Additive effect on hot carrier cooling in a hybrid perovskite†
Yuanju
Zhao‡
a,
Peng
Wang‡
a,
Tai
Wu
a,
Rongjun
Zhao
*b,
Lin
Xie
 *a and
Yong
Hua
*a and
Yong
Hua
 *a
*a
aSchool of Materials and Energy, Yunnan University, Kunming 650091, China. E-mail: huayong@ynu.edu.cn
bDepartment of Physics, Center for Optoelectronics Engineering Research, Yunnan University, Kunming 650091, China
First published on 21st November 2023
Abstract
Slowing hot carrier (HC) cooling in lead halide perovskites is important to further improve the efficiency of perovskite solar cells (PSCs). Herein, we found that HC cooling can be efficiently prolonged by incorporating an organic small molecule (TDGA) into the perovskite film as an additive through transient absorption spectroscopy measurements, which is conducive to the extraction of the HC energies by the carrier transport layers and reduces charge carrier recombination, consequently improving the efficiency of the TDGA-doped device.
Perovskite solar cells (PSCs) have received great attention due to some remarkable photovoltaic properties, including broad and strong sunlight harvesting, low-cost fabrication techniques, and high power conversion efficiency (PCE).1–5 At present, the certified record PCE of PSCs has exceeded 26%,6 which can compete with the commercial silicon thin-film solar cells. Such rapid progress in PSCs is strongly ascribed to numerous works including perovskite composition regulation, device architecture optimization, perovskite defect passivation, and charge transporting material development.7–13 In spite of these indisputable achievements, the PCEs of single-junction PSCs still trail the Shockley–Queisser (S–Q) theoretical limit of around 33.7%.14 To push the current efficiency of PSCs towards the S–Q limit and above, it is significantly important to fully understand the basic physical processes of photogenerated carriers in devices and how to regulate these carriers.
Under photoexcitation with energy above the band gap of a perovskite-based light-absorber, perovskites can produce HC with excess energies.15–17 Theoretical calculation studies have found that harnessing the energies of HCs can push the efficiency of a single-junction solar cell up to 66% under one sunlight illumination,18,19 which far exceeds the current record PCE of PSCs. However, these HCs quickly decay back to the band edge of the perovskite material and lose their excess energy via carrier-phonon scattering on a sub-picosecond time scale.20,21 This process is termed HC cooling, which is supposed to result in the energy loss in solar cells. Therefore, slowing down the HC cooling process and harnessing their energies are very critical for enhancing PSCs efficiency. Recently, additive engineering based on organic functional molecules has been proven to enhance PSCs performance.22–26 However, the effect of organic functional additives on HC cooling dynamics in perovskite thin films has received less attention thus far.
In this work, a thiodiglycolic acid-based additive organic molecule (TDGA) is introduced into perovskite films to tune the PSCs performance, and to gain an insight into the effect of additives on the hot carrier cooling dynamics. TDGA contains two carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) functional groups, which are expected to chemically interact with PbI2 to regulate the perovskite crystallization process.27,28 Moreover, the C
O) functional groups, which are expected to chemically interact with PbI2 to regulate the perovskite crystallization process.27,28 Moreover, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups as electron donors can also bind with unsaturated Pb ions and passivate perovskite defect sites.29,30 It was found that perovskite defects can be effectively reduced by TDGA. More importantly, fs-transient absorption spectroscopy (TA) measurements demonstrated that the addition of TDCA into perovskite films can slow the HC cooling process significantly, which helps in enhancing hot carrier extraction by the charge transport layers, and thus improves device efficiency. This work provides valuable insight for understanding the hot carrier dynamics in halide perovskites for developing highly efficient photovoltaics.
O groups as electron donors can also bind with unsaturated Pb ions and passivate perovskite defect sites.29,30 It was found that perovskite defects can be effectively reduced by TDGA. More importantly, fs-transient absorption spectroscopy (TA) measurements demonstrated that the addition of TDCA into perovskite films can slow the HC cooling process significantly, which helps in enhancing hot carrier extraction by the charge transport layers, and thus improves device efficiency. This work provides valuable insight for understanding the hot carrier dynamics in halide perovskites for developing highly efficient photovoltaics.
X-ray diffraction (XRD) was conducted to verify the crystallization of the perovskite layer. As shown in Fig. 1a, the characteristic peak of PbI2 at 12.7° in the perovskite films with TDGA nearly disappeared compared with the control film, indicating that TDGA can promote the complete conversion of PbI2 to perovskite. By comparison, the peak intensity of the (110) crystal plane at 14.13° is enhanced obviously when the concentration of TDGA is 2.0 mg mL−1, confirming the best perovskite crystallization, which can be verified by the ultraviolet-visible spectra (Fig. S1, ESI†). The morphology of the perovskite films was investigated using scanning electron microscopy (SEM) images, as shown in Fig. S2 (ESI†). It is found that larger perovskite crystal sizes are observed in the TDGA-treated films, confirming the improved crystallization. X-ray photoelectron spectroscopy (XPS) analysis was used to study the interaction mechanism between the perovskite and TDGA, as shown in Fig. 1b. In comparison with the pristine perovskite film, the two Pb 4f peaks in the TDGA-treated perovskite films move to lower binding energies, which is ascribed to Pb–O coordination between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of TDGA and Pb2+ of the perovskite.31,32 FTIR spectroscopy was further employed to confirm the coordination interaction. As shown in Fig. 1c, the 1683 cm−1 peak for the pure TDGA sample corresponds to the asymmetric stretching vibration of C
O of TDGA and Pb2+ of the perovskite.31,32 FTIR spectroscopy was further employed to confirm the coordination interaction. As shown in Fig. 1c, the 1683 cm−1 peak for the pure TDGA sample corresponds to the asymmetric stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and it shifts to 1697 cm−1 after mixing with perovskite films. Thus, the XPS and FTIR results confirm the chemical interaction between the perovskite and TDGA.
O, and it shifts to 1697 cm−1 after mixing with perovskite films. Thus, the XPS and FTIR results confirm the chemical interaction between the perovskite and TDGA.
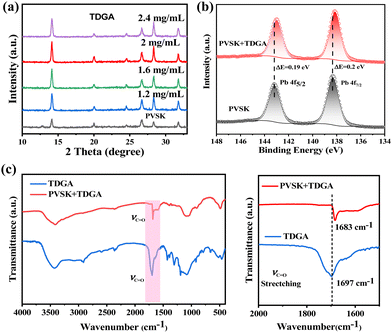 | ||
| Fig. 1 (a) XRD patterns of perovskite films with TDGA. (b) XPS core-level spectra for Pb 4f of perovskite films without and with TDGA. (c) FTIR spectra of the TDGA powder and perovskite-TDGA powder. | ||
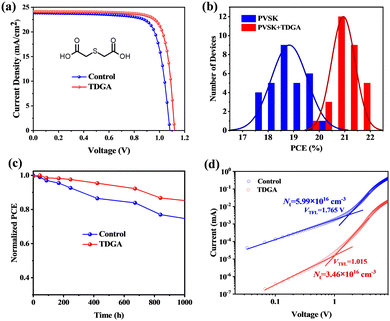
We fabricated PSCs with a n–i–p device structure of FTO/TiO2/perovskite/Spiro-OMeTAD/Ag to study the effect of TDGA on the photovoltaic performance of the devices. The photocurrent density–voltage (J–V) curves of the devices without and with TDGA are displayed in Fig. 2a. The control device shows a PCE of 19.92% with a photocurrent density (JSC) of 23.8 mA cm−2, a voltage (VOC) of 1.08 V, and a fill factor (FF) of 77.5%. After the addition of TDGA, the efficiency of the device is enhanced to 21.62%, with a JSC of 24.1 mA cm−2, a VOC of 1.12 V, and a FF of 80.1%. Fig. 2b and Fig. S3–S5 (ESI†) show the histograms of the photovoltaic parameter distributions of 30 devices. Clearly, the PSCs with TDGA exhibit better photovoltaic performance and reproducibility than that of the control one. Fig. S6 (ESI†) shows the monochromatic incident photon-to-electron conversion efficiency (IPCE) spectra of these devices. The calculated integrated JSC values of devices without and with TDGA are matched well with the JSC values from the measured J–V curves. As shown in the steady-state power output curves (Fig. S7, ESI†), both devices exhibit excellent stability under light-soaking for 300 s. Then, we further evaluated the long-term stability of these devices stored under nitrogen, as shown in Fig. 2c. The TDGA-treated device can retain 88% of its original efficiency after 1000 hours, while the efficiency of the control device decreases to 74%.
To understand the enhanced efficiency of the TDGA-treated device, steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL) were studied. As shown in Fig. S8 (ESI†), an enhanced PL intensity of perovskite films is observed after the addition of TDGA compared with the control perovskite films, suggesting lower defect states in TDGA-treated films. Then, we further measured the defect density of perovskite films by using space charge limited current (electron-only device structure: FTO/TiO2/perovskite/PCBM/Ag). Fig. 2d displays the J–V curves in the dark. The defect density of the pristine perovskite films is 5.99 × 1016 cm−3, which is decreased to 3.46 × 1016 cm−3 after using TDGA as an additive, supporting that the TDGA-based additive can efficiently decrease the defect density of perovskite films. The reduced defect sites in the perovskite films are conducive to minimizing the non-radiative recombination loss as well as facilitating charge carrier transfer in devices. Fig. S9 (ESI†) shows the TRPL spectra of the perovskite films, and their fitted data are listed in Table S1 (ESI†). For the control perovskite film, the average carrier lifetime (τavg) is 195.1 ns, which is significantly enhanced to 300.7 ns when TDGA is incorporated into the perovskite films, clearly indicating the inhibition of non-radiation recombination in the perovskite films. To better understand the charge recombination process, light intensity (Plight)-dependent VOC and JSC characterizations were measured for these devices, which are fitted by the equations of VOC ∝ (nkT/q)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(Plight) and JSC ∝ Pαlight, respectively.33,34 As displayed in Fig. S10 (ESI†), the TDGA-based device exhibits a smaller ideality factor value (nid = 1.39kT/q) than that of the control device (1.51kT/q). Fig. S11 (ESI†) shows the dependence of JSC on light intensity, where the device with TDGA shows an enhanced slope value (α = 0.994) compared with the device without TDGA (α = 0.989). Thus, the smaller nid and larger α values in the TDGA-based device demonstrate that the charge carrier recombination can be suppressed more efficiently.35,36 Finally, we further employed transient photovoltage (TPV) decay to assess quantitatively the charge carrier lifetimes of these devices, as shown in Fig. S12 (ESI†). Clearly, the TDGA-treated device shows a decay lifetime of 237 ms that is much longer than that of the control device (151 ms), clearly confirming that the charge carrier recombination is efficiently suppressed after the introduction of TDGA into the perovskite layer, which is responsible for boosting the VOC in devices.
ln(Plight) and JSC ∝ Pαlight, respectively.33,34 As displayed in Fig. S10 (ESI†), the TDGA-based device exhibits a smaller ideality factor value (nid = 1.39kT/q) than that of the control device (1.51kT/q). Fig. S11 (ESI†) shows the dependence of JSC on light intensity, where the device with TDGA shows an enhanced slope value (α = 0.994) compared with the device without TDGA (α = 0.989). Thus, the smaller nid and larger α values in the TDGA-based device demonstrate that the charge carrier recombination can be suppressed more efficiently.35,36 Finally, we further employed transient photovoltage (TPV) decay to assess quantitatively the charge carrier lifetimes of these devices, as shown in Fig. S12 (ESI†). Clearly, the TDGA-treated device shows a decay lifetime of 237 ms that is much longer than that of the control device (151 ms), clearly confirming that the charge carrier recombination is efficiently suppressed after the introduction of TDGA into the perovskite layer, which is responsible for boosting the VOC in devices.
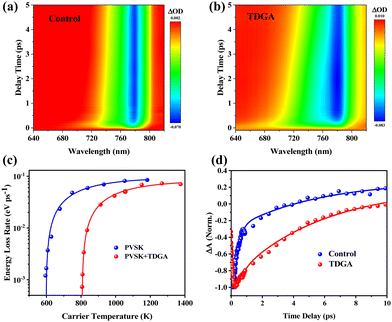
Understanding of photoexcited hot carrier transfer processes is crucial for achieving highly efficient devices, which will be comprehensively investigated by employing femtosecond transient absorption spectroscopy (fs-TA) in this work. Fig. 3a and b show the 2D pseudo-color TA plots of perovskite films without and with TDGA under a photoexcitation wavelength of 475 nm. It is found that both samples have a strong photobleaching (PB) peak located at around 780 nm arising from the band-filling effect of free carriers.37,38 Fig. S13 and S14 (ESI†) display the normalized TA spectra at delay times from 0.1 to 3.4 ps extracted from 2D pseudo-color TA plots. Clearly, the high-energy PB tails (marked with black arrows) for both samples become narrow with an increase in the delay time owing to the hot carrier cooling. Compared with the control perovskite films, the TDGA-treated films show obvious broadening of the PB signal because of the different cooling times of the hot carriers, which can be evaluated by the carrier temperature (TC).39 Fig. S15 (ESI†) displays the curves of TC with the delay times for these samples. The TDGA-doped perovskite films show a higher initial TC value of 1380 K and longer cooling time of the carrier temperature (τLO = 513 fs) than that of the control films (TC = 1184 K and τLO = 395 fs), implying a slower HC cooling process with lower energy loss rate in the TDGA-doped perovskite films. The higher initial TC in the TDGA-based perovskite films is due to the enhanced non-equilibrium LO phonon populations.40 Then, we further investigated the energy loss rate (Jr) per hot carrier. As shown in Fig. 3c, the Jr value for the TDGA-modified perovskite films is 0.057 eV ps−1, which is much higher than that of the control film (0.021 eV ps−1), suggesting that the addition of TDGA can efficiently slow down the hot carrier cooling process, thus, reducing the energy loss and improving the PSCs’ photovoltaic performance. Fig. 3d displays the kinetic profiles of perovskite films at 780 nm, and the fitted results are summarized in Table S2 (ESI†). The average HC lifetime of the perovskite films is enhanced from 4.31 ps to 5.41 ps after the addition of TDGA, which is good for the extraction of these HCs by the carrier transport layers, and meanwhile reduces the charge carrier recombination, consequently enhancing the PSC performance. As shown in Fig. S16 (ESI†), the higher amplitude for the perovskite + TDGA/Spiro-OMeTAD sample (∼30%) compared to the control perovskite/Spiro-OMeTAD sample (∼19%) confirms the enhanced hot hole extraction and transfer from the TDGA-treated perovskite layer into the Spiro-OMeTAD-based hole transport layer.
In this work, we have introduced an organic small molecule, TDCA, into perovskite films to study its effect on the PSC performance and hot carrier cooling process. It was found that TDGA as an additive can effectively reduce the perovskite defect density owing to the formation of the chemical interaction between TDGA and the perovskite. The fs-TA results revealed that the incorporation of TDGA into the perovskite layer can result in a slower HC cooling process and lower energy loss rate of HCs compared with the control films without TDGA. The slow HC cooling helps the HC extraction by the carrier transport layers, thus reducing the charge carrier recombination in devices. Consequently, the TDGA-doped PSCs achieve an enhanced PCE of 21.62% compared with the control device (19.92%). These findings could provide some insights for understanding hot carrier cooling dynamics in lead halide perovskites, which is very critical for further improving the PSC efficiency.
Y. Hua thanks the National Natural Science Foundation of China (22065038), High-Level Talents Introduction in Yunnan Province (C619300A010), the Fund for Excellent Young Scholars of Yunnan (202001AW070008), and Spring City Plan: the High-level Talent Promotion and Training Project of Kunming (2022SCP005) for financial support. R. J. Zhao acknowledges support from the Postdoctoral Foundation of Department of Human Resources and Social Security of Yunnan Province (No. C615300504046) and Postdoctoral Research Foundation of Yunnan University (No. W8223004). L. Xie thanks the National Natural Science Foundation of China (22209144), and the Project of Natural Science Foundation of Yunnan (202101AU070034 and 202101AT070337). The authors thank the Electron Microscopy Center, the Advanced Analysis and Measurement Center of Yunnan University for the sample testing and service.
Conflicts of interest
There are no conflicts to declare.References
- K. A. Bush, A. F. Palmstrom, Z. J. Yu, M. Boccard, R. Cheacharoen, J. P. Mailoa, D. P. McMeekin, R. L. Z. Hoye, C. D. Bailie, T. Leijtens, I. M. Peters, M. C. Minichetti, N. Rolston, R. Prasanna, S. Sofia, D. Harwood, W. Ma, F. Moghadam, H. J. Snaith, T. Buonassisi, Z. C. Holman, S. F. Bent and M. D. McGehee, Nat. Energy, 2017, 2, 17009 CrossRef CAS.
- S. Bai, P. Da, C. Li, Z. Wang, Z. Yuan, F. Fu, M. Kawecki, X. Liu, N. Sakai, J. T.-W. Wang, S. Huettner, S. Buecheler, M. Fahlman, F. Gao and H. J. Snaith, Nature, 2019, 571, 245 CrossRef CAS PubMed.
- Q. Jiang, J. Tong, Y. Xian, R. A. Kerner, S. P. Dunfield, C. Xiao, R. A. Scheidt, D. Kuciauskas, X. Wang, M. P. Hautzinger, R. Tirawat, M. C. Beard, D. P. Fenning, J. J. Berry, B. W. Larson, Y. Yan and K. Zhu, Nature, 2022, 611, 278 CrossRef CAS.
- Z. Li, B. Li, X. Wu, S. A. Sheppard, S. Zhang, D. Gao, N. J. Long and Z. Zhu, Science, 2022, 376, 416 CrossRef CAS PubMed.
- X. Zheng, B. Chen, J. Dai, Y. Fang, Y. Bai, Y. Lin, H. Wei, X. C. Zeng and J. Huang, Nat. Energy, 2017, 2, 17102 CrossRef CAS.
- Best Research Cell Efficiency Chart, https://www.nrel.gov/pv/cell-efficiency.html.
- R. X. Lin, J. Xu, M. Y. Wei, Y. R. Wang, Z. Y. Qin, Z. Liu, J. L. Wu, K. Xiao, B. Chen, S. M. Park, G. Chen, H. R. Atapattu, K. R. Graham, J. Xu, J. Zhu, L. D. Li, C. F. Zhang, E. H. Sargent and H. R. Tan, Nature, 2022, 603, 73 CrossRef CAS PubMed.
- N. X. Li, S. X. Tao, Y. H. Chen, X. X. Niu, C. K. Onwudinanti, C. Hu, Z. W. Qiu, Z. Q. Xu, G. H. J. Zheng, L. G. Wang, Y. Zhang, L. Li, H. F. Liu, Y. Z. Lun, J. W. Hong, X. Y. Wang, Y. Q. Liu, H. P. Xie, Y. L. Gao, Y. Bai, S. H. Yang, G. Brocks, Q. Chen and H. P. Zhou, Nat Energy, 2019, 4, 408 CrossRef CAS.
- R. M. Zhao, L. Xie, R. S. Zhuang, T. Wu, R. J. Zhao, L. Q. Wang, L. C. Sun and Y. Hua, ACS Energy Lett., 2021, 6, 4209 CrossRef CAS.
- Q. Fu, H. Liu, S. T. Li, T. Zhou, M. Q. Chen, Y. Yang, J. Wang, R. Wang, Y. S. Chen and Y. S. Liu, Angew. Chem., Int. Ed., 2022, 61, e202210356 CrossRef CAS.
- T. Q. Niu, W. Y. Zhu, Y. H. Zhang, Q. F. Xue, X. C. Jiao, Z. J. Wang, Y. M. Xie, P. Li, R. F. Chen, F. Huang, Y. Li, H. L. Yip and Y. Cao, Joule, 2021, 5, 249 CrossRef CAS.
- M. Kim, I. Choi, S. J. Choi, J. W. Song, S. I. Mo, J. H. An, Y. Jo, S. Ahn, S. K. Ahn, G. H. Kim and D. S. Kim, Joule, 2021, 5, 659 CrossRef CAS.
- X. G. Sun, Z. L. Zhu and Z. A. Li, Front. Optoelectron., 2022, 15, 46 CrossRef.
- K. Wang, L. Y. Zheng, Y. C. Hou, A. Nozariasbmarz, B. Poudel, J. Yoon, T. Ye, D. Yang, A. V. Pogrebnyakov, V. Gopalan and S. Priya, Joule, 2022, 6, 756 CrossRef CAS.
- J. H. Fu, S. Ramesh, J. W. M. Lim and T. C. Sum, Chem. Rev., 2023, 13, 8154 CrossRef PubMed.
- T. Zhu, Y. Wan and L. B. Huang, Acc. Chem. Res., 2017, 50, 1725 CrossRef CAS.
- P. P. Joshi, S. F. Maehrlein and X. Y. Zhu, Adv. Mater., 2019, 31, 1803054 CrossRef CAS.
- R. T. Ross and A. J. Nozik, J. Appl. Phys., 1982, 53, 3813 CrossRef CAS.
- W. Shockley and H. J. Queisser, J. Appl. Phys., 1961, 32, 510 CrossRef CAS.
- T. C. Sum, N. Mathews, G. C. Xing, S. S. Lim, W. K. Chong, D. Giovanni and H. A. Dewi, Acc. Chem. Res., 2016, 49, 294 CrossRef CAS.
- G. Ghosh, K. Marjit, S. Ghosh, A. Ghosh, R. Ahammed, A. D. Sarkar and A. Patra, J. Phys. Chem. C, 2021, 125, 5859 CrossRef CAS.
- C. F. Qiu, J. X. Song, Y. Wu, X. X. Yin, L. Hu, Z. Su, Y. Z. Jin, W. T. Wang, H. Wang and Z. F. Li, Chem. Commun., 2023, 59, 6414 RSC.
- R. Y. Wang, M. H. Li, Z. W. Ma, Z. W. He, Y. M. Dong, Y. L. Zhang, Z. Y. Xu, G. F. Su and Z. Tan, Chem. Commun., 2023, 59, 6255 RSC.
- M. F. Zhu, Y. R. Xia, L. N. Qin, K. Q. Zhang, J. C. Liang, C. Zhao, D. C. Hong, M. H. Jiang, X. M. Song, J. Wei, P. B. Zhang, Y. X. Tian and Z. Jin, Nano Res., 2023, 16, 6849 CrossRef CAS.
- Y. R. Xia, M. F. Zhu, L. N. Qin, C. Zhao, D. C. Hong, Y. X. Tian, W. S. Yan and Z. Jin, Energy Mater., 2023, 3, 300004 CrossRef CAS.
- Y. R. Xia, C. Zhao, P. Y. Zhao, L. Y. Mao, Y. C. Ding, D. C. Hong, Y. X. Tian, W. S. Yan and Z. Jin, J. Power Sources, 2021, 494, 229781 CrossRef CAS.
- L. Chao, Y. Xia, X. Duan, Y. Wang, C. Ran, T. T. Nie, L. Gu, D. L. Li, J. F. Hu, X. Y. Gao, J. Zhang and Y. H. Chen, Joule, 2022, 6, 2203 CrossRef CAS.
- R. Wang, A. Altujjar, N. Zibouche, X. L. Wang, B. F. Spencer, Z. Y. Jia, A. G. Thomas, M. Z. Mokhtar, R. S. Cai, S. J. Haigh, J. M. Saunders, M. S. Islam and B. R. Saunders, Energy Environ. Sci., 2023, 16, 2646 RSC.
- Z. F. Wu, S. R. Raga, E. J. Juarez-Perez, X. Y. Yao, Y. Jiang, L. K. Ono, Z. J. Ning, H. Tian and Y. B. Qi, Adv. Mater., 2018, 30, 1703670 CrossRef PubMed.
- D. Q. Bi, C. Y. Yi, J. S. Luo, J. D. Décoppet, F. Zhang, S. M. Zakeeruddin, X. Li, A. Hagfeldt and M. Grätzel, Nat. Energy, 2016, 1, 16142 CrossRef CAS.
- J. K. Zhang, Z. P. Li, F. J. Guo, H. J. Jiang, W. J. Yan, C. Peng, R. X. Liu, L. Wang, H. T. Gao, S. P. Pang and Z. M. Zhou, Angew. Chem., Int. Ed., 2023, e202305221 Search PubMed.
- T. Wu, R. j Zhao, D. L. Jia, L. Q. Wang, X. L. Zhang, L. C. Sun and Y. Hua, J. Energy Chem., 2023, 77, 514 CrossRef CAS.
- R. S. Zhuang, L. Q. Wang, J. M. Qiu, L. Xie, X. H. Miao, X. L. Zhang and Y. Hua, Chem. Eng. J., 2023, 463, 142449 CrossRef CAS.
- Q. Lai, R. S. Zhuang, K. Zhang, T. Wu, L. Xie, R. J. Zhao, L. Yang, Y. Wang and Y. Hua, Angew. Chem., Int. Ed., 2023, 62, e202305670 CrossRef PubMed.
- X. P. Duan, X. Li, L. C. Tan, Z. Q. Huang, J. Yang, G. L. Liu, Z. J. Lin and Y. W. Chen, Adv. Mater., 2020, 32, 2000617 CrossRef CAS.
- C. Z. Xu, Z. Zhang, S. C. Zhang, H. N. Si, S. F. Ma, W. Q. Fan, Z. Z. Xiong, Q. L. Liao, A. Sattar, Z. Kang and Y. Zhang, Adv. Funct. Mater., 2021, 31, 2009425 CrossRef CAS.
- M. Li, J. Fu, Q. Xu and T. C. Sum, Adv. Mater., 2019, 31, 1802486 CrossRef CAS.
- I. Dursun, P. Maity, J. Yin, B. Turedi, A. A. Zhumekenov, K. Jae Lee, O. F. Mohammed and O. M. Bakr, Adv. Energy Mater., 2019, 9, 1900084 CrossRef.
- J. H. Fu, Q. Xu, G. F. Han, B. Wu, C. H. A. Huan, M. L. Leek and Tz. C. Sum, Nat. Commun., 2017, 8, 1300 CrossRef PubMed.
- Q. Wei, J. Yin, O. M. Bakr, Z. Wang, C. H. Wang, O. F. Mohammed, M. J. Li and G. C. Xing, Angew. Chem., Int. Ed., 2021, 133, 11052 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc04001a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |