Open Access Article
Open Access ArticlePhotonic Si microwell architectures for rapid antifungal susceptibility determination of Candida auris†
Christopher
Heuer
 abc,
Xin
Jiang
abc,
Xin
Jiang
 a,
Gali
Ron
a,
Orna
Ternyak
d,
Thomas
Scheper
b,
Janina
Bahnemann
a,
Gali
Ron
a,
Orna
Ternyak
d,
Thomas
Scheper
b,
Janina
Bahnemann
 c and
Ester
Segal
c and
Ester
Segal
 *a
*a
aDepartment of Biotechnology and Food Engineering, Technion – Israel Institute of Technology, Haifa, 3200003, Israel. E-mail: esegal@technion.ac.il
bInstitute of Technical Chemistry, Leibniz University Hannover, Hannover 30167, Germany
cInstitute of Physics, University of Augsburg, Augsburg 86159, Germany
dMicro- and Nanofabrication and Printing Unit, Technion – Israel Institute of Technology, Haifa, 3200003, Israel
First published on 27th December 2023
Abstract
We present the application of a photonic silicon chip-based optical sensor system for expeditious and phenotypic antifungal susceptibility testing. This label-free diagnostic assay optically monitors the growth of Candida auris at varying antifungal concentrations on a microwell-structured silicon chip in real-time, and antifungal susceptibility is detected within 6 h, four times faster than in the current gold standard method.
In recent years, multidrug-resistant fungal pathogens have emerged as a global health threat, with high mortality rates resulting in over 1.6 million deaths annually.1,2 The severity of this situation is underscored by the World Health Organization's first-ever fungal priority pathogens list published in 2022, aiming to guide public health strategies against pathogenic fungi.3 The excessive use of antifungals, together with climate change, accelerates the emergence and evolution of resistant fungal pathogens.4,5 The spread of fungal infections in the last three years is mainly ascribed to the increasing number of immunocompromised and vulnerable patients,3 which is closely linked to the COVID-19 pandemic.6 The yeast Candida auris (C. auris), well known for its pathogenicity and associated morbidity, is now classified as an urgent threat by the United States Centers for Disease Control and Prevention.7C. auris is not only difficult to eradicate from clinical settings but is often multidrug-resistant, with some isolates being recognized as pan-resistant (resistant to antifungals of all drug classes).3,8 Thus, there is a paramount need for diagnostic methods that can expeditiously determine the correct antifungal prescription, improving therapy outcomes and minimizing the spread of resistance.9 Specifically, antifungal susceptibility testing (AFST), in which pathogenic fungi are exposed to varying antifungals at increasing concentrations, and the minimum inhibitory concentration (MIC) is determined, can help physicians to guide treatment decisions.10 However, current AFST methods, like agar-based tests or the gold standard broth microdilution (BMD), are lengthy and require >24 h.10 Also, commercially available state-of-the-art automated methods such as the Vitek2 (bioMérieux) typically provide results only within 12–18 h,11,12 a limited set of antifungals is available, and MIC values have not always been accurately determined.13 Thus, there is an urgent clinical need for rapid and reliable novel AFST methods for Candida species.
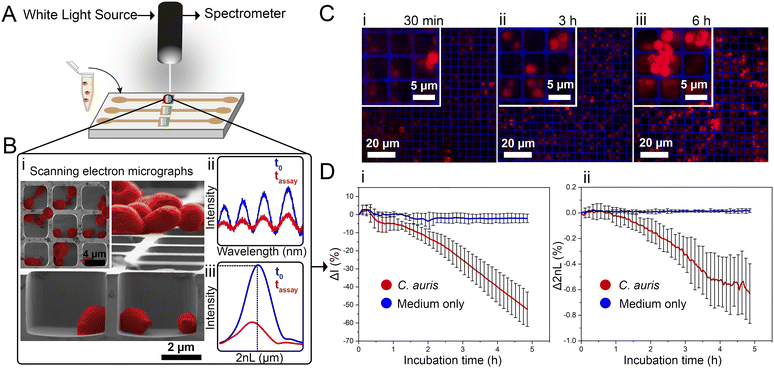
Herein, we present the use of a photonic silicon chip with microwell diffraction gratings that serves as a sensitive sensor for label-free fungal growth monitoring by detecting changes in white light reflectance. Such silicon-based materials are characterized by their biocompatibility and have been widely employed for diagnostic, therapeutic and biosensing applications.14–16 Specifically, we have previously shown that photonic silicon chips, with different dimensions and microtopologies,17 can be used for rapid susceptibility testing of the bacterial species Escherichia coli (E. coli) within 90 minutes18–20 and the mould Aspergillus niger (A. niger) within 10–12 h21 using phase-shift reflectometric interference spectroscopic measurements (PRISM) and intensity-based PRISM (iPRISM) tests. In these optical assays, the photonic silicon chips are individually fixated into heat-controlled microfluidic channels, and suspensions of yeast cells in growth medium are introduced into these channels (see Fig. 1A). The chips consist of arrays of microwells (width: ∼4 μm and depth: ∼4 μm) that were specifically designed to fit the majority of C. auris cells (having spherical to oval shape with characteristic dimensions of 2–3 × 2.5–5 μm22) within the individual wells, see Fig. 1B-i for cross-section and top view (insert) scanning electron microscopy (SEM) images. Yet, C. auris can also form cell aggregates which are mainly found on top of the microwells. Continuous reflectance measurements are used to obtain characteristic spectra (Fig. 1B-ii), which are further processed by Fast Fourier Transform (FFT) frequency analysis. The latter results in a single peak (Fig. 1B-iii), where the peak amplitude corresponds to the intensity of the reflected light and the peak position corresponds to 2nL (n refers to the refractive index of a medium within the grating and L is the microwells' depth). Please also refer to Fig. S1 (ESI†) and to our previous works19,23,24 for a detailed explanation of the involved optical principles.
Monitoring both parameters over time allows tracking C. auris growth in a label-free manner in real-time. Fig. 1D depicts growth curves (intensity and 2nL) for C. auris suspensions at McFarland value of 2.0 (which corresponds to ∼107 cells mL−1, as routinely used for AFST of Candida species by commercial automated systems such as the Vitek2;12 for experimental details and further discussion on C. auris cell density see the ESI,† Fig. S2). For both parameters, a general trend of decreasing signals (intensity slope: −10.8 ΔI (%) h−1 and 2nL slope: −0.13 Δ2nL (%) h−1) is observed (see Fig. 1D-i and ii). The major decrease in the intensity signal is ascribed to C. auris cells growing on the microstructured silicon surface19,23 and Fig. S3 (ESI†) depicts the correlation between cell concentrations and the obtained intensity signal (Fig. S4 (ESI†) presents the corresponding raw reflectance spectra). Fig. 1C shows corresponding confocal laser scanning micrographs of C. auris at different time points (30 min, 3 h, 6 h), demonstrating that at the beginning of the assay, most cells reside within the microwells. Whereas at later times, the cells tend to grow out of the wells and are found on the top of the microstructure, as also revealed by SEM studies presented in Fig. S5 (ESI†). Moreover, similar behaviour is also observed for a second yeast species – the industrial-relevant Saccharomyces cerevisiae where a characteristic reduction in both intensity and 2nL signals is obtained, see Fig. S6 (ESI†).
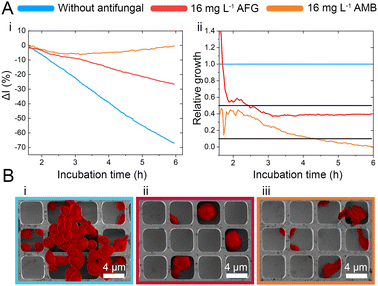
While the observed intensity decrease is in agreement with our previous studies, where varying microorganisms were grown on microstructured silicon gratings,17,19,21 the obtained trend of 2nL reduction (Fig. 1D-ii) is different from the typical behaviour we have observed for bacteria.19,21 In general, we found that bacterial cells tend to reside and proliferate within the microwell structure (see Fig. S7, ESI†) and, as such, induce an increase in the 2nL value over time. The latter is ascribed to an increase in the average refractive index within the microstructure.17–19 We were expecting a similar behaviour for C. auris, as most cells are found to reside in the wells at the beginning of the assay. Yet, within 6 h C. auris forms dense cell aggregates on the top of the microstructure, as depicted in the electron micrographs included in Fig. S5 (ESI†). To further investigate this behaviour, C. auris was studied in a growth medium designated for bacteria (CAMHB), where growth is observed to be impaired compared to that in RPMI (designated for yeasts). The slope of the intensity signal is found to be five times lower in CAMHB, while the 2nL slope is mainly unchanged, see Fig. S8 (ESI†). Moreover, SEM studies show that C. auris do not form aggregates on top of the silicon microstructure in CAMHB, as also depicted in Fig. S8 (ESI†). This may suggest that the cells emerging from the wells and forming dense cell clusters on the top of the microstructure potentially impede light interference from the chip, resulting in a reduction of the 2nL signal. However, this phenomenon should be further investigated as we also observed it in the case of biofilm formation by the bacterial pathogen Pseudomonas aeruginosa.17 Herein, we will mainly focus on monitoring the intensity changes for sensitive growth detection at varying antifungal concentrations, following the iPRISM assay principle.21 The iPRISM concept for AFST of C. auris was first established with high antibiotic concentrations (see Fig. 2) of two clinically relevant antifungal agents with different modes of action, namely anidulafungin (echinocandin: inhibition of cell wall biosynthesis)25 and amphotericin B (polyene: cell membrane damage).26,27 The tested concentrations of these drugs (both 16 mg L−1) are at the upper range of the recommended concentrations for AFST of Candida species according to BMD protocols by the European Society of Antimicrobial Susceptibility Testing (EUCAST).28 Thus, fungal growth is inhibited at these concentrations (Fig. 2A-i), and the relative growth compared to the drug-free control falls below the 50% (anidulafungin) and 10% (amphotericin B) growth values, as depicted in Fig. 2A-ii. The latter values also serve as the MIC threshold for these respective antifungals (anidulafungin MIC: lowest drug concentration with ≥50% growth inhibition and amphotericin B MIC: lowest drug concentration with ≥90% growth inhibition) according to EUCAST.28 Scanning electron micrographs (Fig. 2Bi–iii) reveal that in the absence of an antifungal drug, C. auris grow in dense networks within and on top of the silicon microstructure, while when exposed to an antifungal, fewer cells are observed on the chip surface (Fig. 2B-ii and iii). Moreover, cells exposed to anidulafungin (Fig. 2B-ii) appear round and swollen – characteristic of morphological changes induced by this type of antifungal agent.29 In the case of amphotericin B, cells exhibit a deformed appearance, which is ascribed to cell membrane damage (Fig. 2B-iii).30
C. auris cells were grown at various concentrations of anidulafungin and amphotericin B in order to determine MIC values, and Fig. 3 depicts the corresponding relative growth curves. Increased concentrations of both antifungal agents result in reduced growth compared to the untreated control. According to the MIC definitions (as explained above), the iPRISM MIC values are 0.25 mg L−1 for both drugs. Statistical analysis of iPRISM results obtained from different chips (as shown in Fig. S9, ESI†) reveals that MIC value determination is feasible within 3.5 h and 6 h for anidulafungin and amphotericin B, respectively. Thus, the iPRISM platform provides a significantly reduced assay time compared to the gold-standard BMD (time: 24 h) and the commercially available Vitek2 system (time: 12–18 h).11,12,28 Please also refer to Fig. S11 (ESI†), demonstrating that standard BMD-based MIC value determination (C. auris and amphotericin B) can be reduced to 19 h using continuous microplate reader measurements and statistical analysis. Thus, the presented iPRISM assay still provides a significantly faster MIC determination.
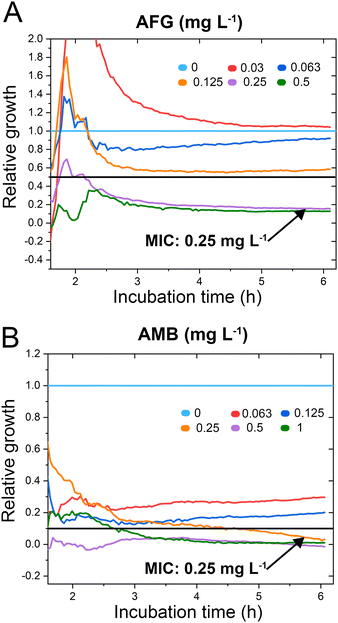 | ||
| Fig. 3 C. auris iPRISM AFST. Relative growth of C. auris at varying concentrations of (A) anidulafungin (AFG) and (B) amphotericin B (AMB). The underlying intensity changes and also the corresponding 2nL growth curves are presented in Fig. S10 (ESI†), demonstrating that the intensity is superior in its quantitative sensitivity for growth at varying antifungal concentrations while the 2nL is still sensitive qualitatively. | ||
To test the accuracy of the obtained MIC values, we compare the latter concentrations to the outcome of gold standard BMD performed according to EUCAST protocols, see Table S1 in the ESI.† The iPRISM MIC values for anidulafungin (0.25 mg L−1vs. 0.0625 mg L−1) and amphotericin B (0.25 mg L−1vs. 0.125–0.25 mg L−1) are one to two dilutions higher than the BMD value. Such a difference between two dilutions is considered as an essential agreement.31 Furthermore, the obtained MIC values do not exceed the tentative epidemiological cut-off values (ECOFFs) determined by different approaches (Table S1, ESI†).32 As such, for both drugs, this C. auris isolate is correctly classified into the wild-type population without acquired drug resistance. It should be noted that MIC value deviations between different methods and protocols are often encountered in susceptibility testing.33 As previously observed, MIC values determined by (i)PRISM for E. coli18,19 and A. niger21 tend to be higher in comparison to BMD MICs. These deviations are mainly ascribed to the different assay procedures. While in the BMD, the fungal cells are suspended and grow in a liquid medium, in the iPRISM assay, the behaviour of the cell-silicon substrate interface is monitored.
A diagnostic platform for rapid and phenotypic AFST of C. auris as a model yeast pathogen is shown, demonstrating and extending the applicability of this label-free assay to a wide variety of clinically relevant species (bacteria, filamentous fungi, yeast). The yeast cells grow on a microstructured silicon surface that also serves as a sensitive sensing element allowing the detection of changes in the white light reflectivity. The latter changes are correlated to fungal growth and used to study the behaviour of C. auris upon exposure to anidulafungin and amphotericin B – two clinically relevant antifungals from two distinct antifungal classes. MIC values for these antifungals can be obtained within 6 h and agree with values determined by classical BMD. Thus, iPRISM provides a novel method for AFST of Candida species that is significantly faster (time reduction: ≥18 h) than gold standard methods.
This work was supported by the Israel Science Foundation (grant No. 2458/21). We also acknowledge funding by the German Research Foundation (DFG) via the Emmy Noether program (project ID346772917), and grant SCHE 279/32-2, as well as by the VolkswagenStiftung via the program “Niedersächsisches Vorab: Research Cooperation Lower Saxony—Israel”. The authors thank the staff of the Micro- and Nanofabrication and Printing Unit (MNFPU) at the Technion for the fabrication of the photonic silicon chips.
Conflicts of interest
There are no conflicts to declare.References
- K. Benedict, M. Richardson, S. Vallabhaneni, B. R. Jackson and T. Chiller, Lancet Infect. Dis., 2017, 17, e403–e411 CrossRef PubMed.
- A. Rokas, Nat. Microbiol., 2022, 7, 607–619 CrossRef CAS PubMed.
- WHO fungal priority pathogens list to guide research, development and public health action, https://www.who.int/publications/i/item/978924006024, (accessed March 2023).
- U. Hofer, Nat. Rev. Microbiol., 2019, 17, 588 CrossRef CAS PubMed.
- N. A. R. Gow, C. Johnson, J. Berman, A. T. Coste, C. A. Cuomo, D. S. Perlin, T. Bicanic, T. S. Harrison, N. Wiederhold, M. Bromley, T. Chiller and K. Edgar, Nat. Commun., 2022, 13, 5352 CrossRef CAS PubMed.
- CDC special report 2022: COVID-19 U.S. impact on antimicrobial resistance.
- CDC report: antibiotic resistance threats in the United States 2019.
- B. O’Brien, J. Liang, S. Chaturvedi, J. L. Jacobs and V. Chaturvedi, Lancet Microbe, 2020, 1, e193–e194 CrossRef PubMed.
- D. S. Perlin, R. Rautemaa-Richardson and A. Alastruey-Izquierdo, Lancet Infect. Dis., 2017, 17, e383–e392 CrossRef PubMed.
- C. Heuer, J. Bahnemann, T. Scheper and E. Segal, Small methods, 2021, 5, 2100713 CrossRef CAS PubMed.
- M. Cuenca-Estrella, A. Gomez-Lopez, A. Alastruey-Izquierdo, L. Bernal-Martinez, I. Cuesta, M. J. Buitrago and J. L. Rodriguez-Tudela, J. Clin. Microbiol., 2010, 48, 1782 CrossRef PubMed.
- E. Borghi, R. Iatta, R. Sciota, C. Biassoni, T. Cuna, M. T. Montagna and G. Morace, J. Clin. Microbiol., 2010, 48, 3153–3157 CrossRef CAS PubMed.
- S. Kathuria, P. K. Singh, C. Sharma, A. Prakash, A. Masih, A. Kumar, J. F. Meis and A. Chowdhary, J. Clin. Microbiol., 2015, 53, 1823–1830 CrossRef CAS PubMed.
- Y. Chen, M. Alba, T. Tieu, Z. Tong, R. Singh Minhas, D. Rudd, N. H. Voelcker, A. Cifuentes-Rius and R. Elnathan, Adv. NanoBiomed Res., 2021, 1, 2100002 CrossRef CAS.
- T. Tieu, M. Alba, R. Elnathan, A. Cifuentes-Rius and N. H. Voelcker, Adv. Ther., 2018, 2, 1800095 CrossRef.
- C. Pacholski, Sensors, 2013, 13, 4694–4713 CrossRef CAS PubMed.
- H. Leonard, X. Jiang, S. Arshavsky-Graham, L. Holtzman, Y. Haimov, D. Weizman, S. Halachmi and E. Segal, Nanoscale Horiz., 2022, 7, 729–742 RSC.
- C. Heuer, J.-A. Preuss, M. Buttkewitz, T. Scheper, E. Segal and J. Bahnemann, Lab Chip, 2022, 22, 4950–4961 RSC.
- H. Leonard, S. Halachmi, N. Ben-Dov, O. Nativ and E. Segal, ACS Nano, 2017, 11, 6167–6177 CrossRef CAS PubMed.
- X. Jiang, T. Borkum, S. Shprits, J. Boen, S. Arshavsky-Graham, B. Rofman, M. Strauss, R. Colodner, J. Sulam, S. Halachmi, H. Leonard and E. Segal, Adv. Sci., 2023, e2303285 CrossRef PubMed.
- C. Heuer, H. Leonard, N. Nitzan, A. Lavy-Alperovitch, N. Massad-Ivanir, T. Scheper and E. Segal, ACS Infect. Dis., 2020, 6, 2560–2566 CrossRef CAS PubMed.
- J. Osei Sekyere, MicrobiologyOpen, 2018, 7, e00578 CrossRef PubMed.
- Y. Mirsky, A. Nahor, E. Edrei, N. Massad-Ivanir, L. M. Bonanno-Young, E. Segal and A. Sa’ar, Appl. Phys. Lett., 2013, 103, 033702 CrossRef.
- N. Massad-Ivanir, Y. Mirsky, A. Nahor, E. Edrei, L. M. Bonanno-Young, N. Ben Dor, A. Sa’ar and E. Segal, Analyst, 2014, 139, 3885–3894 RSC.
- J. A. Vazquez and J. D. Sobel, Clin. Infect. Dis., 2006, 43, 215 CrossRef PubMed.
- A. Zumbuehl, D. Jeannerat, S. E. Martin, M. Sohrmann, P. Stano, T. Vigassy, D. D. Clark, S. L. Hussey, M. Peter, B. R. Peterson, E. Pretsch, P. Walde and E. M. Carreira, Angew. Chem., 2004, 116, 5293–5297 CrossRef.
- S. L. Regen, JACS Au, 2021, 1, 3–7 CrossRef CAS PubMed.
- EUCAST DEFINITIVE DOCUMENT E.DEF 7.3.2 Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts.
- N. Yagüe, L. Gómez-Delgado, M. Á. Curto, V. S. D. Carvalho, M. B. Moreno, P. Pérez, J. C. Ribas and J. C. G. Cortés, Pharmaceuticals, 2021, 14, 1332 CrossRef PubMed.
- E. Grela, A. Zdybicka-Barabas, B. Pawlikowska-Pawlega, M. Cytrynska, M. Wlodarczyk, W. Grudzinski, R. Luchowski and W. I. Gruszecki, Sci. Rep., 2019, 9, 17029 CrossRef PubMed.
- N. P. Wiederhold, Open Forum Infect. Dis., 2021, 8, ofab444 CrossRef PubMed.
- M. C. Arendrup, A. Prakash, J. Meletiadis, C. Sharma and A. Chowdhary, Antimicrob. Agents Chemother., 2017, 61, e00485-17 CrossRef PubMed.
- C. B. Terwee, L. D. Roorda, J. Dekker, S. M. Bierma-Zeinstra, G. Peat, K. P. Jordan, P. Croft and H. C. W. de Vet, J. Clin. Epidemiol., 2010, 63, 524–534 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc04446g |
| This journal is © The Royal Society of Chemistry 2024 |