Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and supramolecular properties of all-cis-2,4,6-trifluorocyclohexane-1,3,5-triol†
Shyamkumar V.
Haridas
and
Max
von Delius
 *
*
Institute of Organic Chemistry, Ulm University, Albert-Einstein-Allee 11, Ulm 89081, Germany. E-mail: max.vondelius@uni-ulm.de
First published on 11th December 2023
Abstract
We report the synthesis of all-cis fluorinated cyclohexanes bearing three hydroxy, ether or ester functionalities in the non-fluorinated positions. These tripodal molecules have a high dipole moment of up to 6.3 debye and were successfully used to bind anions and form gels.
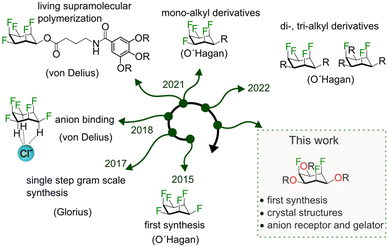
Tripodal molecules are valuable building blocks in supramolecular chemistry. Structural motifs with three-fold symmetry such as amines,1,2 orthoesters,3–5 1,3,5 substituted benzenes6,7 and cyclohexanes8 are therefore used for the synthesis of a variety of cryptands, cages or supramolecular polymers. For the synthesis of receptors and cages it is advantageous when the tripodal molecule provides both a suitable geometry and additional binding affinity with the guest. The orthoester cryptands developed in our lab are ideal examples, because the orthoester bridgehead not only provides the macrobicyclic architecture but also contributes to the binding of cationic guests.
With this in mind, we thought of developing a tripodal architecture based on all-cis-1,2,3,4,5,6-hexafluorocyclohexane (all-cis C6H6F6).9,10 All-cis C6H6F6 with its positive and negatively polarized regions and world-record (aliphatic) dipole moment (6.2 debye) holds potential for applications in supramolecular chemistry,11 material chemistry12–15 and medicinal chemistry16,17 (Fig. 1). In 2015 David O’Hagan and co-workers reported the first synthesis of all-cis C6H6F6.9 Following Glorius’ one-step synthesis of all-cis C6H6F6 and its derivatives,18 we have studied the anion affinity of this compound class in solution and the solid state19 and we have explored the living supramolecular polymerization of fluorinated cyclohexanes.20,21
The O’Hagan lab synthesized mono-, di-, and tri-alkyl derivatives of all-cis hexafluorocyclohexane.22–24 From the solid state structures and thorough physical organic studies the authors demonstrated that it is the three axial fluorine atoms, which are predominantly responsible for the high dipole moment and the solid state packing. With these interesting properties in mind, we wondered whether symmetric tri-oxo derivatives of all-cis hexafluorocyclohexanes could be synthesized. In such a molecule, the triaxial fluorine conformation would provide a large dipole moment, while three oxygens on the same face would further increase the dipole moment and represent an opportunity to attach up to three side arms forming a new tripodal motif. Herein, we report the first synthesis of all-cis-2,4,6-trifluorocyclohexane-1,3,5-triol and demonstrate the application of this molecule as a gelator and anion receptor.
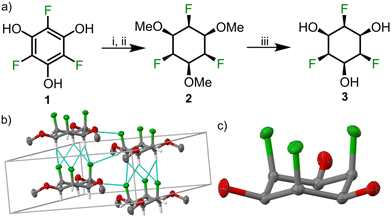
The trifluorophloroglucinol precursor (1) was synthesized by C–H activation of 1,3,5-trifluorobenzene using bis(pinacolato)diboron (B2Pin2), (1,5-cyclooctadiene)(methoxy)iridium(I) dimer as catalyst and 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbPy) as ligand according to a reported procedure.25 The obtained triborylated compound was then oxidized using oxone to obtain the trifluorophloroglucinol 1.26 The hydroxy groups were protected by treatment with methyl iodide. The crucial face-selective hydrogenation step was carried out in a steel reactor using the rhodium–cyclic (alkyl)(amino)carbene (RhCAAC) catalyst18 and hydrogen (60 bar) at 50 °C for 14 days to give 2 in 20–40% yield. In a typical reaction, we obtained ca. 30% yield of the product and recovered ca. 30% of the starting material. Additionally, we observed the formation of some side products (possibly partially hydrogenated/defluorinated compounds18), but an exact identification of these minor side products is not straightforward. Single crystal XRay diffraction (SCXRD) of trimethoxy ether 2 revealed an axial conformation of the three fluorine atoms and conversely the equatorial conformation for the methoxy groups with parallel stacks of cyclohexanes in the P63 space group (Fig. 2). The axial fluorine atoms in one molecule point towards the center of two axial hydrogen atoms (Fig. 2b) in a neighbouring molecule with a CF–HC distance of 2.46 Å, which is below the sum of vdW radii of the two atoms (2.67 Å) and in accordance with a related structure.22 The non-centrosymmetric nature of the space group points toward potential applications of this compound class in ferroelectric and piezoelectric materials.
To complete the synthesis of the desired all-cis-2,4,6-trifluorocyclohexane-1,3,5-triol 3, the methoxy ethers were cleaved using aluminium trichloride and n-butanethiol. SCXRD of 3 revealed a solid-state structure in which stacks of cyclohexanes interact via axial fluorine atoms, axial hydrogen atoms and equatorial oxygen atoms. A molecule of water was present in the asymmetric unit cell and involved in hydrogen bonding with the hydroxyl group of 3. Due to the centrosymmetric nature of the space group Pnma, stacks of the fluorinated cyclohenaxes are arranged in opposite direction, which is contrast to the packing observed for 2, where all stacks are arranged in the same direction (see Fig. S1 and S2, ESI†).
Having successfully synthesized compounds 2 and 3 we were interested in their dipole moments and “ring-flip” equilibria, which is why we performed high-level DFT calculations (B3LYP-D3/def2-TZVP and PBE0-D3/def2-TZVP) and VT-NMR experiments (details are provided in the ESI† file, including link to the raw DFT data). As summarized in Fig. 3, we found that 2 in the F-axial conformation has a dipole moment of 6.3 debye, which is very similar to the parent molecule all-cis hexafluorocyclohexane.9 The dipole moment of 2 is significantly lower in the F-equatorial conformation, which is expected from the difference of electronegativity between fluorine and oxygen. For compound 3 we observed the opposite trend, however, i.e. higher dipole moment for the F-equatorial conformer and we propose that this is due to the presence of relatively strong intramolecular hydrogen bonds between neighbouring OH and F groups, as described in reports on related systems.27,28 The hypothesis is further corroborated by the calculated free energies of axial-vs.-equatorial conformers in the gas-phase (F-axial 3 is an outlier that exhibits six F–HO hydrogen bonds, see Fig. S3, ESI†). Since the calculated gas-phase energy difference between the conformers of 2 was only 0.8 kcal mol−1, we attempted to observe the ring flip isomers by low temperature NMR spectroscopy. However, even at a temperature of −80 °C in acetone, when coalescence should have occurred,9,10 we could only observe one signal in the 19F NMR spectrum (Fig. S9, ESI†). This finding indicates that the DFT calculations that take solvent into account, correctly predict the strong thermodynamic preference for the F-axial isomers.
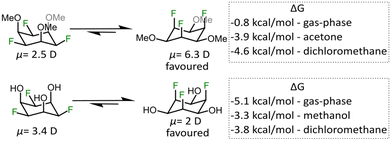 | ||
| Fig. 3 Calculated dipole moments for “ring flip” conformers of 2 and 3 and the free energy difference in the gas-phase and two implicit solvents. Level of theory: DFT, PBE0-D3/def2-TZVP (for dipole moment and single point energy calculations), in line with previous computational work on related molecules.23,24 | ||
Having understood key structural aspects of the title compounds, we decided to explore supramolecular applications of compound 3 and its simple derivatives. We thought that simple amphiphiles could be made by alkylating one of the hydroxyl groups with a non-polar alkyl chain. Amphiphiles29 are used as gelators since they are known to form cylindrical micelles, and further bundling will lead to the formation of supramolecular gels.30–33 We synthesized monoalkyl derivative 4 by reacting 3 with the pentafluorophenol (PFP) active ester of dodecanoic acid (Fig. 4a). As expected, 4 formed a gel in toluene (0.75 wt%) and scanning electron microscopic (SEM) revealed a fibrous morphology (Fig. 4e). The role of hydroxyl groups and the fluorines in self assembly was confirmed by the SCXRD data of a model compound 5 (shorter alkyl chain (Fig. 4b and c)). We propose that such networks could give rise to interesting ferroelectric34 behaviour.
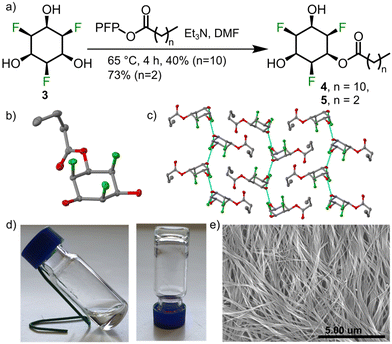 | ||
| Fig. 4 (a) Synthesis of esters 4 and 5. (b) Solid state structure of 5 (single crystal obtained from methanol layered with n-hexane, space group p21/n; colour code and ellipsoids as in Fig. 2). (c) Hydrogen bonds (cyan lines) observed in the solid state packing of 5. (d) 4 forms a gel at 0.75 wt% in toluene. (e) SEM image of the toluene gel of 4 showing fibrous morphology. | ||
We also decided to synthesize simple anion receptors35–42 from triol 3, in order to make use of the known anion affinity of all-cis fluorinated cyclohexanes (binding constants 400 M−1 for the parent compound and 170 M−1 for a mono-derivative determined in acetone19). For the tris-methoxyether 2 we determined a chloride binding constant of 25 M−1 in acetone (Fig. S8 and Table S5, ESI†) which shows that alkoxy-substitution leads to decreased binding affinity. To investigate whether higher binding constants can be obtained in tripodal architectures, we synthesized new receptors 8 and 9 (Fig. 5). We used CuAAC “click” chemistry, because the resulting triazole motifs can provide additional C–X (X = H or I) hydrogen or halogen bonding43,44 to the anion. The hydroxyl groups of 3 were functionalized using propargyl bromide and sodium hydride in DMF to obtain the trialkyne 6 (Fig. 5a). 6 was treated with phenyliodine(III)diacetate (PIDA) and tetrabutylammonium iodide (TBAI) in acetonitrile furnishing the triiodo alkyne compound 7. Tris-triazole receptor 8 and tris-iodotriazole receptor 9 were obtained by reacting benzyl azide with 6, 7 respectively. NMR host–guest titrations (ESI†) were carried out with both hosts (8, 9) in CH2Cl2 using tetrabutylammonium chloride (TBACl) as chloride ion source. Both receptors showed anion affinity higher than 2 (in CH2Cl2), which is most likely due to the presence of additional C–X bonds to the anion. Receptor 8 binds to Cl− with a Ka of 29 M−1 while receptor 9 binds with a Ka of 142 M−1 (Fig. 5c). We propose that the higher Ka observed for 9 (compared to 8) is due to halogen bonding and the larger size of the three iodine atoms, which probably helps the XB donor to “reach” the chloride ions in the tripodal binding cavity. Overall, the relatively low association constants can be explained by the open and relatively flexible geometry of the receptors.
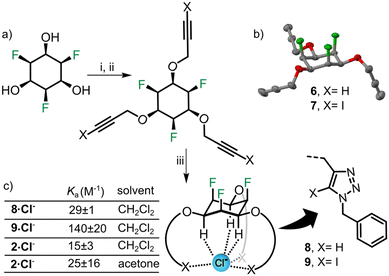 | ||
| Fig. 5 (a) (i) for the synthesis of 6-NaH, propargyl bromide, DMF, 0 °C to rt, overnight, 62% (ii) for the synthesis of 7-alkyne 6, PIDA, TBAI, ACN, rt 2 days, 91%, (iii) (Cu(CH3CN)4)PF6, TBTA, benzyl azide, THF, rt, overnight, 8 in 63% and 9 in 55%. (b) Crystal structure of 6; colour code and ellipsoids as in Fig. 2 and 4. (c) Association constants of 8, 9 and 2 for the chloride ion (as TBACl salt) in different solvents (standard error based on triplicate experiments). | ||
In conclusion, we synthesized all-cis-2,4,6-trifluorocyclohexane-1,3,5-triol and demonstrate its potential applications as a tripodal molecule. DFT calculations showed the dipole moment is especially large, when three axial fluorine atoms are complemented by three equatorial OR groups (R ≠ H) on the same face. We were able to make use of this dipole moment in proof-of-principle studies on a simple gelator and a tripodal receptor for the chloride ion (whose binding affinity was enhanced by hydrogen/halogen bonding with triazole/iodotriazole side arms). These results show the potential of this tripodal and highly polar platform in a variety of supramolecular applications. Future work will focus on the synthesis of rigid dynamic covalent45 as well as metalla cages46 and the exploration of ferroelectric34 behaviour.
This work was supported by the European Union (ERCstg 802428 “SUPRANET”). The authors acknowledge the infrastructure provided by the state of Baden-Württemberg through bwHPC and DFG through grant no. INST 40/575-1 FUGG (JUSTUS 2 cluster) and Mohammed Siddhique for his valuable suggestions in DFT calculations.
Conflicts of interest
There are no conflicts to declare.References
- Y. Liu, W. Zhao, C.-H. Chen and A. H. Flood, Science, 2019, 365, 159–161 CAS.
- B. Dietrich, J. M. Lehn and J. P. Sauvage, Tetrahedron Lett., 1969, 10, 2889–2892 CrossRef.
- R.-C. Brachvogel, F. Hampel and M. von Delius, Nat. Commun., 2015, 6, 7129 CrossRef PubMed.
- O. Shyshov, R.-C. Brachvogel, T. Bachmann, R. Srikantharajah, D. Segets, F. Hampel, R. Puchta and M. von Delius, Angew. Chem., Int. Ed., 2017, 56, 776–781 CrossRef CAS PubMed.
- S. Hollstein, O. Shyshov, M. Hanževački, J. Zhao, T. Rudolf, C. M. Jäger and M. von Delius, Angew. Chem., Int. Ed., 2022, 61, e202201831 CrossRef CAS PubMed.
- C. Kulkarni, E. W. Meijer and A. R. A. Palmans, Acc. Chem. Res., 2017, 50, 1928–1936 CrossRef CAS PubMed.
- S. Cantekin, T. F. A. de Greef and A. R. A. Palmans, Chem. Soc. Rev., 2012, 41, 6125–6137 RSC.
- J. Boekhoven, J. M. Poolman, C. Maity, F. Li, L. van der Mee, C. B. Minkenberg, E. Mendes, J. H. van Esch and R. Eelkema, Nat. Chem., 2013, 5, 433–437 CrossRef CAS PubMed.
- N. S. Keddie, A. Z. Slawin, T. Lebl, D. Philp and D. O’Hagan, Nat. Chem., 2015, 7, 483–488 CrossRef CAS PubMed.
- D. O’Hagan, Chem. Rec., 2023, 23, e202300027 CrossRef PubMed.
- B. E. Ziegler, M. Lecours, R. A. Marta, J. Featherstone, E. Fillion, W. S. Hopkins, V. Steinmetz, N. S. Keddie, D. O’Hagan and T. B. McMahon, J. Am. Chem. Soc., 2016, 138, 7460–7463 CrossRef CAS PubMed.
- S. M. Pratik, A. Nijamudheen and A. Datta, ChemPhysChem, 2016, 17, 2373–2381 CrossRef CAS PubMed.
- A. Theodoridis, G. Papamokos, M. P. Wiesenfeldt, M. Wollenburg, K. Müllen, F. Glorius and G. Floudas, J. Phys. Chem. B, 2021, 125, 3700–3709 CrossRef CAS PubMed.
- C. Fischer, S. Das, Q. Zhang, Y. Liu, L. Weinhardt, D. O’Hagan, M. Zharnikov and A. Terfort, Nano Res., 2023, 16, 11030–11041 CrossRef CAS.
- N. Al-Maharik, P. Kirsch, A. M. Z. Slawin, D. B. Cordes and D. O’Hagan, Org. Biomol. Chem., 2016, 14, 9974–9980 RSC.
- J. L. Clark, R. M. Neyyappadath, C. Yu, A. M. Z. Slawin, D. B. Cordes and D. O’Hagan, Chem. – Eur. J., 2021, 27, 16000–16005 CrossRef CAS PubMed.
- Y. Wang, W. Lee, Y.-C. Chen, Y. Zhou, E. Plise, M. Migliozzi and J. J. Crawford, ACS Med. Chem. Lett., 2022, 13, 1517–1523 CrossRef CAS PubMed.
- M. P. Wiesenfeldt, Z. Nairoukh, W. Li and F. Glorius, Science, 2017, 357, 908–912 CrossRef CAS PubMed.
- O. Shyshov, K. A. Siewerth and M. von Delius, Chem. Commun., 2018, 54, 4353–4355 RSC.
- O. Shyshov, S. V. Haridas, L. Pesce, H. Qi, A. Gardin, D. Bochicchio, U. Kaiser, G. M. Pavan and M. von Delius, Nat. Commun., 2021, 12, 3134 CrossRef CAS PubMed.
- S. V. Haridas, O. Shyshov and M. von Delius, Org. Mater., 2023, 5, 166–174 CrossRef CAS.
- J. L. Clark, A. Taylor, A. Geddis, R. M. Neyyappadath, B. A. Piscelli, C. Yu, D. B. Cordes, A. M. Z. Slawin, R. A. Cormanich, S. Guldin and D. O’Hagan, Chem. Sci., 2021, 12, 9712–9719 RSC.
- T. J. Poskin, B. A. Piscelli, K. Yoshida, D. B. Cordes, A. M. Z. Slawin, R. A. Cormanich, S. Yamada and D. O’Hagan, Chem. Commun., 2022, 58, 7968–7971 RSC.
- C. Yu, B. A. Piscelli, N. Al-Maharik, D. B. Cordes, A. M. Z. Slawin, R. A. Cormanich and D. O’Hagan, Chem. Commun., 2022, 58, 12855–12858 RSC.
- H. Takata, K. Ono and N. Iwasawa, Chem. Commun., 2020, 56, 5613–5616 RSC.
- R. E. Maleczka, F. Shi, D. Holmes and M. R. Smith, J. Am. Chem. Soc., 2003, 125, 7792–7793 CrossRef CAS PubMed.
- G. T. Giuffredi, V. Gouverneur and B. Bernet, Angew. Chem., Int. Ed., 2013, 52, 10524–10528 CrossRef CAS PubMed.
- A. Zapata, B. Bernet and A. Vasella, Helv. Chim. Acta, 1996, 79, 1169–1191 CrossRef CAS.
- M. A. Greenfield, J. R. Hoffman, M. Olvera de la Cruz and S. I. Stupp, Langmuir, 2010, 26, 3641–3647 CrossRef CAS PubMed.
- C.-W. Chu and C. A. Schalley, Org. Mater., 2021, 3, 25–40 CrossRef CAS.
- P. R. A. Chivers and D. K. Smith, Nat. Rev. Mater., 2019, 4, 463–478 CrossRef CAS.
- E. R. Draper and D. J. Adams, Chem, 2017, 3, 390–410 CAS.
- J. Mayr, C. Saldías and D. D. Díaz, Chem. Soc. Rev., 2018, 47, 1484–1515 RSC.
- A. S. Tayi, A. Kaeser, M. Matsumoto, T. Aida and S. I. Stupp, Nat. Chem., 2015, 7, 281–294 CrossRef CAS PubMed.
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef CAS PubMed.
- D. A. McNaughton, W. G. Ryder, A. M. Gilchrist, P. Wang, M. Fares, X. Wu and P. A. Gale, Chem, 2023, 9, 3045–3112 CAS.
- L. K. Macreadie, A. M. Gilchrist, D. A. McNaughton, W. G. Ryder, M. Fares and P. A. Gale, Chem, 2022, 8, 46–118 CAS.
- Y. Hua and A. H. Flood, Chem. Soc. Rev., 2010, 39, 1262–1271 RSC.
- A. Cataldo, K. Norvaisa, L. Halgreen, S. E. Bodman, K. Bartik, S. J. Butler and H. Valkenier, J. Am. Chem. Soc., 2023, 145, 16310–16314 CrossRef CAS PubMed.
- M. J. Langton, C. J. Serpell and P. D. Beer, Angew. Chem., Int. Ed., 2015, 55, 1974–1987 CrossRef PubMed.
- P. A. Gale, J. T. Davis and R. Quesada, Chem. Soc. Rev., 2017, 46, 2497–2519 RSC.
- C.-L. Deng, J. P. Bard, J. A. Lohrman, J. E. Barker, L. N. Zakharov, D. W. Johnson and M. M. Haley, Angew. Chem., Int. Ed., 2019, 58, 3934–3938 CrossRef CAS PubMed.
- G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478–2601 CrossRef CAS PubMed.
- L. C. Gilday, S. W. Robinson, T. A. Barendt, M. J. Langton, B. R. Mullaney and P. D. Beer, Chem. Rev., 2015, 115, 7118–7195 CrossRef CAS PubMed.
- G. Montà-González, F. Sancenón, R. Martínez-Máñez and V. Martí-Centelles, Chem. Rev., 2022, 122, 13636–13708 CrossRef PubMed.
- T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001–7045 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2306049, 2306050, 2306058 and 2306069. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc05510h |
| This journal is © The Royal Society of Chemistry 2024 |