Pope, Dalton Trans., 2019, 48, 1599–1612 RSC.
- W. A. Herrmann, M. Elison, J. Fischer, C. Köcher and G. R. J. Artus, Chem. – Eur. J., 1996, 2, 772–780 CrossRef CAS.
- D. Zhang and G. Zi, Chem. Soc. Rev., 2015, 44, 1898–1921 RSC.
- C. Lorber and L. Vendier, Dalton Trans., 2009, 6972–6984 RSC.
- T. C. Lovell, C. E. Colwell, L. N. Zakharov and R. Jasti, Chem. Sci., 2019, 10, 3786–3790 RSC.
- E. R. Darzi and R. Jasti, Chem. Soc. Rev., 2015, 44, 6401–6410 RSC.
- J. M. van Raden, B. M. White, L. N. Zakharov and R. Jasti, Angew. Chem., Int. Ed., 2019, 58, 7341–7345 CrossRef CAS PubMed.
- J. M. van Raden, N. N. Jarenwattananon, L. N. Zakharov and R. Jasti, Chem. – Eur. J., 2020, 26, 10205–10209 CrossRef CAS PubMed.
- T. C. Lovell, S. G. Bolton, J. P. Kenison, J. Shangguan, C. E. Otteson, F. Civitci, X. Nan, M. D. Pluth and R. Jasti, ACS Nano, 2021, 15, 15285–15293 CrossRef CAS PubMed.
- C. W. Patrick, J. F. Woods, P. Gawel, C. E. Otteson, A. L. Thompson, T. D. W. Claridge, R. Jasti and H. L. Anderson, Angew. Chem., 2022, 61, e202116897 CrossRef CAS PubMed.
- J. Zhang, H. Cheng, Z. Meng, H. Zhang, Z. Luo, M. Muddassir, X. Li and Y. Sha, Polym. Chem., 2023, 14, 5029–5033 RSC.
- F. Bernt and H. A. Wegner, Chem. – Eur. J., 2023, 29, e202301001 CrossRef CAS PubMed.
- A. Chartoire, C. Claver, M. Corpet, J. Krinsky, J. Mayen, D. Nelson, S. P. Nolan, I. Peñafiel, R. Woodward and R. E. Meadows, Org. Process Res. Dev., 2016, 20, 551–557 CrossRef CAS.
- H. M. J. Wang and I. J. B. Lin, Organometallics, 1998, 17, 972–975 CrossRef CAS.
- R. Visbal, A. Laguna and M. C. Gimeno, Chem. Commun., 2013, 49, 5642–5644 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic protocols, spectra, crystallographic data, and details on calculations. CCDC 2303780 and 2310446. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc06225b |
| This journal is © The Royal Society of Chemistry 2024 |

