Open Access Article
Open Access ArticleMolecular baskets form inclusion complexes with phenethylamine drugs in water†
Tyler J.
Finnegan‡
,
Christopher
Mortensen‡
and
Jovica D.
Badjić
 *
*
The Ohio State University, Department of Chemistry & Biochemistry, 100 W 18th Avenue, Columbus, OH 43210, USA. E-mail: badjic.1@osu.edu
First published on 16th January 2024
Abstract
Molecular basket 16− comprising a nonpolar cavity and an anionic nest of six carboxylates at its rim was found to form inclusion complexes with (1R, 2S)-ephedrine, (1R, 2R)-pseudoephedrine, and (1S, 2R)-tranylcypromine. Experimental results (NMR) and theory (MM/DFT) suggest the basket encapsulates phenethylamines in unique and predictable fashion.
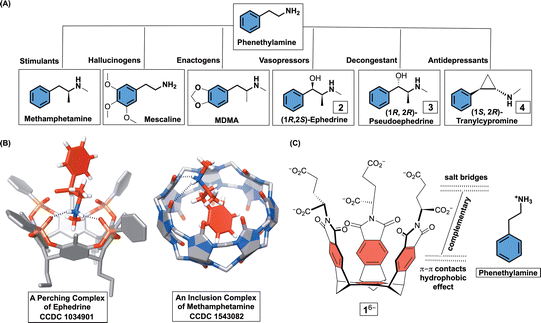
Substituted phenethylamines (Fig. 1A) are organic molecules with the 2-phenylethan-1-amine framework and a broad range of biological activities. To mention a few, phenethylamines include stimulants (e.g., methamphetamine), hallucinogens (e.g., mescaline), entactogens (e.g. MDMA), vasopressors (e.g., ephedrine), decongestants (e.g., pseudoephedrine), bronchodilators (e.g., albuterol), and antidepressants (e.g., tranylcypromine).1 With facile access to a great number of phenethylamines,2 there has been a considerable interest toward derivatizing their framework and examining potentially novel pharmacological effects.3 In this vein, development of more potent but also cheap hallucinogens and stimulants4 is having a negative impact on society by expanding the drug epidemic and illicit activities.5 Accordingly, supramolecular chemists have used macrocyclic hosts (i.e., cavitands) for examining facile detection,6 resolution,1e,7 and sequestration8 of phenethylamine drugs. The rationale behind these studies is to use functionalized but also conformationally restricted cavitands for complementing ammonium and/or phenyl units within targeted compound. In the cases of resorcin[4]arenes1e and cucurbit[6]urils,1d,6c,9 the formation of perching complexes ensued in polar solvents and/or solid state with cavitands’ polar rim holding onto the ammonium site of phenethylamines via hydrogen bonding (Fig. 1B). With cucurbit[7]urils,6a calix[4]arenes8b β,γ-cyclodextrins,10 pillar[5]arenes,11 and pillar[6]arenes,8a the phenyl group was found to, in aqueous media, insert into each host's nonpolar cavity thereby placing the ammonium at the polar rim to form hydrogen bonds (Fig. 1B). We wondered, would molecular basket 16− (Fig. 1C), comprising a nonpolar cavity and an anionic nest of six carboxylates at the rim (i.e., three glutamic acid residues),12 form an inclusion complex with positively charged phenethylamines in water? In this regard, easily accessible and enantiopure (1R, 2S)-ephedrine 2, (1R, 2R)-pseudoephedrine 3, and (1S, 2R)-tranylcypromine 4 (Fig. 1A) seemed suitable for the study. While these drugs appeared to have structural complementarity to 16− (Fig. 1C), we were curious about the exact mode of interaction (i.e., stoichiometry and docking geometry)13 as well as the stability of these complexes. In fact, host 16− was previously shown to form inclusion complexes with organophosphorus agents (OPs)14 and α,ω-diammonium alkanes13,15 with millimolar (or even greater) stabilities. While nonpolar P–CH3/P–OCH3 groups from OPs occupy the inner space of 16−, polar P = O units reside at the rim of binary [OPs⊂1]6−. In the case of diammonium alkanes, two RNH3+ groups would form salt bridges with α- and γ-carboxylates from two molecules of 16− surrounding the guest to give a ternary complex.15
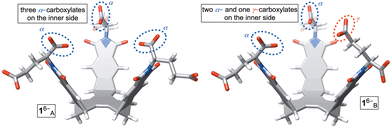
Molecular basket 1 was obtained by following an earlier described synthetic protocol.12 This molecule can be dissolved in aqueous 30 mM phosphate buffer solution (PBS) at pH = 7.0 to give hexaanionic 16−. At concentrations of 5.0 mM or lower, amphiphilic 16− was shown to be monomeric with results from DLS, UV-Vis, MS, and NMR experiments all suggesting the absence of aggregation.12 Importantly, 1H NMR spectrum of 16− (Fig. 3A) comprises a set of sharp signals corresponding to, on average, a C3 symmetric compound. A Monte Carlo conformational search (OPLS3) in implicit water solvent showed the presence of two conformers 1A/B6− within 1 kcal mol−1 (Fig. 2; Table S3, ESI†); each conformer was additionally optimized at a higher level of theory (DFT:B3LYP/6-31+G*). While 1A6− has all three α-carboxylates from glutamic acid residues pointing to the concave side of the host, 1B6− has one of these carboxylates at the convex face. Along with computational results, we reasoned that a rapid interconversion of 1A6− to 1B6− (Fig. 2), in addition to each molecule's conformational dynamics, contributed to the observed 1H NMR spectrum of 16−.
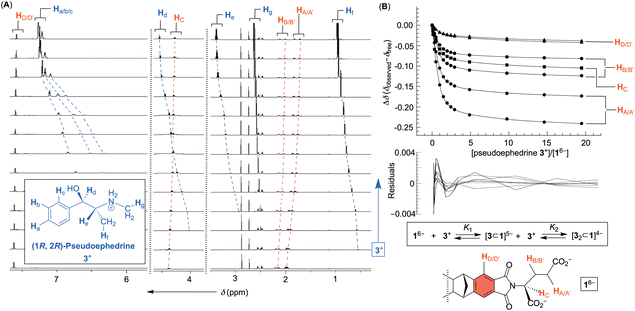
An incremental addition of a standard solution of phenethylamines 2+–4+ to 16− was monitored (30 mM PBS at pH = 7.0) with 1H NMR spectroscopy (Fig. 3 and Fig. S1–S3, ESI†); each titration was repeated three times. Importantly, additions prompted a steady change (i.e., magnetic shielding) of the basket's resonances (Fig. 3A). We reasoned that noncovalent complexation ought to be taking place with the host residing in the shielding region of aromatic guests (Fig. 3B). To determine the stoichiometry of the complexation, the change in chemical shift of seven resonances from 16− as a function of the concentration of 2+–4+ was subjected to nonlinear regression analyses using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding models (Fig. S4–S6, ESI†).16 In this regard, it is important to note that 1H NMR resonances of drugs 2+–4+ remained constant as a function of their variable concentrations (Fig. S7–S9, ESI†) so no competing dimerization or aggregation of drug molecules took place during supramolecular titrations. The curve fitting result for the titration of pseudoephedrine 3+ to 16− using 1
1 binding models (Fig. S4–S6, ESI†).16 In this regard, it is important to note that 1H NMR resonances of drugs 2+–4+ remained constant as a function of their variable concentrations (Fig. S7–S9, ESI†) so no competing dimerization or aggregation of drug molecules took place during supramolecular titrations. The curve fitting result for the titration of pseudoephedrine 3+ to 16− using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding model (i.e., [3⊂1]5− and [32⊂1]4− complexes) is shown in Fig. 3B. The model is accepted given a somewhat random distribution of residuals17 and the small covariance of the fit (i.e., covfit = 3 × 10−4; Fig. S5, ESI†).18,19 When the same criteria were used for evaluating the goodness of fit (GOF) for 1
2 binding model (i.e., [3⊂1]5− and [32⊂1]4− complexes) is shown in Fig. 3B. The model is accepted given a somewhat random distribution of residuals17 and the small covariance of the fit (i.e., covfit = 3 × 10−4; Fig. S5, ESI†).18,19 When the same criteria were used for evaluating the goodness of fit (GOF) for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding models, we could disqualify both 1
1 binding models, we could disqualify both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (covfit = 3 × 10−3) and 2
1 (covfit = 3 × 10−3) and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexations (covfit = 6 × 10−4).19 For all three drugs, the covariance of the fit slightly favoured 1
1 complexations (covfit = 6 × 10−4).19 For all three drugs, the covariance of the fit slightly favoured 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 over 2
2 over 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 models of association (Fig. S4–S6, ESI†). Additionally, we decided to examine Akaike's information criterion (AIC).20 This information-theoretic approach was recently introduced20 to the field of host–guest chemistry as a superior alternative for evaluating the GOF. To calculate AIC, one needs to use the formula AIC = N
1 models of association (Fig. S4–S6, ESI†). Additionally, we decided to examine Akaike's information criterion (AIC).20 This information-theoretic approach was recently introduced20 to the field of host–guest chemistry as a superior alternative for evaluating the GOF. To calculate AIC, one needs to use the formula AIC = N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln (SSR/N) + 2k in which N is the number of fitted points, SSR is the sum of the squared residuals and k is the number of fitted parameters. The smaller the AIC the better the GOF. After computing AIC values for three independent titrations of each drug to 16− (Table 1 and Tables S1 and S2, ESI†), in addition to Akaike weights wi, we compared the data. First, 1
ln (SSR/N) + 2k in which N is the number of fitted points, SSR is the sum of the squared residuals and k is the number of fitted parameters. The smaller the AIC the better the GOF. After computing AIC values for three independent titrations of each drug to 16− (Table 1 and Tables S1 and S2, ESI†), in addition to Akaike weights wi, we compared the data. First, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexations are uniformly showing the least favorable AIC scores (i.e., the most positive numbers). Between the other two models, 1
1 complexations are uniformly showing the least favorable AIC scores (i.e., the most positive numbers). Between the other two models, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 gave lower AIC values than 2
2 gave lower AIC values than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in two out of three titrations for each drug. We conclude that 1
1 in two out of three titrations for each drug. We conclude that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 association ought to be dominating under the experimental conditions. To corroborate statistical considerations of the fit, ESI† mass spectrometry measurements of a mixture of phenethylamine drugs 2+–4+ with basket 16− were taken in 1
2 association ought to be dominating under the experimental conditions. To corroborate statistical considerations of the fit, ESI† mass spectrometry measurements of a mixture of phenethylamine drugs 2+–4+ with basket 16− were taken in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 10
1, and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios (Fig. S10–S12, ESI†). At all ratios, only the presence of 1
1 ratios (Fig. S10–S12, ESI†). At all ratios, only the presence of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes were observed. While the electrostatic repulsion between two anionic 16− disfavors 2
2 complexes were observed. While the electrostatic repulsion between two anionic 16− disfavors 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation, we hypothesize that 1
1 complexation, we hypothesize that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex comprises the first guest occupying the cavity of 16− while the second one undergoes an exo-complexation.
2 complex comprises the first guest occupying the cavity of 16− while the second one undergoes an exo-complexation.
Model 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Model 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Model 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||
|---|---|---|---|---|
| Titration I | AIC | −1039.7 | −![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) ![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) . .![[6 with combining low line]](https://www.rsc.org/images/entities/char_0036_0332.gif) |
−1113.3 |
| w i | 4 × 10−67 | ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) |
2 × 10−26 | |
| Titration II | AIC | −1108.8 | −![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) ![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif) . .![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) |
−1232.5 |
| w i | 7 × 10−57 | ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) |
4 × 10−3 | |
| Titration III | AIC | −1541.3 | −1619.2 | −![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) ![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif) ![[7 with combining low line]](https://www.rsc.org/images/entities/char_0037_0332.gif) ![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif) . .![[5 with combining low line]](https://www.rsc.org/images/entities/char_0035_0332.gif) |
| w i | 1 × 10−190 | 9 × 10−157 | ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) |
After elucidating the binding stoichiometry, we went on to analyse the thermodynamic stability of binary [drug⊂1]5− and ternary [drug2⊂1]4− complexes (Table 2; see also Fig. S4–S6, ESI†). Evidently, the formation of binary complexes dominates each equilibria with K1 ≫ K2. This bodes well with the earlier notion that [drug⊂1]5− is an inclusion and more stable complex than [drug2⊂1]4− in which the association takes place on the basket's outer side. The stability of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes are in the millimolar range, but interestingly the affinity of tranylcypromine 4+ toward 16− is an order of magnitude greater than ephedrine 2+ and pseudoephedrine 3+.
1 complexes are in the millimolar range, but interestingly the affinity of tranylcypromine 4+ toward 16− is an order of magnitude greater than ephedrine 2+ and pseudoephedrine 3+.
| Phenethylamine drug | K 1 (M−1) | K 2 (M−1) |
|---|---|---|
| (1R, 2S)-ephedrine 2+ | 7 ± 1 × 103 | 54 ± 11 |
| (1R, 2R)-pseudoephedrine 3+ | 6 ± 2 × 103 | 58 ± 11 |
| (1S, 2R)-tranylcypromine 4+ | 2 ± 1 × 104 | 39 ± 14 |
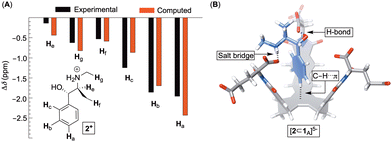
After quantifying the thermodynamic parameters characterizing the binding, we sought out to learn more about the mode of complexation and intermolecular interactions driving the association. First, the magnetic perturbation of resonances from each drug undergoing complexation show phenyls’ Ha/b/c experiencing the largest magnetic shielding (Δδ = δbound − δfree, Fig. 4A; Fig. S1–S3, ESI†). Inclusion of the phenyl ring into the basket's cavity with Ha reaching for the cavity's bottom would explain the observation.13 Along with such docking of the aromatic, the ammonium site from 2–4+ ought to reside in the anionic nest (Fig. 1C). If so, what is the exact role of α- and γ-carboxylates in the complexation and what is the dominant pose of each guest with respect to the basket host? To answer these questions, we developed a computational protocol to estimate a pose which best represents the observed NMR shielding effect. First, the magnetic environments of 1A6− and 1B6− were mapped by computing nucleus independent chemical shift values (NICS) for a grid around the molecules (Fig. S14 and S15, ESI†).21 Next, we ran Monte-Carlo conformational searches (OPLS3) for inclusion complexes [2–4⊂1A]5− and [2–4⊂1B]5−. During MC searches, we froze conformational motions of 1A/B6− but allowed guests to change their position and conformation, akin to docking protocols. Finally, we algorithmically assigned shielding values (Δδcomp) for proton nuclei of all MC conformers of [2–4⊂1A]5− and [2–4⊂1B]5− using the NICS maps from 1A6− and 1B6−. Having computed (Δδcomp) and experimental (Δδexp) values of proton shifts, we analysed the data in two different ways. For the first approach, we determined root mean squared error 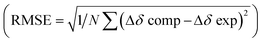 for each computed pose and the pose with the lowest RMSE was assumed to be the best representation of the experimental result (Table 3, Fig. 4B; see also Fig. S19–S21, ESI†). As for the second approach, we Boltzmann weighted Δδcomp values for each proton from all computed poses and summed each poses contribution to the ensemble average 〈Δδcomp〉. Next, RMSEs for [2–4⊂1A]5− and [2–4⊂1B]5− were determined using the 〈Δδcomp〉 values (Table 3). From Table 3, one notes that the ensemble of poses is, in more cases than not, giving lower RMSEs than single structures. It follows that the ensemble is more effective than a single pose at describing the experimental result. Furthermore, the values for RMSEs from complexes of 1A6− and 1B6− are comparable to indicate that both conformers likely participate in the binding; however, we noted that 1A6− showed a closer agreement to experimental NMR shielding values (Fig. S16–S18, ESI†). For (1R, 2S)-ephedrine 2+ occupying 1A6−, RMSE values for the ensemble of poses (0.52) and a single pose (0.79) are similar (Table 3).
for each computed pose and the pose with the lowest RMSE was assumed to be the best representation of the experimental result (Table 3, Fig. 4B; see also Fig. S19–S21, ESI†). As for the second approach, we Boltzmann weighted Δδcomp values for each proton from all computed poses and summed each poses contribution to the ensemble average 〈Δδcomp〉. Next, RMSEs for [2–4⊂1A]5− and [2–4⊂1B]5− were determined using the 〈Δδcomp〉 values (Table 3). From Table 3, one notes that the ensemble of poses is, in more cases than not, giving lower RMSEs than single structures. It follows that the ensemble is more effective than a single pose at describing the experimental result. Furthermore, the values for RMSEs from complexes of 1A6− and 1B6− are comparable to indicate that both conformers likely participate in the binding; however, we noted that 1A6− showed a closer agreement to experimental NMR shielding values (Fig. S16–S18, ESI†). For (1R, 2S)-ephedrine 2+ occupying 1A6−, RMSE values for the ensemble of poses (0.52) and a single pose (0.79) are similar (Table 3).
| Phenethylamine drug | Type | 1 A 6− (RMSE) | 1 B 6− (RMSE) |
|---|---|---|---|
| (1R, 2S)-ephedrine 2+ | Ensemble | 0.52 | 0.43 |
| Single | 0.79 | 0.89 | |
| (1R, 2R)-pseudoephedrine 3+ | Ensemble | 0.86 | 0.88 |
| Single | 0.65 | 0.70 | |
| (1S, 2R)-tranylcypromine 4+ | Ensemble | 0.50 | 0.78 |
| Single | 0.95 | 1.0 |
With an assumption that this single pose presents an average picture of the ensemble, the structure of [2⊂1A]5− in Fig. 4B shows ephedrine anchoring its benzene in the basket's cavity (via C–H⋯π contacts) while using hydroxyl and ammonium groups to form a hydrogen bond and salt bridge with α-carboxylates. Importantly, all of the best fit single poses of 2–4+ bound to 1A/B6− show the benzene from phenethylamines occupying the basket's cavity to contribute to non-classical hydrophobic effect22 with ΔH° < 0 (Fig. S13, ESI†) while OH and NH+ groups hydrogen bond/salt bridge α- and to a smaller degree γ-carboxylates (Fig. S19–S21, ESI†).
In conclusion, molecular basket 16− forms inclusion complexes with phenethylamines in water. The results of both experimental and computational studies suggest that the probed drug molecules anchor their phenyl ring in the basket's cavity as driven by C–H⋯π contacts and the hydrophobic effect while forming hydrogen bonds and/or salt bridges with primarily α-carboxylates at the rim. With a well characterized mode of binding, the opportunity to install alternative amino acids or peptides atop our basket scaffold is clear. In addition to the investigation of structure function relationships, further derivatization may indeed furnish a better agent for the preparation of chemosensors or sequesters of phenethylamines.
This study was financially supported with funds obtained from the National Science Foundation under CHE-2304883.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) M. D. Anglin, C. Burke, B. Perrochet, E. Stamper and S. Dawud-Noursi, J. Psychoact. Drugs, 2000, 32, 137–141 CrossRef CAS PubMed; (b) G. K. Aghajanian and G. J. Marek, Neuropsychopharmacology, 1999, 21, 16S–23S CrossRef CAS PubMed; (c) D. J. Heal, J. Gosden, S. L. Smith and C. K. Atterwill, Neuropharmacology, 2023, 225, 109375 CrossRef CAS PubMed; (d) O. Danylyuk, CrystEngComm, 2018, 20, 7642–7647 RSC; (e) E. Biavardi, F. Ugozzoli and C. Massera, Chem. Commun., 2015, 51, 3426–3429 RSC.
- S. Freeman and J. F. Alder, Eur. J. Med. Chem., 2002, 37, 527–539 CrossRef CAS PubMed.
- D. E. Nichols and W. E. Fantegrossi, Emerging designer drugs, 2014, pp. 575–596 Search PubMed.
- M. E. Nelson, S. M. Bryant and S. E. Aks, Emerg. Med. Clin. North Am., 2014, 32, 1–28 CrossRef PubMed.
- R. Gonzales, L. Mooney and R. A. Rawson, Ann. Rev. Public Health, 2010, 31, 385–398 CrossRef PubMed.
- (a) Y. Jang, M. Jang, H. Kim, S. J. Lee, E. Jin, J. Y. Koo, I.-C. Hwang, Y. Kim, Y. H. Ko, I. Hwang, J. H. Oh and K. Kim, Chemistry, 2017, 3, 641–651 CrossRef CAS; (b) D. King, C.-L. Deng and L. Isaacs, Tetrahedron, 2023, 145, 133607 CrossRef CAS; (c) E. Biavardi, S. Federici, C. Tudisco, D. Menozzi, C. Massera, A. Sottini, G. G. Condorelli, P. Bergese and E. Dalcanale, Angew. Chem., Int. Ed., 2014, 53, 9183–9188 CrossRef CAS PubMed.
- J. Vachon, S. Harthong, E. Jeanneau, C. Aronica, N. Vanthuyne, C. Roussel and J.-P. Dutasta, Org. Biomol. Chem., 2011, 9, 5086–5091 RSC.
- (a) A. T. Brockett, W. Xue, D. King, C.-L. Deng, C. Zhai, M. Shuster, S. Rastogi, V. Briken, M. R. Roesch and L. Isaacs, Chemistry, 2023, 9, 881–900 CrossRef CAS PubMed; (b) S. Ganapati, S. D. Grabitz, S. Murkli, F. Scheffenbichler, M. I. Rudolph, P. Y. Zavalij, M. Eikermann and L. Isaacs, ChemBioChem, 2017, 18, 1583–1588 CrossRef CAS PubMed.
- O. Danylyuk, V. P. Fedin and V. Sashuk, Chem. Commun., 2013, 49, 1859–1861 RSC.
- M. T. Garcia-Valverde, M. L. Soriano, R. Lucena and S. Cardenas, Anal. Chim. Acta, 2020, 1126, 133–143 CrossRef CAS PubMed.
- R. Zhang, Y. Ren, Q. Zhang, W. Huang, H. Bai and X. Zeng, New J. Chem., 2022, 46, 20909–20917 RSC.
- S. E. Border, R. Z. Pavlovic, L. Zhiquan, M. J. Gunther, H. Wang, H. Cui and J. D. Badjic, Chem. – Eur. J., 2019, 25, 273–279 CrossRef CAS PubMed.
- R. Z. Pavlovic, S. E. Border, T. J. Finnegan, L. Zhiquan, M. J. Gunther, E. Munoz, C. E. Moore, C. M. Hadad and J. D. Badjic, J. Am. Chem. Soc., 2019, 141, 16600–16604 CrossRef CAS PubMed.
- S. E. Border, R. Z. Pavlovic, Z. Lei, M. J. Gunther, H. Wang, H. Cui and J. D. Badjic, Chem. Commun., 2019, 55, 1987 RSC.
- R. Z. Pavlovic, S. E. Border, Y. Li, X. Li and J. D. Badjic, Chem. Commun., 2020, 56, 2987–2990 RSC.
- P. Thordarson, Supramolecular Chemistry: From Molecules to Nanomaterials, 2012, vol. 2, pp. 239–274 Search PubMed.
- (a) F. Ulatowski, K. Dabrowa, T. Balakier and J. Jurczak, J. Org. Chem., 2016, 81, 1746–1756 CrossRef CAS PubMed; (b) D. Brynn Hibbert and P. Thordarson, Chem. Commun., 2016, 52, 12792–12805 RSC.
- J. E. A. Webb, M. J. Crossley, P. Turner and P. Thordarson, J. Am. Chem. Soc., 2007, 129, 7155–7162 CrossRef CAS PubMed.
- P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305–1323 RSC.
- K. Ikemoto, K. Takahashi, T. Ozawa and H. Isobe, Angew. Chem., Int. Ed., 2023, 62, e202219059 CrossRef CAS PubMed.
- Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. V. R. Schleyer, Chem. Rev., 2005, 105, 3842–3888 CrossRef CAS PubMed.
- L. Zhiquan, S. M. Polen, C. M. Hadad, T. V. RajanBabu and J. D. Badjic, Org. Lett., 2017, 19, 4932–4935 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc05485c |
| ‡ These two authors contributed equally to this study. |
| This journal is © The Royal Society of Chemistry 2024 |