Open Access Article
Open Access ArticleSynthesis of polyvinylethylene glycols (PVEGs) via polyetherification of vinylethylene carbonate by synergistic catalysis†
Fan
Yang
ab,
Minghang
Wang
ab and
Yong Jian
Zhang
 *ab
*ab
aShanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Frontiers Science Center for Transformative Molecules, and School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China. E-mail: yjian@sjtu.edu.cn
bSichuan Research Institute, Shanghai Jiao Tong University, Chengdu 610042, P. R. China
First published on 2nd March 2024
Abstract
An efficient and controllable polyetherification of vinylethylene carbonate (VEC) using diols as initiators is developed. By using a synergistic catalysis with palladium and boron reagents under mild conditions, the polymerization process enables the regioselective production of a series of polyvinylethylene glycols (PVEGs) bearing pendent vinyl groups in high yields with accurate molecular weight control and narrow molecular weight distribution. The utility of PVEGs is demonstrated by the production of functional polyurethanes and post-polymerization modification via thiol–ene photo-click chemistry.
The application of design criteria and practical technologies for molecular level control in organic synthesis to prepare well-defined macromolecules plays an important role in the discovery of new polymer materials with distinctive properties and functions. In particular, the application of these technologies in the polymerization of functional monomers to access polymers with pendent functional groups is a valuable tool for the variation of the structure and function of the macromolecules by post-polymerization modifications.1 Nevertheless, the synthesis of functional polymers with precisely defined molecular weight, composition, and architecture still remains a formidable challenge. These polymerizations require not only efficient and accurate control, but also higher functional group tolerance. In addition, the introduction of versatile and reactive functional groups into the side chains of polymers is essential for the post-polymerization modifications. Therefore, the development of direct polymerization reactions using readily accessible monomers to prepare polymers with valuable pendent functional groups is still highly appealing.
Polyether polyols, mainly synthesized through ring-opening polymerization (ROP) of epoxides,2 are widely used in the production of polyurethanes (PUs), surfactants and lubricants, as well as in the biomedical and cosmetic domains.3 Although most of the functional groups are incompatible with the harsh ROP conditions, successful examples of synthesizing functional polyethers have been achieved using glycidyl ether derivatives as functional monomers.2,4 Notably, allyl glycidyl ether (AGE) and ethoxyl vinyl glycidyl ether (EVGE) stand out as representative functional monomers, which allow the versatile vinyl group to be introduced into the chain of the polymers.5 The pendent vinyl group can react with various thiols via thiol–ene click chemistry6 to decorate polyethers with diverse pendant functionalities, thereby enabling the modification of both structure and function (Fig. 1A).7
Palladium-catalyzed allylic substitution is one of the most potent methods for carbon–carbon and carbon–heteroatom bond formation.8 This useful organic transformation has been successfully applied to the polymerization by Nomura and colleagues.9 However, this polymerization approach has generally been limited to polycondensation between bis-allylic monomers and malonate-type carbon nucleophiles to provide polymers bearing a carbon–carbon double bond on the main chain. Most recently, we developed regio- and stereoselective allylic etherification of substituted vinylethylene carbonates (VECs)10 with water and alcohols under the cooperative catalysis system.11 The catalytic reaction furnished functional ethylene glycols in almost quantitative yields with complete branched-regioselectivities. This efficient catalytic organic transformation inspired us to develop a novel polymerization approach for the synthesis of functional polyethylene glycols bearing a vinyl group on the side chain. We realized that commercially available VEC is an appropriate functional monomer. Our hypothesis is that VEC reacts with a palladium catalyst to afford the zwitterionic allylpalladium intermediate I, which can convert into boronate intermediate II by reaction with the boron reagent and capture alcohol as an initiator (Fig. 1B). This species would subsequently undergo nucleophilic addition with branched selectivity to afford reactive intermediate III to finish the initiation step.11 Intermediate III would react with intermediate I generated from the reaction of VEC with a palladium catalyst to afford intermediate IV which undergoes regioselective etherification to give intermediate V. In the same manner under the cooperative catalysis system, leading to chain growth, the termination step would be achieved by releasing the borate with alcohol, resulting in the desired polyvinylethylene gylcols (PVEG). The boron co-catalyst will play an important role for the enhancement of the nucleophilicity of the alcohols and control the branched-selectivity of the etherification step.11a Herein, we report the successful implementation of this allylic etherification polymerization strategy and present a first efficient and controllable polyetherification approach that allows rapid access to vinyl-functional polyether diols with accurate molecular weight control and narrow molecular weight distribution. In addition, the utility of the obtained PVEGs is demonstrated by the synthesis of functional PU and the post-polymerization modification via thiol–ene photo-click chemistry.
Our initial studies focused on the optimization of allylic etherification polymerization of VEC 1 and 1,4-phenylenedimethanol (2a) as an initiator (Table 1). The identification of appropriate ligands for the polymerization of 1 (1.0 mmol, 10 equiv.) with 2a (0.1 mmol, 1 equiv.) was firstly examined using a catalytic amount of Pd2(dba)3·CHCl3 (0.2 mol% for 1) as a palladium precursor and BEt3 (0.4 mol% for 1) in THF as a solvent at 20 °C (entries 1–4). As a result, we inspiringly found that the polymerization can finish within 2 h by using bis(2-diphenylphosphinophenyl)ether (DPEphos) as a ligand to produce the desired polyether diol P1 in 86% isolated yield (entry 4). The solvent effect was next surveyed by using DPEphos as a ligand (entries 5–9), but no further improvement in the reaction efficiency was observed. It was found that the polymerization reaction is not sensitive to the reaction temperature (entries 10–13). These results indicated that the polyetherification is very reactive and almost no β-hydride elimination occurs.10c The best results were found at 10 °C to afford the product P1 in up to 95% yield (entry 10). The polymerization proceeded smoothly when the palladium precursor was reduced to 0.15 mol% (entry 14). Finally, the optimal conditions were determined for synergistic catalysis including a palladium complex generated in situ from Pd2(dba)3·CHCl3 (0.15 mol% for 1) and DPEphos (0.3 mol% for 1) and BEt3 (0.3 mol% for 1) in THF at 10 °C for 2 h (entry 14).
| Entry | Ligand | Solvent | Temp. (°C)/time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (1.0 mmol, 10 equiv.), 2a (0.1 mmol, 1 equiv.), Pd2(dba)3·CHCl3 (0.2 mol% for 1), ligand (0.4 mol% for 1), BEt3 (0.4 mol% for 1), solvent (1.0 mL). b Isolated yields. c The reaction carried out using Pd2(dba)3·CHCl3 (0.15 mol% for 1), DPEphos (0.3 mol% for 1) and BEt3 (0.3 mol% for 1) under otherwise identical conditions. | ||||
| 1 | dppp | THF | 20/12 | 5 |
| 2 | dppb | THF | 20/12 | 20 |
| 3 | BINAP | THF | 20/12 | 80 |
| 4 | DPEphos | THF | 20/2 | 86 |
| 5 | DPEphos | 1,4-Dioxane | 20/2 | 61 |
| 6 | DPEphos | MTBE | 20/2 | 75 |
| 7 | DPEphos | Toluene | 20/2 | 78 |
| 8 | DPEphos | CH2Cl2 | 20/2 | 73 |
| 9 | DPEphos | CH3CN | 20/2 | 76 |
| 10 | DPEphos | THF | 10/2 | 95 |
| 11 | DPEphos | THF | 50/2 | 91 |
| 13 | DPEphos | THF | 70/2 | 92 |
| 14c | DPEphos | THF | 10/2 | 95 |
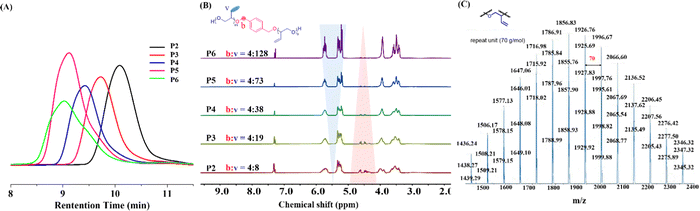
With the optimized conditions in hand, the catalytic polyetherification of VEC 1 with 10 times scale up by using various diol initiators was investigated (Fig. 2). The obtained polyether diol PVEGs (P2–P12) were characterized by nuclear magnetic resonance (NMR) and gel permeation chromatography (GPC) spectroscopies, and their hydroxyl values and molecular weight distributions were also assayed. Firstly, the different ratio of VEC 1 to initiator 2a (1.0 mmol scale) for the polymerization under the optimized conditions was studied. To our delight, all polymerization reactions proceeded smoothly, yielding the desired PVEGs in almost quantitative yields. In addition, the molecular weight could be accurately controlled by the variation of the ratio of 1 to 2a, and the produced polyether diols P2–P6 showed narrow molecular weight distributions (PDI, 1.18–1.31). The molecular weight increased significantly with the increase of the ratio of 1 to 2b, whereas the hydroxyl value decreased, albeit the chain growth was slowed down when the polymerization of 1 and 2a was conducted at a ratio of 160 to 1 as determined by GPA analysis, as shown in Fig. 3A. The controlled polymerization could also be proved clearly by integration of the ratio of benzylic and vinylic protons in 1H NMR spectrograms (Fig. 3B). The ratio of benzylic and vinyl protons increased remarkably from P2 to P6. In addition, the repeat unit was assayed as 70 g mol−1 by the determination of P4 using MALDI-TOF MS spectroscopy (Fig. 3C), which further proved the correctness of the structure of the obtained PVEGs.
Next, the catalytic polyetherifications of VEC 1 with other diol initiators 2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 = 40) were investigated. As shown in Fig. 2, various diols could be accommodated under the polymerization conditions, yielding the corresponding PVEGs, namely P7–P12, in high yields with accurate molecular weight control and narrow molecular weight distributions. Notably, the ROP of propylene oxide produced polyether polyols with predominantly secondary hydroxyls,12 which often lack sufficient reactivity with isocyanates for the production of PUs. As a distinct contrast, our catalytic polyetherification provided polyether diols with complete primary hydroxyl content.
2 = 40) were investigated. As shown in Fig. 2, various diols could be accommodated under the polymerization conditions, yielding the corresponding PVEGs, namely P7–P12, in high yields with accurate molecular weight control and narrow molecular weight distributions. Notably, the ROP of propylene oxide produced polyether polyols with predominantly secondary hydroxyls,12 which often lack sufficient reactivity with isocyanates for the production of PUs. As a distinct contrast, our catalytic polyetherification provided polyether diols with complete primary hydroxyl content.
In order to demonstrate the utility of the obtained PVEGs, the polyaddition of PVEG P4 and 4,4′-diphenylmethane diisocyanate (MDI) was conducted using dibutyltin dilaurate (DBTDL) as a catalyst and 1,4-butadiol (BDO) as a chain extender in THF (Scheme 1). The polymerization proceeded smoothly to afford vinyl-functional thermoplastic polyurethane (VFTPU) in high yield with 20.9 kDa number average molecular weights (Mn). (For the details regarding the characterization of the obtained VFTPU, see the ESI†). Thermal properties of the VFTPU determined by thermal gravimetric analysis (TGA) showed that the thermal degradation behavior is consistent with the thermal properties for usual TPUs.13 The thermo-mechanical properties of the VFTPU were next assessed by dynamic mechanical analysis (DMA). The results showed that the storage modulus (G′) gradually increased at temperatures above 100 °C, indicating that the pendent vinyl group of VFTPU might undergo some degree of crosslinking at high temperature (Fig. S35c and Table S1, ESI†). Finally, the mechanical properties of the obtained VFTPU were also characterized by tensile tests (Fig. S35d, ESI†). The VFTPU exhibited the typical elastomer behavior, with a tensile strength of ∼780 Pa and a strain at break of ∼2490%.
The post-polymerization modification of PVEG P4 was subsequently conducted via thiol–ene photo-click chemistry.6 The post-modification was straightforwardly carried out by the UV (365 nm) irradiation of P4 with excess 1-dodecanethiol in the presence of 2-hydroxy-2-methylpropiophenone (1 wt%) as a photoinitiator for 60 seconds, thus providing thioether-functional polyglycol (TEFPG) with very high efficiency. As shown in Fig. 4, the 1H NMR spectrograms clearly indicated that the vinyl protons of P4 disappeared completely after post-modification by the photo-click reaction. Correspondingly, the proton signals of –CH2SCH2– and alkyl chains appeared in the 1H NMR spectrogram of the produced TEFPG. These observations provide incontrovertible evidence of successful post-polymerization modification of pendent vinyl groups of PVEGs through efficient photo-click chemistry.
In conclusion, we have developed for the first time an efficient and controllable polyetherification of VEC 1 with diols 2 as initiators by a synergistic catalysis. By using a palladium complex and triethyl borane as a cooperative catalyst under mild neutral conditions, this polyetherification process effectively produced vinyl-functional PVEGs in high yields with complete branched-regioselectivities, accurate molecular weight control and narrow molecular weight distribution. The utility of the vinyl-functional PVEGs was demonstrated by the production of functional TPU and post-polymerization modification of the pendant vinyl group of PVEG using thiol–ene photo-click chemistry.
This work was supported by the National Natural Science Foundation of China (22171182) and the Sichuan Tianfu Emei Plan. We thank the Instrumental Analysis Center of Shanghai Jiao Tong University for GPC and MALDI-TOF MS analysis.
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- (a) A. Shahrokhiia, P. Biswas and J. F. Reuther, J. Polym. Sci., 2021, 59, 1748 CrossRef; (b) E. Blasco, M. B. Sims, A. S. Goldmann, B. S. Summerlin and C. Barner-Kowollik, Macromolecules, 2017, 50, 5215 CrossRef CAS; (c) M. A. Gauthier, M. I. Gibson and H. A. Klok, Angew. Chem., Int. Ed., 2009, 48, 48 CrossRef CAS PubMed.
- J. Herzberger, K. Niederer, H. Pohlit, J. Seiwert, M. Worm, F. R. Wurm and H. Frey, Chem. Rev., 2016, 116, 2170 CrossRef CAS PubMed.
- (a) R. Klein and F. R. Wurm, Macromol. Rapid Commun., 2015, 36, 1147 CrossRef CAS PubMed; (b) H. W. Engels, H. G. Pirkl, R. Albers, R. W. Albach, J. Krause, A. Hoffmann, H. Casselmann and J. Dormish, Angew. Chem., Int. Ed., 2013, 52, 9422 CrossRef CAS PubMed; (c) P. Pouyan, M. Cherri and R. Haag, Polymers, 2022, 14, 2684 CrossRef CAS PubMed.
- (a) J. Zhang and G. Wang, Sci. China: Chem., 2015, 58, 1674 CrossRef CAS; (b) C. Mangold, F. Wurm and H. Frey, Polym. Chem., 2012, 3, 1714 RSC; (c) B. Obermeier, F. Wurm, C. Mangold and H. Frey, Angew. Chem., Int. Ed., 2011, 50, 7988 CrossRef CAS PubMed.
- (a) J. Lee, S. Han, M. Kim and B. S. Kim, Macromolecules, 2020, 53, 355 CrossRef CAS; (b) B. F. Lee, M. Wolffs, K. T. Delaney, J. K. Sprafke, F. A. Leibfarth, C. J. Hawker and N. A. Lynd, Macromolecules, 2012, 45, 3722 CrossRef CAS PubMed; (c) B. F. Lee, M. J. Kade, J. A. Chute, N. Gupta, L. M. Campos, G. H. Fredrickson, E. J. Kramer, N. A. Lynd and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 4498 CrossRef CAS PubMed; (d) M. Erberich, H. Keul and M. Müller, Macromolecules, 2007, 40, 3070 CrossRef CAS; (e) A. Sunder, H. Türk, R. Haag and H. Frey, Macromolecules, 2000, 33, 7682 CrossRef CAS.
- (a) C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540 CrossRef CAS PubMed; (b) C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355 RSC.
- (a) K. W. Oleske, K. P. Barteau, M. Z. Turker, P. A. Beaucage, L. A. Estroff and U. Wiesner, Macromolcules, 2017, 50, 542 CrossRef CAS; (b) E. Sokolovskaya, J. Yoon, A. C. Misra, S. Brase and J. Lahann, Macromol. Rapid Commun., 2013, 34, 1554 CrossRef CAS PubMed; (c) C. Mangold, C. Dingels, B. Obermeier, H. Frey and F. Wurm, Macromolecules, 2011, 44, 6326 CrossRef CAS; (d) B. Obermeier and H. Frey, Bioconjugate Chem., 2011, 22, 436 CrossRef CAS PubMed.
- (a) Z. Lu and S. Ma, Angew. Chem., Int. Ed., 2008, 47, 258 CrossRef CAS PubMed; (b) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921 CrossRef CAS PubMed.
- (a) N. Nomura, S. Komiyama, H. Kasugai and M. Saba, J. Am. Chem. Soc., 2008, 130, 812 CrossRef CAS PubMed; (b) N. Nomura, K. Tsurugi, T. V. RajanBabu and T. Kondo, J. Am. Chem. Soc., 2004, 126, 5354 CrossRef CAS PubMed; (c) N. Nomura, K. Tsurugi and M. Okada, Angew. Chem., Int. Ed., 2001, 40, 1932 CrossRef CAS; (d) N. Nomura, K. Tsurugi and M. Okada, J. Am. Chem. Soc., 1999, 121, 7268 CrossRef CAS.
- (a) C. Zhao, I. Khan and Y. J. Zhang, Chem. Commun., 2020, 56, 12431 RSC; (b) I. Khan, C. Zhao and Y. J. Zhang, Chem. Commun., 2018, 54, 4708 RSC; (c) K. Liu, I. Khan, J. Cheng, Y. J. Hsueh and Y. J. Zhang, ACS Catal., 2018, 8, 11600 CrossRef CAS; (d) A. Khan, L. Yang, J. Xu, L. Y. Jin and Y. J. Zhang, Angew. Chem., Int. Ed., 2014, 126, 11439 CrossRef; (e) A. Khan, R. Zheng, Y. Kan, J. Ye, J. Xing and Y. J. Zhang, Angew. Chem., Int. Ed., 2014, 126, 6557 CrossRef; (f) M. Quan, N. Butt, J. Shen, K. Shen, D. Liu and W. Zhang, Org. Biomol. Chem., 2013, 11, 7412 RSC.
- (a) A. Khan, S. Khan, I. Khan, C. Zhao, Y. Mao, Y. Chen and Y. J. Zhang, J. Am. Chem. Soc., 2017, 139, 10733 CrossRef CAS PubMed; (b) S. Khan, H. Li, C. Zhao, X. Wu and Y. J. Zhang, Org. Lett., 2019, 21, 9457 CrossRef CAS PubMed.
- (a) T. Miyajima, K. Nishiyama, M. Satake and T. Tsuji, Polym. J., 2015, 47, 771 CrossRef CAS; (b) A. Raghuraman, D. Babb, M. Miller, M. Paradkar, B. Smith and A. Nguyen, Macromolecules, 2016, 49, 6790 CrossRef CAS.
- (a) M. Chen, X. Pan, T. Tian, J. Li, Q. Tan, M. Jin and J. Ren, Prog. Org. Coat., 2023, 182, 107695 CrossRef CAS; (b) J. Fu, H. Yu, L. Wang, L. Lin and R. U. Khan, Prog. Org. Coat., 2020, 144, 105635 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc05580a |
| This journal is © The Royal Society of Chemistry 2024 |