Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Boosting the photo-switchability of double-responsive water-soluble polymers by incorporating arylazopyrazole dyes†
René
Steinbrecher
 a,
Peiran
Zhang
a,
Peiran
Zhang
 b,
Christine M.
Papadakis
b,
Christine M.
Papadakis
 b,
Peter
Müller-Buschbaum
b,
Peter
Müller-Buschbaum
 bc,
Andreas
Taubert
bc,
Andreas
Taubert
 a and
André
Laschewsky
a and
André
Laschewsky
 *ad
*ad
aInstitute of Chemistry, University of Potsdam, Potsdam-Golm, Germany. E-mail: laschews@uni-potsdam.de
bPhysics Department, TUM School of Natural Sciences, Technical University of Munich, Garching, Germany
cHeinz Maier-Leibnitz Zentrum (MLZ), Technical University of Munich, Garching, Germany
dFraunhofer Institute for Applied Polymer Research IAP, Potsdam-Golm, Germany
First published on 22nd January 2024
Abstract
Dual thermo- and light-responsive water-soluble copolymers that respond to exclusively non-invasive triggers are obtained by functionalising poly(N,N-dimethylacrylamide) with arylazopyrazole side chains. The light-induced E–Z (trans–Z) photo isomerisation of these dyes provides an exceptionally effective photo-switch, which can reversibly shift the LCST-type phase transition temperatures by almost 25 K.
Stimuli-responsive, often called ‘smart’, polymeric materials can adapt to changes of the surrounding environment.1 Typically, large and reversible property changes are induced upon applying a small trigger signal. This may be a small change of the pH, of electrolyte content, of temperature, or a photo stimulus, to name a few. Stimuli-responsive polymers in aqueous media are of special interest, where they typically undergo reversible changes of their hydration. In particular, when involving a coil-to-globule collapse transition of the polymer chains, stimuli responsive polymers are attractive for a wealth of applications, specifically in the biomedical field, e.g., as sensors, for controlled release, or artificial muscles.2,3 An inherent problem of many triggers – such as changes of the pH or ionic strength – is that substances must be added to the system to induce the property changes. These substances must then be removed again to induce the reverse transition in the polymer. Therefore, non-invasive triggers such as temperature changes or light are most advantageous for systems that are supposed to repeatedly switching back and forth. While temperature changes are a well-studied and versatile trigger,4–6 reversible photo-responsive systems are scarce and have been much less explored.7–9
Aqueous reversible light responsive systems generally use photo-active moieties which undergo a structural change to alter their polarity and thereby also the overall hydrophilicity of the polymer.10 This approach allows for modulating the phase transition temperature (Ttrans) of thermo-responsive polymers,7,8 and thus, enables their isothermal switching by irradiation as alternative to their switching by temperature. Common systems are based on spiropyrans,11 cinnamates12 or azobenzenes.13 Azo dyes are particularly advantageous as their E–Z (trans–Z) photo-isomerisation which increases their dipole moment and polarity, and consequently their solubility in water, is virtually free of side reactions and thus fully reversible,14 showing no fatigue.
Still, their use suffers from two shortcomings. First, the Z-state tends to relax rapidly to the more stable E-state.15 This makes it difficult to implement bi-stable switching scenarios. Second, the shifts of Ttrans achieved have been mostly limited to a few degrees.13,16–31 For many of the envisaged uses, this is too small. As an exception, Menzel and coworkers32 succeeded to shift Ttrans of azobenzene-bearing polyacrylamides upon irradiation by up to 14 °C.29 Still, the rather short half-life time (τ½) of the Z-state and the marked overlap of the main absorption bands of E- and Z-states that lead only to a mixed photo stationary state (PSS), limited the potential of such systems.33 Also, a singular study reported cloud point shifts by up to 35 °C of branched poly(ethylene imine) that was weakly functionalised with azobenzenecarboxylic acid and propionyl groups.34 Exciting as it appears, the implications of the report are difficult to assess as the polymers were chemically ill-defined, the reported shifts seem inconsistent (rather suggesting changes <15 °C), any information about τ½ of the Z-state is missing, and superposed pH-effects on these weak polyelectrolytes cannot be ruled out. In any case, to the best of our knowledge no follow-up studies have been published.
Therefore, new types of azobenzene and azoheteroarene chromophores14,35 have been developed in recent years. In particular, the aryl azopyrazole (AAP) photo switch introduced by Fuchter and coworkers36 excels by effectively separating the absorbance bands of the E- and Z-isomers as well as by featuring long τ½ of the Z-state. Up to now, AAP-based compounds have been used to modify various surfaces37–40 or to realise photo-responsive gelators,41 enzyme inhibitors,42 or shape memory materials.43 Inspired by these reports, we have now incorporated the AAP-chromophore as photo-trigger into thermo-responsive polymers, for controlling their lower critical solution temperature (LCST)-type coil-to-globule collapse (transition) temperature.
Adapting an established synthesis route,41 the new AAP-bearing acrylamide 6 was synthesised in 5 steps with a total yield of 38% after chromatographic purification (Scheme 1, steps (a)–(e); for details see the ESI†). The orange solid is soluble in lower alcohols including methanol, but not in water. In agreement with the study on a closely related AAP dye by Fuchter and coworkers,36 the overlap of the absorbance spectra of the E- and Z-isomers is minimized, and a photo stationary state with a minimum content of 97% of Z-isomer is reached in methanol solution upon irradiation with 365 nm light (see ESI†). Moreover, the thermal relaxation of the Z- to the E-isomer is advantageously slow (τ½ = 29 h, at 20 °C in methanol).
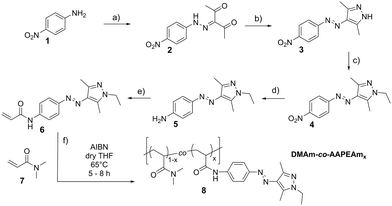 | ||
| Scheme 1 Synthesis of the azo dye-functionalised monomer AAPEAm 6, and its copolymerisation (cf. ESI†). (a) Diazotation of 1 with NaNO2/HCl, and reaction with pentane-2,4-dione. (b) Cyclisation of 2 with hydrazine. (c) Nucleophilic substitution of 3 with ethyl bromide. (d) Reduction of the nitro group of 4 with Na2S. (e) Amidation of the aniline 5 with acryloyl chloride. (f) Statistical copolymerisation of AAPEAm 6 with DMAm 7 to yield thermo- and light-responsive polyacrylamides 8. | ||
Thermo- and photo-responsive polymers were prepared via conventional free radical copolymerisation of the AAP-functionalised acrylamide 6 with N,N-dimethylacrylamide 7 (DMAm). DMAm was chosen as major constituent for the copolymers because its homopolymer is very hydrophilic and soluble in the full temperature window of 0–100 °C under ambient pressure.44 Hence, we envisaged to obtain copolymers that are still water-soluble for rather high AAP contents (see below) despite monomer 6 being insoluble in water (see above). The hydrophobicity and concurrent low water solubility of most photo-responsive groups is a general problem and has inherently limited their maximum incorporation into water-soluble thermo-responsive polymers in the past.17,24,29,32 Also, DMAm is an N-substituted acrylamide alike 6, so that both monomers were expected to exhibit comparable reactivities in their copolymerisation and to incorporate equally well into the growing polymer chain. By engaging increasing amounts of 6, a set of copolymers DMAm-AAPEAmx8 with varying AAP-content x (mol%) was successfully produced (Scheme 1 step f, Table 1). The key features of the copolymers are summarised in Table 1. More details of the polymerisation and the polymer characterisation are provided in the ESI.†
| Code | AAP-monomer 6 in feed/mol% | Polymerization time/h | Monomer conversiona/% | M appn /104 g mol−1 | Đ | AAP content in polymerc/mol% |
|---|---|---|---|---|---|---|
| a Determined by 1H NMR in the reaction mixture (decay of the signal of the acrylic protons). b Determined for the purified copolymers by SEC in N-methyl-2-pyrrolidone, calibration by polystyrene standards. c Precision of 10 rel%, but not better than ±0.5 mol% for low contents of azodye (determined by 1H NMR). | ||||||
| DMAm-AAPEAm1.5 | 2 | 8 | 98 | 1.2 | 2.9 | 1.5 ± 0.5 |
| DMAm-AAPEAm4.5 | 5 | 8 | 97 | 1.3 | 3.1 | 4.5 ± 0.5 |
| DMAm-AAPEAm7 | 8 | 6.5 | 94 | 1.8 | 2.8 | 7.0 ± 0.7 |
| DMAm-AAPEAm8 | 9 | 7.5 | 93 | 1.2 | 2.3 | 8.0 ± 0.8 |
| DMAm-AAPEm9.5 | 11 | 6.5 | 93 | 1.7 | 2.7 | 9.5 ± 1.0 |
| DMAm-AAPEAm11 | 14 | 5.0 | 89 | 2.2 | 2.7 | 11.0 ± 1.1 |
Remarkably, the copolymerisation seems to be hardly affected by the content of the AAP-functionalised comonomer. All reactions were quite fast and could be conducted to high monomer conversions. In a marked contrast, structurally similar azobenzene comonomers were reported to significantly reduce polymerisation rates and yields.17,20,24,29,32 Also, the SEC results of the various DMAm-AAPEAm copolymers suggest on the first view that the molar masses even rise with increasing content of 6. This result is unexpected, since generally, in parallel to the polymerisation rates and yields, the molar mass decreases with increasing azodye content for similar systems.17,20,24,29,32 Still, it must be kept in mind that the values represent only apparent, not absolute molar masses. With increasing content of 6, the solvent quality might improve and thereby the hydrodynamic radii of the copolymers, thus only pretending an increase of Mappn. In any case, it seems that the DMAm-AAPEAm copolymers are well comparable to each other, even for increased contents of the AAP dye.
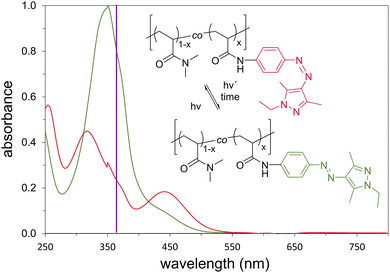
Initially, we verified whether the advantageous spectroscopic properties of monomer 6 regarding the E- to Z-isomerization were preserved in the copolymers. Copolymers were dissolved in methanol as well as in water, stored at ambient temperature for 24 h in the dark, and analyzed via UV-Vis spectroscopy before and after irradiation at 365 nm. As monomer 6 is insoluble in water, its photo-response was only analysed in methanol (Fig. 1 and ESI,† Fig. S31). Compared to the monomer spectra, small blue shifts of both the main band of the E- and Z-isomers from 351 to 348 nm and 317 to 313 nm, respectively, were found. I.e., the π → π* transitions of the E- and Z-states are separated by ca. 35 nm for both the monomer and the copolymer, though the Z-band is somewhat broadened after irradiation. Nevertheless, this enables the specific irradiation of both isomers with a good selectivity.
As the spectral features of the copolymers are virtually the same in methanol and aqueous solution (cf.Fig. 1 and Fig. S32, S33 ESI†), they seem to be linked to the polymeric character, but not to the change of the solvent methanol/water. As the overlap of bands of the E- and Z-states at 365 nm is somewhat higher for the copolymers than for monomer 6, the Z-content in the photo stationary state was somewhat lower, but still very high (>85%). The estimated half-life time τ½ exemplified for copolymer DMAm-co-AAPEAm9.5 was markedly enhanced with 165 h at 20 °C and 19 h 40 °C in water. The polymer scaffold apparently drastically slows down the thermal relaxation of the polymer-bound dye compared to the monomer.
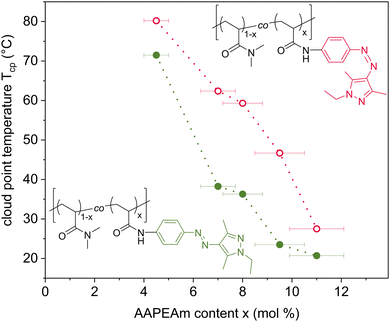
Subsequently, we determined Ttrans, more precisely the cloud points (Tcp) that indicate the beginning phase separation of the polymer solution, for the copolymer set in water (see ESI,† Fig. S34). Tcp values were determined by turbidimetry as the onset of the clouding transition for both the non-irradiated and the irradiated solutions. The results are compiled in Fig. 2.
In agreement with the extremely high Ttrans of homopolymer pDMAm, copolymer DMAm-co-AAPEAm1.5 with a minimal AAP-content (see Table 1) shows no clouding up to 85 °C. For all other copolymers, a cloud point is observed, which steeply decreases with increasing content of the hydrophobic AAP-chromophore, which lowers the hydrophilic–hydrophobic balance of the copolymers, in analogy to many reports on azodye-functionalized water-soluble polymers.17,24,29,32 While in their E states, copolymer DMAm-co-AAPEAm4.5 shows a Tcp of ca. 72 °C, and copolymer DMAm-co-AAPEAm7 a Tcp of 37 °C, the solution of copolymer DMAm-co-AAPEAm11 with the highest AAP content turns turbid already at 21 °C.
For determining the photo-responses, the polymer solutions were irradiated at 365 nm with an LED for 15 min, and immediately analyzed regarding Tcp in the photo stationary state. The photo-responsive copolymers DMAm-co-AAPEAm4.5 and DMAm-co-AAPEAm11 with the lowest and highest contents of incorporated AAP achieve both Tcp shifts of about 7 °C that compare already favorably with many reports on isomerization-induced temperature shifts of polymers by azobenzene groups (see above). Most remarkably, the copolymers with intermediate contents of AAP (7–9 mol%) display Tcp changes of more than 23 °C, with a maximum of around 24 °C for copolymer DMAm-co-AAPEAm7 (Fig. 2). Apart from the single report mentioned above,34 this is the largest light-induced modulation of Ttrans for azo dye-functionalized thermo-responsive polymers that has been reported so far for aqueous solutions. The observed maximum of the effect for intermediate AAP contents leads us to assume that beyond the dye content itself, another effect is responsible for the Tcp shifts. As evoked by the findings of Tribet and coworkers,23 an aggregation of the dye moieties in the E-state could shield them partially from the surrounding water, thus reducing the effect of the polarity difference between aggregated E-state dyes vs. free Z-state dyes. Further, the prolonged τ½ of the polymer-bound dye could also be explained with the formation of aggregates, i.e., an aggregation could reduce the efficiency of the back isomerisation of the Z-AAP group which requires a sufficient free volume for the isomerisation.45 Noteworthy, Menzel and coworkers also observed a maximum Tcp shift at intermediate azo dye contents for their systems.32
In conclusion, new DMAm-co-AAPEAm copolymers were synthesized that contain an azo dye-functionalized monomer from the rather new class of arylazopyrazoles (AAP) as key component. The spectral separation of the main absorption bands of the E- and Z-states of the AAP chromophore in the near UV-Vis range is sufficient for transforming the majority of the AAP chromophore into the Z-isomer in the photo stationary state (>85%), thereby modulating effectively the overall hydrophilicity. The polymers change their LCST-type phase transition temperatures Ttrans in aqueous solution depending on the content of incorporated azodye in a non-monotonous way. Substantial changes of Ttrans are achieved for moderate AAP contents, with outstanding shifts of >23 °C for the DMAm-co-AAPEAm copolymers bearing 7–9 mol% of the dye. Moreover, the lifetime of the Z-state is advantageously long (τ½ = 19 h at 40 °C) compared to previously reported azobenzene-functionalized photo-responsive polymers. Further investigations of this system with scattering methods such as DLS, SAXS or SANS are envisaged for the future, to improve the understanding of this new highly effective dual thermo- and photo-responsive switching system.
Financial support by Deutsche Forschungsgemeinschaft (grants La 611/19-1, MU 1487/42-1, and PA 771/31-1) is gratefully acknowledged, as is the help by Stefan Mies with DSC and Sascha Prentzel with SEC analysis (both University of Potsdam). The manuscript was written with the contributions of all authors. All authors approved the final version of the manuscript.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. A. Cohen Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef CAS PubMed.
- M. Wei, Y. Gao, X. Li and M. J. Serpe, Polym. Chem., 2017, 8, 127–143 RSC.
- H. R. Culver, J. R. Clegg and N. A. Peppas, Acc. Chem. Res., 2017, 50, 170–178 CrossRef CAS PubMed.
- D. Roy, W. L. A. Brooks and B. S. Sumerlin, Chem. Soc. Rev., 2013, 42, 7214–7243 RSC.
- S. Strandman and X. X. Zhu, Prog. Polym. Sci., 2015, 42, 154–176 CrossRef CAS.
- C. M. Papadakis, P. Müller-Buschbaum and A. Laschewsky, Langmuir, 2019, 35, 9660–9676 CrossRef CAS PubMed.
- O. Bertrand and J.-F. Gohy, Polym. Chem., 2017, 8, 52–73 RSC.
- F. D. Jochum and P. Theato, Chem. Soc. Rev., 2013, 42, 7468–7483 RSC.
- L. Li, J. M. Scheiger and P. A. Levkin, Adv. Mater., 2019, 31, e1807333 CrossRef PubMed.
- J. Volarić, W. Szymanski, N. A. Simeth and B. L. Feringa, Chem. Soc. Rev., 2021, 50, 12377–12449 RSC.
- A. Ivanov, N. Eremeev, P.-O. Wahlund, I. Galaev and B. Mattiasson, Polymer, 2002, 43, 3819–3823 CrossRef CAS.
- A. Laschewsky and E. D. Rekaï, Macromol. Rapid Commun., 2000, 21, 937–940 CrossRef CAS.
- D. Kungwatchakun and M. Irie, Makromol. Chem., Rapid Commun., 1988, 9, 243–246 CrossRef CAS.
- A. Mukherjee, M. D. Seyfried and B. J. Ravoo, Angew. Chem., Int. Ed., 2023, 62, e202304437 CrossRef CAS PubMed.
- A. Goulet-Hanssens and C. J. Barrett, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 3058–3070 CrossRef CAS.
- S. Wang, P. Poudel, F. H. Schacher and L. I. Kaberov, Polym. Chem., 2023, 14, 3381–3391 RSC.
- H. Akiyama and N. Tamaoki, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5200–5214 CrossRef CAS.
- T. Yoshida, S. Kanaoka and S. Aoshima, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5337–5342 CrossRef CAS.
- K. Matsubara, M. Watanabe and Y. Takeoka, Angew. Chem., Int. Ed., 2007, 46, 1688–1692 CrossRef CAS PubMed.
- H. Akiyama and N. Tamaoki, Macromolecules, 2007, 40, 5129–5132 CrossRef CAS.
- F. D. Jochum and P. Theato, Polymer, 2009, 50, 3079–3085 CrossRef CAS.
- X. Tao, Z. Gao, T. Satoh, Y. Cui, T. Kakuchi and Q. Duan, Polym. Chem., 2011, 2, 2068–2073 RSC.
- Y.-J. Liu, A. Pallier, J. Sun, S. Rudiuk, D. Baigl, M. Piel, E. Marie and C. Tribet, Soft Matter, 2012, 8, 8446–8455 RSC.
- A. Miasnikova, C. A. Benítez-Montoya and A. Laschewsky, Macromol. Chem. Phys., 2013, 214, 1504–1514 CrossRef CAS.
- L. Li, B. Lu, Y. Zhang, X. Xing, X. Wu and Z. Liu, J. Polym. Res., 2015, 22, 176 CrossRef.
- J. He, L. Tremblay, S. Lacelle and Y. Zhao, Polym. Chem., 2014, 5, 5403–5411 RSC.
- L. Ding, P. Zhang, C. Fu, J. Yin, Y. Mao, N. Liu, S. Li, C. Yang, R. Zhao and K. Deng, Macromol. Chem. Phys., 2019, 220, 1900349 CrossRef CAS.
- R. Colaco, C. Appiah and A. Staubitz, Gels, 2023, 9, 75 CrossRef CAS PubMed.
- H. Menzel, R. Kröger and M. L. Hallensleben, J. Macromol. Sci. A, 1995, 32, 779–787 CrossRef.
- Q. Zhong, M. Lu, S. Nieuwenhuis, B.-S. Wu, G.-P. Wu, Z.-K. Xu, P. Müller-Buschbaum and J.-P. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 5414–5426 CrossRef CAS PubMed.
- C. Hu and A. Pich, Macromolecules, 2023, 56, 4910–4918 CrossRef CAS.
- R. Kröger, H. Menzel and M. L. Hallensleben, Macromol. Chem. Phys., 1994, 195, 2291–2298 CrossRef.
- A. A. Beharry and G. A. Woolley, Chem. Soc. Rev., 2011, 40, 4422–4437 RSC.
- Y. Mok, M. Noh, G. Chan Kim, Y. Song, H. Kim, S. Kim, S. Yi, J.-H. Seo and Y. Lee, Polymer, 2016, 107, 37–43 CrossRef CAS.
- A. Goulet-Hanssens, F. Eisenreich and S. Hecht, Adv. Mater., 2020, 32, e1905966 CrossRef PubMed.
- C. E. Weston, R. D. Richardson, P. R. Haycock, A. J. P. White and M. J. Fuchter, J. Am. Chem. Soc., 2014, 136, 11878–11881 CrossRef CAS PubMed.
- A. Gibalova, N. B. Arndt, L. Burg and B. J. Ravoo, ACS Appl. Mater. Interfaces, 2023, 15, 12363–12371 CrossRef CAS PubMed.
- D. T. Nguyen, M. Freitag, C. Gutheil, K. Sotthewes, B. J. Tyler, M. Böckmann, M. Das, F. Schlüter, N. L. Doltsinis, H. F. Arlinghaus, B. J. Ravoo and F. Glorius, Angew. Chem., Int. Ed., 2020, 59, 13651–13656 CrossRef CAS PubMed.
- N. B. Arndt, F. Schlüter, M. Böckmann, T. Adolphs, H. F. Arlinghaus, N. L. Doltsinis and B. J. Ravoo, Langmuir, 2022, 38, 735–742 CrossRef CAS PubMed.
- C. Honnigfort, L. Topp, N. García Rey, A. Heuer and B. Braunschweig, J. Am. Chem. Soc., 2022, 144, 4026–4038 CrossRef CAS PubMed.
- C.-W. Chu, L. Stricker, T. M. Kirse, M. Hayduk and B. J. Ravoo, Chemistry, 2019, 25, 6131–6140 CrossRef CAS PubMed.
- B. G. Dwyer, C. Wang, D. Abegg, B. Racioppo, N. Qiu, Z. Zhao, D. Pechalrieu, A. Shuster, D. G. Hoch and A. Adibekian, Angew. Chem., Int. Ed., 2021, 60, 3071–3079 CrossRef CAS PubMed.
- G. Davidson-Rozenfeld, L. Stricker, J. Simke, M. Fadeev, M. Vázquez-González, B. J. Ravoo and I. Willner, Polym. Chem., 2019, 10, 4106–4115 RSC.
- F. Fischer, D. Zufferey and R. Tahoces, Polym. Int., 2011, 60, 1259–1262 CrossRef CAS.
- G. S. Kumar and D. C. Neckers, Chem. Rev., 1989, 89, 1915–1925 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc06178g |
| This journal is © The Royal Society of Chemistry 2024 |