Application of biomaterials in the treatment of intracerebral hemorrhage
Wei
Wang
ab,
Xiaowen
Liu
ab,
Yupeng
Wang
 *b,
Dongfang
Zhou
*b,
Dongfang
Zhou
 *abc and
Lukui
Chen
*abc and
Lukui
Chen
 *a
*a
aDepartment of Neurosurgery, Southern Medical University Hospital of Integrated Traditional Chinese and Western Medicine, Southern Medical University, Guangzhou 510310, P. R. China. E-mail: dfzhou@smu.edu.cn; neuro_clk@hotmail.com
bNMPA Key Laboratory for Research and Evaluation of Drug Metabolism & Guangdong Provincial Key Laboratory of New Drug Screening & Guangdong-Hongkong-Macao Joint Laboratory for New Drug Screening, School of Pharmaceutical Sciences, Southern Medical University, Guangzhou, 510515, P. R. China. E-mail: wangyupeng5@i.smu.edu.cn
cKey Laboratory of Mental Health of the Ministry of Education, Southern Medical University, Guangzhou, 510515, P. R. China
First published on 3rd July 2024
Abstract
Although the current surgical hematoma removal treatment saves patients’ lives in critical moments of intracerebral hemorrhage (ICH), the lethality and disability rates of ICH are still very high. Due to the individual differences of patients, postoperative functional improvement is still to be confirmed, and the existing drug treatment has limited benefits for ICH. Recent advances in biomaterials may provide new ideas for the therapy of ICH. This review first briefly describes the pathogenic mechanisms of ICH, including primary and secondary injuries such as inflammation and intracerebral edema, and briefly describes the existing therapeutic approaches and their limitations. Secondly, existing nanomaterials and hydrogels for ICH, including exosomes, liposomes, and polymer nanomaterials, are also described. In addition, the potential challenges and application prospects of these biomaterials for clinical translation in ICH treatment are discussed.
1. Introduction
Stroke remains a leading cause of mortality, second only to ischemic heart disease, and is a major contributor to disability worldwide. Ischemic strokes represent 62.4% of all strokes, followed by intracerebral hemorrhage (ICH) at 27.9%, and subarachnoid hemorrhage at 9.7%.1 During the initial month, the mortality rate can reach 43%–51%.2 Survivors frequently experience lasting effects, with the nature and intensity of neurological impairments dependent on the specific location of the injury. Hemiparesis emerges as the most common deficit, often accompanied by speech difficulties, cognitive impairments, emotional disturbances, challenges in daily activities, as well as sensations of pain and numbness.The rupture of cerebral blood vessels allows blood to seep into the brain parenchyma, causing tissue damage and neurological impairments. Brain damage from ICH can be categorized into primary and secondary injuries.3 Primary injury encompasses the hematoma's mass effect, ischemia, hypoxia, metabolic disruptions, elevated intracranial pressure, brain herniation, and tissue necrosis, along with microglial activation and blood–brain barrier (BBB) disruption due to substances released from the bloodstream and injury site.4,5 Secondary injury involves perihematoma edema, inflammation, and the breakdown of blood components. Perihematoma edema progresses through three stages: the first marked by increased osmotic pressure and cytotoxic edema; the second by heightened BBB permeability and vasogenic edema; and the third by oxidative stress induced by iron, a by-product of hemoglobin breakdown, further exacerbating vasogenic edema.6 The inflammatory response begins soon after ICH, with microglia and astrocytes activating and releasing inflammatory mediators that impact the BBB and neurons.7,8 The complementary system also participates in this response, forming complexes that compromise cell membranes.9 The lysis of erythrocytes releases hemoglobin and iron, leading to oxidative damage that targets DNA, proteins, and lipid membranes, ultimately disrupting cellular functions.10
In the treatment of ICH, the current basic clinical treatment involves pharmacologic and surgical therapies. Pharmacologic therapy focuses on blood pressure control, hemostatic drug use, anticoagulation reversal, and intracranial pressure control. It is usually aimed at controlling the symptoms and complications of ICH rather than directly addressing the bleeding itself, so the effectiveness of pharmacotherapy is generally limited.5 Surgical treatments encompass traditional open hematoma removal and innovative minimally invasive surgical techniques. These approaches aim to decrease the mass effect by extracting the hematoma, reducing intracranial pressure, averting herniation-pressure syndrome, and halting secondary injuries triggered by the hematoma. Surgery is sometimes necessary for patients with large hematomas that produce a mass effect, resulting in a midline shift, or impaired consciousness, as surgical treatment can be life-saving.11,12
However, conservative pharmacologic and surgical treatments are currently limited to targeting the primary injury of ICH. Owing to the selective permeability of the BBB, the systemic administration of most pharmacological agents fails to ensure their efficacious delivery to targeted lesion sites within the central nervous system. The optimal timing and indications for surgery are unclear. There are individual differences and the condition is complex. While surgery can save lives in emergencies by reducing the mass effect and preventing fatal complications like cerebral herniation, its benefits in terms of improving functional outcomes have not yet been demonstrated.5 Moreover, surgical treatment is risky and invasive, and may also lead to complications such as bleeding, infection, and cerebral edema. Overall, despite the success of existing treatments in controlling the pathologic progression of ICH, they are limited in improving the long-term functional prognosis of patients.
Therefore, to enhance the long-term functional prognosis of ICH, new therapeutic strategies need to be developed. These strategies should target various pathophysiological mechanisms, such as secondary brain injury caused by hematotoxicity, including iron overload and neuroinflammation post-ICH. In addition, researchers should focus on creating innovative drug delivery systems capable of effectively transporting drugs to the lesion site to achieve therapeutic effects.13–15
With the development of science and technology, biomaterials are gradually entering people's vision. Developed nanotechnology has also brought significant impetus to the pharmaceutical and medical fields, bringing new diagnosis or treatment methods for many diseases, such as cancer,16–18 acquired immune deficiency syndrome (AIDS),19 tuberculosis,20 osteoarthritis21,22 and infection.23,24 As mentioned earlier, due to the lack of special and effective treatment methods for ICH, it forces researchers to seek new treatment methods. In addition to relying on surgery to reduce the mortality rate due to ICH, many studies are currently seeking breakthroughs in secondary injury and functional recovery after ICH. Hemostatic drugs, iron chelators, neuroprotective agents, and other therapies are gradually being tested in clinical practice.25–30 However, traditional drug delivery methods face challenges due to the unique anatomical structure of the nervous system.31 These challenges include short half-life, low bioavailability, inadequate targeting, and difficulty in crossing the BBB. As a result, drug distribution in the brain is significantly limited, hindering the achievement of desired therapeutic effects. Moreover, systemic administration may also bring unnecessary adverse reactions. The rapid development of biomaterials and nanotechnology and the great advances they have brought to the field of medicine have led us to consider their great potential in ICH treatment. Compared with traditional drug delivery methods, nanomaterials-based delivery systems can help drugs penetrate the BBB, improve drug targeting ability, and also reduce side effects caused by drug residues in other organs. The hydrogel-based delivery system can precisely inject the drug into the lesion site, reducing the side effects of systemic administration, while the hydrogel controls the release of the drug, maintaining and prolonging the efficacy. In the next section, we summarize the nanomaterials and hydrogels commonly used for the treatment of ICH, and finally discuss and look forward to the potential, prospects and challenges of these materials for the treatment of ICH (Fig. 1).
 | ||
| Fig. 1 Application of biomaterials in the treatment of intracerebral hemorrhage. The figure was edited using Figdraw. | ||
2. Application of nanomaterials in the treatment of ICH
The application of nanomaterials including liposomes, exosomes, and diverse nanoparticles in ICH treatment offers several notable advantages. Firstly, their diminutive size enables efficient drug transport across the BBB, thereby optimizing drug bioavailability and therapeutic efficacy, and minimizing adverse effects and toxicity. Secondly, certain biologically derived nanomaterials like exosomes demonstrate reduced immunogenicity and heightened biocompatibility compared to synthetic counterparts. Lastly, nanomaterials can be tailored to co-deliver multiple drugs, allowing for the design of tailored nanomedicines targeting diverse pathogenic mechanisms underlying ICH.32 In addition, nanomaterials can also serve as biological probes for early diagnosis, quantitative monitoring, disease assessment, and therapeutic evaluation of ICH through magnetic resonance imaging.332.1 Lipid nanoparticles
Lipid nanoparticles, have been employed in the management of various diseases, including oncological, anti-inflammatory conditions, and as prophylactic agents in vaccines against infectious diseases, demonstrating their maturation in the field.34 Research has demonstrated that encapsulating therapeutic agents within liposomes can protect them from rapid degradation or inactivation within the bloodstream, thereby substantially improving the pharmacokinetics of the drugs.35In ICH, interleukin-4 (IL-4) is considered a key anti-inflammatory cytokine. It promotes polarization of M2-type microglia, and IL-4 also reduces the inflammatory response by driving Th2 cell differentiation and maintaining M2 polarization, suppressing M1 and Th1 phenotypes.15 Xu et al. designed a lipid nanoparticle to encapsulate IL-4, which was administered intranasally for the treatment of a mouse model of ICH. This approach effectively facilitated hematoma resolution, mitigated brain injury, and enhanced long-term functional recovery. Furthermore, it underscored the significance of the IL-4/STAT6/ST2 signaling pathway in the processes of hematoma resolution and functional recuperation post-ICH.36 Interleukin-10 (IL-10) plays a neuroprotective role in ICH and intracerebral ischemia by triggering phagocytosis by microglia/macrophages to accelerate hematoma clearance and anti-inflammatory polarization, respectively,37,38 but its short half-life39,40 and limited ability to penetrate the BBB may make its efficacy to be less than optimal, and these shortcomings can be ameliorated precisely with the help of drug carriers. It has been demonstrated that phosphatidylserine (PS) as a hallmark of cell apoptosis and necroptosis41 is one of the most prominent “eat-me” signals for apoptotic cells to be recognized by phagocytes.42 Therefore, Toita et al. prepared IL-10-conjugated PSL (PS-containing liposomes-IL-10) for the treatment of HFD-induced obese mice and found that it did have macrophage-targeting ability and observed significant anti-obesity and anti-inflammatory effects.43 Inspired by the above, Han et al. used PSL-IL10 for the first time in the treatment of ICH. They injected the prepared PSL-IL10 intranasally into ICH mice, and the results showed that PSL-IL10 not only improved the efficiency of IL-10 delivery to the hematoma area, accelerated the hematoma clearance, and reduced the size of the lesion, but also improved the neurological function, which effectively improved the prognosis of ICH. The important roles of STAT3 and CD36 in the regulation of microglia/macrophage phagocytosis by PSL-IL10 were also verified.44 Importantly, both drug delivery methods employed transnasal administration, a modality that has garnered research interest for its capacity to circumvent the BBB and enhance drug delivery efficiency.45 Their investigation reaffirms the substantial promise of intranasal delivery in augmenting drug delivery efficacy for ICH. Notably, PSL-IL10 demonstrated effectiveness at lower doses relative to IL-4 lipid nanoparticles, an observation that may be ascribed to PSL's propensity for targeting microglia/macrophages.
The liposomal phospholipid layer structure also enables the stretcher loading and delivery of hydrophobic drugs. Instead of using lipid nanoparticles alone, inspired by biomimetic nanomaterials such as natural cell membranes, Fan et al. synthesized a pH-responsive lipid and neutrophil membrane hybrid nanoparticle to encapsulate hydrophobic desmosterol (LXR agonist) and GW280264X (ADAM17 inhibitor) (Fig. 2). The nanoparticles were able to target bleeding sites in the brain and release the drugs under acidic condition. It was shown that this co-delivery method promotes erythrocyte phagocytosis, modulates the inflammatory microenvironment, and improves neurological function. The excellent brain targeting ability of the nanoparticles was confirmed by in vivo imaging analysis, which improved the targeting efficiency by nearly five times over liposomes alone. This suggests that combining natural cell membranes with nanomaterials is a novel and reliable method for drug delivery.46
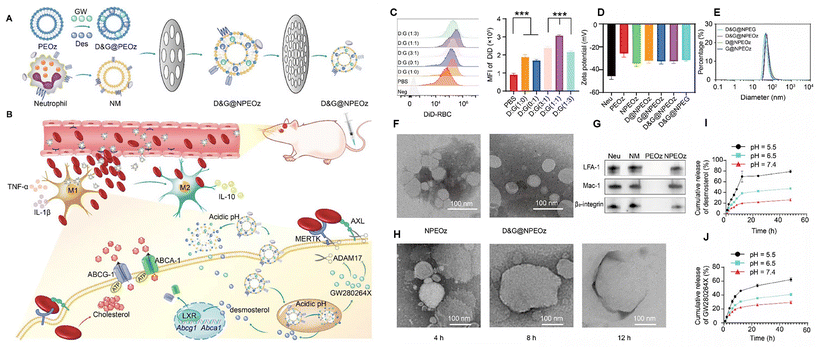 | ||
| Fig. 2 Illustration of D&G@NPEOz therapy for ICH and the characterization of D&G@NPEOz. (A) Schematic representation of the D&G@NPEOz synthesis process, co-extruding drug-carrying pH-responsive liposomes with neutrophil membranes to obtain D&G@NPEOz. (B) Visual depiction of the D&G@NPEOz therapy for erythrophagocytosis and neurological functional recovery following ICH. The camouflage of the neutrophil membrane effectively transports nanoparticles to the site of injury through brain microvascular endothelial cells. D&G@NPEOz releases desmosterol and GW280264X in response to the acidic environment, facilitating erythrophagocytosis by microglia/macrophages via activation of LXR and inhibition of ADAM17. This process promotes the transition of microglia/macrophages from the M1 to M2 phenotype, suppresses inflammation, and consequently triggers neurological recovery post-ICH. (C) Representative flow cytometry histogram and quantity analysis of the mean fluorescence intensity (MFI) of engulfed labeled-erythrocytes in BV2 cells with different radios of desmosterol and GW280264X treatment followed by 2 h incubation with DiD-labeled RBCs (n = 3). (D) Zeta potential of NPEOz, D&G@NPEG, D&G@NPEOz, D@NPEOz and G@NPEOz (n = 3). (E) Hydrodynamic diameter distribution of D&G@NPEG, D&G@NPEOz, D@NPEOz and G@NPEOz. (F) TEM images of NPEOz and D&G@NPEOz (scale bar = 100 nm). (G) Representative western blot showing characteristic protein expression from Neu (neutrophil), NM (neutrophil membrane), PEOz and NPEOz. (H) The morphological changes of TEM images of D&G@NPEOz with time while incubated in PBS at pH 5.5 (scale bar = 100 nm). (I and J) Desmosterol and GW280264X release characteristics of D&W@NPEOz in PBS at pH = 5.5, 6.5 or 7.4 during 48 h incubation (n = 3). Reproduced from ref. 46 with permission from Ivyspring International Publisher, copyright 2024. | ||
Lipid nanoparticles have been extensively studied in the treatment of ischemic stroke due to their excellent drug loading capacity, drug protection and high biocompatibility, and the possibility of modifying specific ligands on their surface to increase their ability to penetrate the BBB.47 However, its potential for ICH treatment has not yet been exploited completely, which is a relatively vacant area. The studies mentioned above are basically based on delivering interleukins or hydrophobic drugs, but in fact, its special structure can carry both hydrophilic and hydrophobic drugs, so further attempts to carry other therapeutic drugs can be made in the future (Table 1).
| Material forms | Active components | Animal model | Method of administration | Ref. |
|---|---|---|---|---|
| Liposomes | IL-10 | Collagenase, mouse | Transnasal drug delivery | 44 |
| Lipid nanoparticles | IL-4 | Autologous blood, mouse/collagenase, mouse | Transnasal drug delivery | 36 |
| Neutrophil cell membrane hybrid liposomes | Desmosterol/GW280264X | Autologous blood, mouse | Intravenous injection (i.v.) | 46 |
2.2 Exosomes
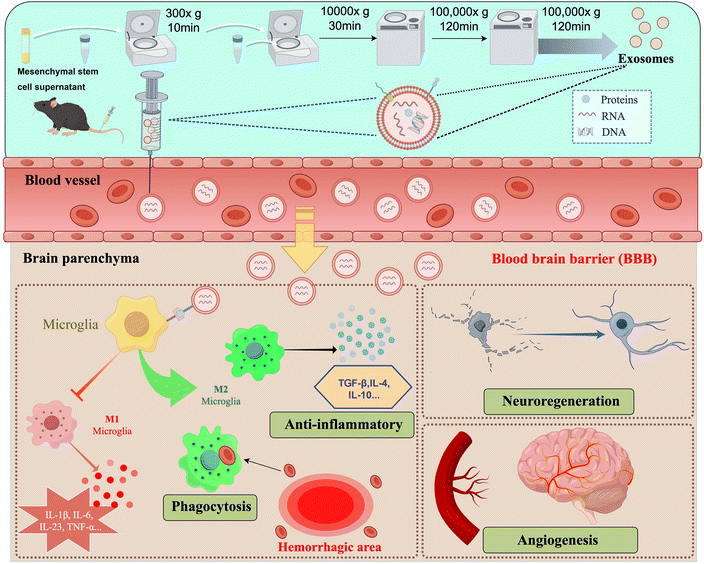
Exosomes are extracellular vesicles with a diameter of 30–150 nm and are essentially endogenous lipid nanoparticles.48 As a means of intercellular communication and signaling, most cells in the human body can secrete exosomes, which are widely distributed in a variety of bodily fluids.49 Studies have shown that exosomes are able to penetrate the BBB in both directions, i.e., from the brain to the blood system and from the blood to the central nervous system, which gives exosomes a natural advantage in the treatment of neurological disorders, but the exact mechanism is not clear.50 Exosomes exert their effects by releasing their contents into the cytoplasm of recipient cells. Exosomal cargoes can be composed of many different molecules, such as nucleic acids, pro- or anti-inflammatory cytokines, enzymes, and various other proteins.51 For example, mesenchymal stem cell (MSC)-derived exosomes contain cytokines such as vascular endothelial growth factor (VEGF), TGF-β, IL-6, and IL-10, which contribute to angiogenesis and immunomodulation. Compared to MSCs, exosomes have lower immunogenicity and higher biosafety and compatibility. The administration of MSC-derived exosomes (MSC-Exosomes) has been shown to enhance neurogenesis and angiogenesis, improve spatial learning ability and recovery of sensorimotor function, and attenuate neuroinflammation after brain injury in the middle cerebral artery occlusion (MCAO) rat model versus the traumatic brain injury (TBI) rat model.52,53Li et al. discovered that suppressing exosome secretion exacerbated neuroinflammation and cerebral injury in mice with ICH. Conversely, exosomes harvested from the brain tissue of donor mice afflicted with ICH ameliorated brain damage in recipient mice. These findings suggest that exosomes could have a beneficial impact on the prognosis of ICH.54 Han et al.'s experiments also demonstrated that MSC-derived exosomes could effectively improve neurological recovery, possibly by promoting endogenous angiogenesis and neurogenesis in rats after ICH (Fig. 3).55
Shen et al. injected MSC-derived exosomes enriched with miR-133b into ICH rats, and the apoptotic and degenerated neurons in rat brain tissues were significantly ameliorated after ICH. The results showed that exosomes provide miR-133b-mediated anti-apoptotic effects, which were involved in attenuating the brain damage. This therapeutic effect may be mediated by up-regulation of miR-133b in the brain tissue to inhibit RhoA and activate the ERK1/2/CREB pathway.56 Meanwhile, Sun et al. found that MSC-derived exosomes containing miR-150-3p could affect ICH injury by modulating the TRAF6/NF-κB axis, gut microbiota, and metabolism to improve neurological function, decrease brain water content, and reduce the expression of inflammatory factors.57 In contrast, Zhang et al. prepared miR-21-overexpressing MSCs and directly injected the MSCs into the brains of ICH rats for treatment, and it was found that the therapeutic effects were actually mediated through exosomes derived from miR-21-overexpressing MSCs.58 Furthermore, Duan et al. observed that exosomes from bone marrow-derived mesenchymal stem cells (BMSCs), enriched with miR-146a-5p, were capable of mitigating neuronal apoptosis. This effect was achieved by downregulating the expression of IRAK1 and NFAT5, which concurrently inhibited the inflammatory response and microglial M1 polarization post-intracerebral hemorrhage in rats.59 Building on the research of Duan et al., Ding et al. postulated that extracellular vesicles (EVs) derived from bone marrow stem cells (BMSC-EV) might play a role in mitigating neuroinflammation following diabetic ICH. They prepared BMSC-EVs with an average size of approximately 110 nm and administered them to db/db-ICH rats. BMSC-EVs, carrying miR-183-5p, significantly improved the behavioral outcomes and reduced neuroinflammation in diabetic ICH-afflicted rats. This suggests a potential new mechanism by which BMSC-EVs could modulate neuroinflammation post-diabetic ICH.60
In addition to miRNA-based studies, Tang et al. found that the exosomal tumor necrosis factor-stimulated gene-6 (TSG-6) secreted by BMSCs also has anti-inflammatory effect, which can regulate activated astrocytes and ameliorate BBB injury after ICH by inhibiting the NF-κB signaling pathway.61
Unlike the delivery of miRNAs or other therapeutic factors using exosomes alone, Gao et al. obtained modified exosomal SIRPα variants by gene editing of MSCs and used them for the treatment of ICH mice. The results showed that the clearance of hematomas was accelerated after SIRPα-v exosome treatment and by ameliorating the damage to white matter, thereby improving long-term neurological dysfunction. It was also verified that SIRPα-v exosomes polarize microglia/macrophages around hematomas toward an anti-inflammatory M2 phenotype by recruiting regulatory T cells (Tregs) to promote the hematoma. Notably, in this experiment, they utilized a high-affinity SIRPα variant as a CD47 antagonist, which was engineered to interfere with CD47-SIRPα signaling between erythrocytes and microglia/macrophages, thus promoting phagocytosis of erythrocytes by macrophages and accelerating hematoma clearance. Their study may lead to a new way of thinking about exosome therapy, in which exosomes can not only deliver miRNAs or other cytokines, but also be designed to deliver specific antibodies or drugs to treat ICH.62
In addition to MSCs, gene-edited microglia-derived exosomes can also be used to treat ICH. Guo et al. transfected microglia and prepared miRNA-124-enriched microglia-derived exosomes, which were treated by intranasal administration to attenuate neurological deficits, brain edema, BBB leakage and cell death, and reduced inflammatory cytokine levels in the brain after ICH, suggesting that it can modulate neuroinflammation to mitigate the outcome of ICH.63 In summary, stem cell-derived exosomes hold promise as a novel cell-free therapy for ICH.
Interestingly, plasma-derived exosomes can also improve functional recovery from ICH by attenuating iron death. Yang et al. extracted exosomes from the plasma of young healthy humans, which were firstly utilized for the treatment of ICH mice by ventricular injection. The results showed that exosomes from young human plasma reduced the area of brain damage and promoted neurological recovery in ICH mice. In contrast, exosomes from plasma sources of the elderly did not have similar therapeutic effects. An in-depth study revealed that miR-25-3p expression was more abundant in plasma exosomes from young people, which could mediate neuroprotective effects by regulating the P53/SLC7A11/GPX4 signaling pathway to attenuate iron death in ICH.64
In summary, exosomes hold promise for ICH therapy, given their capacity to traverse the BBB and their proven safety in preclinical models. They are envisioned as potential agents for cell-free therapy and as vehicles for drug delivery. Nonetheless, the path to clinical application is fraught with challenges, including the imperative to delineate the heterogeneity of exosomes secreted by various cell types, which differ in their contents and resultant effects. For instance, inflammatory cells may release exosomes that aggravate pathology, such as when activated microglia transmit miR-383-3p to neurons, precipitating neuronal death by suppressing the expression of activating transcription factor 4 (ATF4).65 Consequently, an exhaustive elucidation of the origins and characteristics of exosomes is vital prior to their clinical deployment, encompassing the influence of disparate physicochemical conditions on the generation of exosomes and their molecular payload. This understanding is indispensable for the judicious employment of exosomes in disease management (Table 2).
| Material forms | Sources of exosomes/EVs | Active components | Animal model | Method of administration | Ref. |
|---|---|---|---|---|---|
| Exosomes | BMSCs | miR-150-3p | Collagenase, mouse | Intravenous injection (i.v.) | 57 |
| Exosomes | Microglia | miRNA-124 | Collagenase, mouse | Transnasal drug delivery | 60 |
| Exosomes | Young human plasma | miR-25-3p | Autologous blood, mouse | In situ injection | 64 |
| Exosomes | BMSCs | SIRPα variants | Autologous blood, mouse | Intravenous injection (i.v.) | 62 |
| Exosomes | BMSCs | Tumor Necrosis Factor-stimulated Gene-6 (TSG-6) | Collagenase, rat | Intravenous injection (i.v.) | 61 |
| Extracellular vesicles | BMSCs | miR-183-5p | Collagenase, db-rat | Intravenous injection (i.v.) | 63 |
| Exosomes | BMSCs | miRNA-146a-5p | Collagenase, rat | Intravenous injection (i.v.) | 59 |
| BMSCs | — | miR-21 | Collagenase, rat | In situ injection | 58 |
| Exosomes | BMSCs | — | Autologous blood, rat | Intravenous injection (i.v.) | 55 |
| Exosomes | BMSCs | miR-133b | Autologous blood, rat | Intravenous injection (i.v.) | 56 |
2.3 Polymer nanoparticles
Polymer nanoparticles (polymer NPs) exhibit excellent biocompatibility and low toxicity, enhancing their safety profile for clinical applications. Notably, certain polymer NPs can be engineered to be responsive to external stimuli, such as changes in pH, temperature, or light exposure. Such responsiveness facilitates the controlled release of drugs under specific physiological conditions, thereby heightening the precision of treatment. The targeted release mechanism ensures that therapeutic agents are deployed precisely when needed, augmenting treatment efficacy while concurrently minimizing undesirable side effects. These characteristics position polymer NPs with promising application prospects in drug delivery and therapy. In the context of ICH, polymer NPs serve as carriers for drugs that cannot traverse the BBB, enabling their delivery to the brain. This mechanism allows for therapeutic actions like nerve protection, modulation of the inflammatory response, and reduction of hematoma volume, ultimately leading to effective ICH treatment.Among natural polymers, chitosan nanoparticles (CS NPs) are often used as drug carriers because of their high functionalization possibilities, biocompatibility, and biodegradability.66 Nicardipine hydrochloride is a calcium channel blocker with an anti-neuroinflammatory neuroprotective effect. HAMC is a rapid gel injection material made from a mixture of hyaluronan (HA) and methyl cellulose (MC). By using CS NPs as carriers for nicardipine and combining them with HAMC gel, this composite can cross the BBB by passage. This occurs after transnasal administration, allowing nicardipine to exert its therapeutic effects more effectively. The generation of brain edema and apoptosis of neuronal cells were significantly reduced in a mouse model of ICH.67
Synthetic polymer nanoparticles can carry existing drugs or multiple molecules, and with further modifications, they enable some drugs to exert their efficacy across the BBB. For example, curcumin (Cur) and resveratrol (Res) possess antioxidant, anti-inflammatory, and neuroprotective activities. However, their therapeutic effects are limited by physiological barriers, including poor aqueous solubility, low oral bioavailability, and difficulty in effectively crossing the BBB. Cur-NPs and Res-NPs, obtained by encapsulating Cur and Res in the polymers PEG-PTMC and MPEG-PLGA, respectively, promoted the distribution of these drugs in plasma and facilitated their crossing of the BBB. Oral administration of Cur-NPs significantly improved symptoms, reduced hematoma volume, attenuated neurological damage, improved behavior, and decreased iron overdeposition in ICH mice.68,69 In addition, poly(butylcyanoacrylate) (PBCA) NPs with relatively low toxicity are one of the fastest biodegradable synthetic polymers, which are effective carriers for the delivery of macromolecules to the injured brain.70 BCA NPs facilitated the intracellular transport of plasmid neurotrophic factor-3 (NT-3), which contains a hormone response element (HRE) driven by the cytomegalovirus (CMV) promoter, aiding in the differentiation of induced pluripotent stem cells (iPSCs). Treatment with PBCA NP/cmvNT-3-HRE complexes increased the ability of iPSCs to express NT-3, TrkC and MAP-2. In addition, PBCA NPs shielded cmvNT-3-HRE from degradation by EcoRI/PstI and DNase I in vitro, and augmented the overall BBB delivery rate in vivo. The NPs also suppressed the expression of apoptosis-inducing factor, cleaved caspase-3, and DNA fragmentation, thereby diminishing cell death after ICH in vivo.71 Platelet membrane modified polydopamine (Menp@PLT) nanoparticles can precisely target intracranial hematoma sites, scavenging ROS, and improve the neuroinflammatory milieu of ICH, attributed to their superior anti-ROS properties.72 In addition, a metal ion-responsive nanocarrier based on phosphonated cupric[4] aryl derivatives has been developed. It delivers taurine to the ICH injury site and enhances drug release in an iron environment, contributing to neuroprotection, ROS generation inhibition, apoptosis reduction, inflammation mitigation, and partial restoration of the BBB (Fig. 4).73 By synthesizing polymer NPs, two pharmacological effects can be exerted simultaneously. For example, poly(DFO-PEGm) NPs were engineered by combining high-density deferoxamine (DFO) with functional groups like catechol moieties. This formulation not only effectively eliminates iron overload but also significantly reduces the ROS level, potentially mitigating secondary cellular damage after ICH. Consequently, it presents a novel approach for addressing iron deficiency following ICH.32
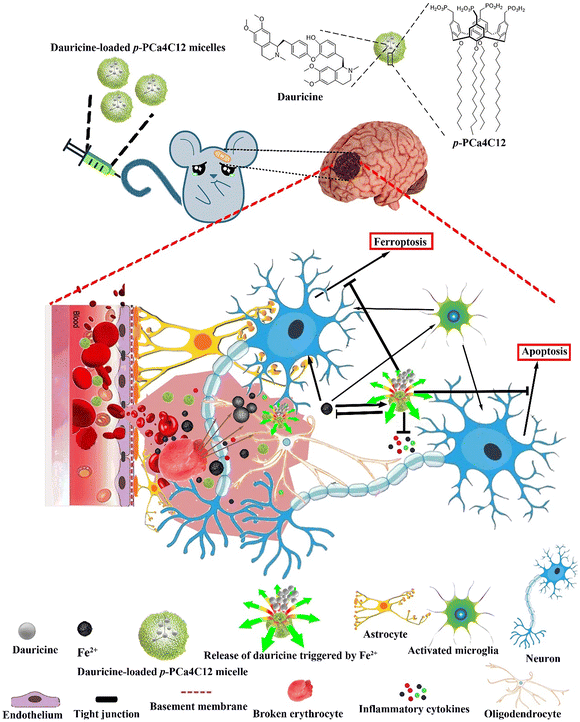 | ||
| Fig. 4 llustration depicting the structure and responsive nature of metal ion-triggered nanocarriers utilizing a phosphonated calix[4]arene derivative encapsulating the neuroprotective compound dauricine. These micelles deliver dauricine upon encountering high levels of Fe2+ present at both primary and secondary brain injury sites. Reproduced from ref. 73 with permission from Springer Nature, copyright 2020. | ||
To enhance the performance and functionality of polymer NPs, various modifications can be made to the polymers. For instance, polyethylene glycol (PEG), an FDA-approved non-toxic hydrophilic polymer, can form a hydrophilic layer around the particles to increase dispersibility and can greatly extend the half-life by delaying the conditioning effect. The synthesis of DEF-HCC-PEG, through the covalent attachment of deferoxamine to polyethylene glycol-conjugated hydrophilic carbon clusters (PEG-HCCs), has shown to prevent hemoglobin- and iron-mediated toxicity more efficiently than standalone or combined treatments. DEF-HCC-PEG inhibited hemoglobin- and iron-induced neurotoxicity in both cultured cells and experimental ICH mouse models, where iron overload contributes to ICH and subsequent neurodegenerative conditions.74
Furthermore, statins have been identified as a potential neuroprotective agent to target the inflammatory response after ICH. However, the clinical application of statins is usually constrained by their inherent limitations. Primarily, their poor water solubility results in malabsorption and reduced bioavailability when administered orally. Secondly, high doses of statins are associated with numerous complications, including heightened risks of myopathy/myalgia, ICH and diabetes. Statin nanomicelles, formulated using a poly(ethylene glycol)-block-poly(ε-caprolactone) (PEG-PCL) copolymer, have been shown to inhibit inflammatory cell infiltration, attenuate cerebral edema, modulate microglial cell/macrophage polarization, decrease the expression of IL-1b and TNF-a, and increase the expression of IL-10, and decrease neuronal degeneration, thus promoting the recovery of neurological function. Overall, the use of nanomicelles to deliver statins may have the potential to target neuroinflammation and improve their efficacy in the treatment of ICH (Table 3).75
| Polymeric NP types | Encapsulating agents | Animal model | Method of administration | Ref. |
|---|---|---|---|---|
| Chitosan nanoparticles | Nicardipine | Autologous whole blood, rat | Transnasal drug delivery | 67 |
| Polymer-based nanoparticles (NPs) | Curcumin (Cur) | Collagenase, mouse | Oral medication | 68 |
| Polymer-based nanoparticles (NPs) | Resveratrol (Res) | Collagenase, rat | Oral medication | 69 |
| Polybutylcyanoacrylate (PBCA) nanoparticles (NPs) | Plasmid neurotrophin-3 (NT-3) containing hormone response element (HRE) | Collagenase, rat | Intravenous injection (i.v.) | 71 |
| Platelet-membrane-modified polydopamine (Menp@PLT) nanoparticles | — | Collagenase, mouse | Intravenous injection (i.v.) | 72 |
| Ion-responsive nanocarrier based on a phosphonated calix[4]arene derivative | Dauricine (DRC) | Autologous whole blood, mouse | Intravenous injection (i.v.) | 73 |
| Carbon nanoparticle | Poly(ethylene glycol)-conjugated hydrophilic carbon clusters (PEG-HCCs), deferoxamine | Collagenase, rat | Intraperitoneal injection | 74 |
| Nanocellular micelles | Rosuvastatin | Collagenase, mouse | Intravenous injection (i.v.) | 75 |
| Poly(catechol)-DFO nanoscavengers | Deferoxamine and Catechol | Collagenase, mouse | Intravenous injection (i.v.) | 32 |
2.4 Inorganic nanoparticles
Inorganic nanoparticles offer several advantages in the treatment of ICH. Firstly, the unique design of inorganic nanoparticles enables them to circumvent BBB, facilitating the direct conveyance of therapeutic agents to the cerebral lesions. Moreover, inorganic nanoparticles can be amalgamated with alternative therapeutic modalities, such as photothermal or magnetic therapy, to yield a multimodal treatment strategy that amplifies therapeutic effectiveness. This synergistic therapeutic potential paves the way for a more tailored and comprehensive treatment regimen for diverse types of cerebral hemorrhage.Mesoporous silicon dioxide nanoparticles have a tunable mesoporous structure, high specific surface area, and large pore volume. Such properties confer a distinct advantage of encapsulating various therapeutic drugs and targeting these deliveries to specific sites.76 For example, selenium (Se), a cofactor for antioxidant enzymes like glutathione peroxidase (GSH-Px) and thioredoxin reductase, plays a crucial role in defending against oxidative stress and maintaining redox balance, thereby shielding neurons from damage. However, selenium's toxicity cannot be overlooked in therapeutic applications. The development of porous Se@SiO2 nanocomposites allows for a gradual and sustained release of selenium, optimizing its beneficial effect while minimizing toxicity. By oral administration in a mouse ICH model, Se can be helped to function across the BBB to ameliorate ICH-induced neurological deficits and perihematoma oxidative stress, protect the BBB integrity, and attenuate cerebral edema.77 In addition, cerium oxide nanoparticles (CeNPs) were modified with a PEG coating for better biocompatibility and to avoid interparticle agglomeration. PEG-CeNPs exhibited better colloidal stability than normal CeNPs after storage in purified water at 4 °C. Treatment with PEG-CeNPs markedly decreased cerebral edema 3 days after ICH, reduced ROS accumulation, fostered myelin regeneration, and enhanced neurological function.78 Minocycline (MC) exhibits superior CNS penetration among tetracyclines, boasting capabilities such as inflammation inhibition and free radical reduction. Despite this, its clinical trial outcomes are limited due to the high doses required for therapeutic onset. Xu et al. synthesized minocycline-loaded cerium oxide nanoparticles by incorporating MC into polyethylene glycol (PEG)-coated cerium oxide nanoparticles (CeO2-MC). This nanocomposite enables precise targeting of MC to the hematoma, enhancing therapeutic efficacy while minimizing drug dosage. In comparison with MC alone, CeO2-MC more effectively induces microglial conversion to the M2 phenotype and mitigates iron-induced cell death post-ICH. It also enhances spatial learning and sensorimotor functions in mice, offering innovative prospects for ICH clinical management (Fig. 5).79
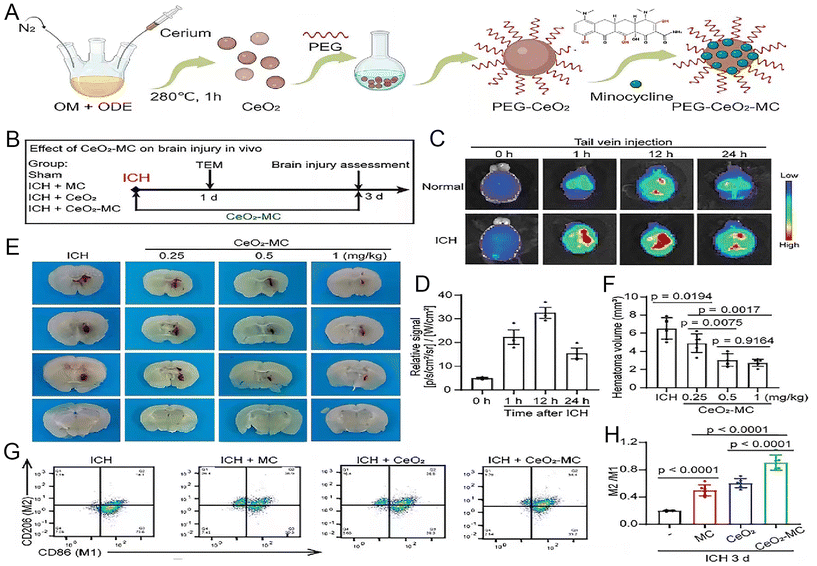 | ||
| Fig. 5 The synthesis of CeO2-MC demonstrate that it mitigates brain edema by modulating the polarization of M1/M2 macrophages. (A) Illustration depicting the creation process of CeO2-MC. (B) A timeline and design for the in vivo study. (C) Fluorescence imaging tracking Cy5.5-tagged CeO2-MC in mice, pre and post intravenous (IV) administration at specified time points. (D) Quantified fluorescence intensity in the brain tissues of Intracerebral Hemorrhage (ICH) model mice. (E and F) Visual and numerical analysis of hematoma size in ICH models treated with varying doses of CeO2-MC. (G and H) Flow cytometry analysis of macrophage markers in ICH and various treatment groups. Reproduced from ref. 79 with permission from Wiley-VCH GmbH, copyright 2024. | ||
Magnetic NPs can be used as drug carriers to target lesions of diseases. For example, magnetic targeting nanocarriers can transport neural stem cells and PPARγ agonists to the site of ICH for therapeutic intervention. Spherical neural mass (SNM) is an intermediate generation process derived from embryonic stem cells prior to differentiation into neural precursor cells, and exhibits stem cell trophic effects and immunomodulatory capacity. It can also regenerate neural networks and restore neurovascular function after stroke (but only a small number of stem cells can migrate to the target organ by intravenous injection, and the efficacy of delivery is limited). Using ferromagnetic iron oxide nanocubes (FION) loaded with SNM and guided by magnetically embedded helmets, it was confirmed that their distribution in the brain increased substantially and effectively reduced hematoma area, atrophy of the injured hemisphere, recruitment of inflammatory cells, and enhanced early neurological function recovery after ICH.80 In addition, magnetic targeting nanocarriers loaded with PPARγ agonist can be targeted and enriched in the hematoma area, and focused ultrasound (FUS) was applied to enhance the drug diffusion. PPARγ is a transcription factor with anti-inflammatory and hematoma-clearing effects, which can attenuate brain injury and neuritis after ICH. The nanocarrier, when administered intravenously, benefits from efficient magnetic targeting and ultrasound responsiveness, enhancing hematoma clearance. It also contributes to the attenuation of brain injury reduction of neuroinflammation, minimization of intracerebral hematoma, and improvement of locomotor behavioral sciences in mice with ICH (Fig. 6).81
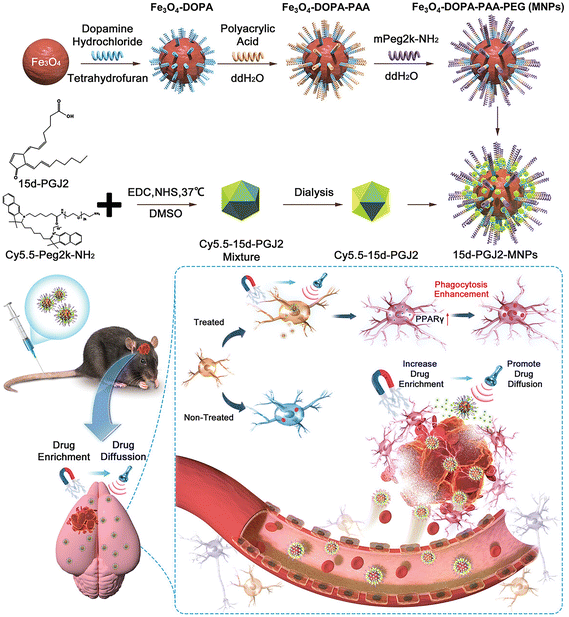 | ||
| Fig. 6 The synthesis process of Fe3O4-DOPA-PAA-PEG (MNPs) and 15d-PGJ2-MNPs, along with a hypothetical mechanism of administering 15d-PGJ2-MNPs using a magnet and focused ultrasound. Reproduced from ref. 81 with permission from Wiley-VCH GmbH, copyright 2023. | ||
Unlike other nanomaterials, magnetic nanomaterials can be used as contrast agents for imaging. Magnetic particle imaging (MPI), a radiation-free, tissue background-free tomographic imaging method for the direct three-dimensional detection of superparamagnetic iron oxide particles (SPIO), has excellent contrast, sensitivity, spatial and temporal resolution, safety, and biocompatible tracers. MPI can be operated at the patient's bedside, and bedside monitoring has become possible, as well as has a great advantage over traditional detection modalities.82 It has also been demonstrated that magnetic particle imaging can reliably and rapidly detect ICH in mouse models. Proper use of developers and tracers can detect ICH within 2.5 minutes, compared to the evaluation examination time of 11–13 minutes for CT and MRI. Experimentally, multicontrast MPI differentiated between areas of fluid and coagulated blood within the hematoma, which was not possible with other imaging techniques. With MPI, it is possible to detect a decrease in cerebral perfusion and adjust treatment to control cerebral pressure (Table 4).33
| Inorganic nanoparticle types | Encapsulating agents | Animal model | Method of administration | Ref. |
|---|---|---|---|---|
| Cerium oxide nanoparticles | Minocycline (MC) | Autologous whole blood, mouse | Intravenous injection (i.v.) | 79 |
| Selenium nanocomposite | — | Collagenase, mouse | Intraperitoneal injection | 77 |
| Iron oxide nanoparticle | Neural masses | Collagenase, rat | Intravenous injection (i.v.) | 80 |
| Magnetic targeting nanocarriers | PPARγ agonist | ICH mouse | Intravenous injection (i.v.) | 81 |
| Ceria nanoparticles (CeNPs) | — | Collagenase, mouse | Intravenous injection (i.v.) | 78 |
| Superparamagnetic iron oxide particles | — | Collagenase, mouse | Intravenous injection (i.v.) | 33 |
3. Application of hydrogels in the treatment of ICH
Hydrogel is a kind of polymeric material with high water content and a three-dimensional network structure, which has good biocompatibility, biodegradability, and mechanical elasticity, and thus has a wide range of applications in biomedical fields.83,84 As quintessential biomaterials for tissue regeneration, hydrogels present unique benefits in the therapeutic management of ICH. Distinct from other nanomaterials, hydrogel precursors can be formulated as injectable solutions. These solutions can be directly administered into the ICH lesion site and undergo in situ solidification to form a gel. This gel assumes a gelatinous consistency with mechanical properties akin to the brain tissue, effectively filling the irregular cavities resultant from ICH damage. Moreover, hydrogels can be engineered to emulate the native extracellular matrix (ECM), which provides structural support during tissue repair. This biomimicry facilitates the infiltration of CNS cells into the hydrogel, fostering local tissue regeneration, mitigating glial scar formation, and establishing a conducive microenvironment for the restoration of nerve tissue and axon regeneration.85 Second, another great advantage of it as a drug carrier is that it can effectively avoid the BBB.47 By stereotactic injection into the focal area and slow release of the loaded drug, not only can the local drug be maintained at a stable concentration for a long period of time, reducing the dosage of the administered drug, but also avoid the side effects caused by systemic administration of the drug. In general, hydrogels also have stable biodegradability, thus adapting to the regeneration of the brain tissue in the later stages of the disease (Fig. 7).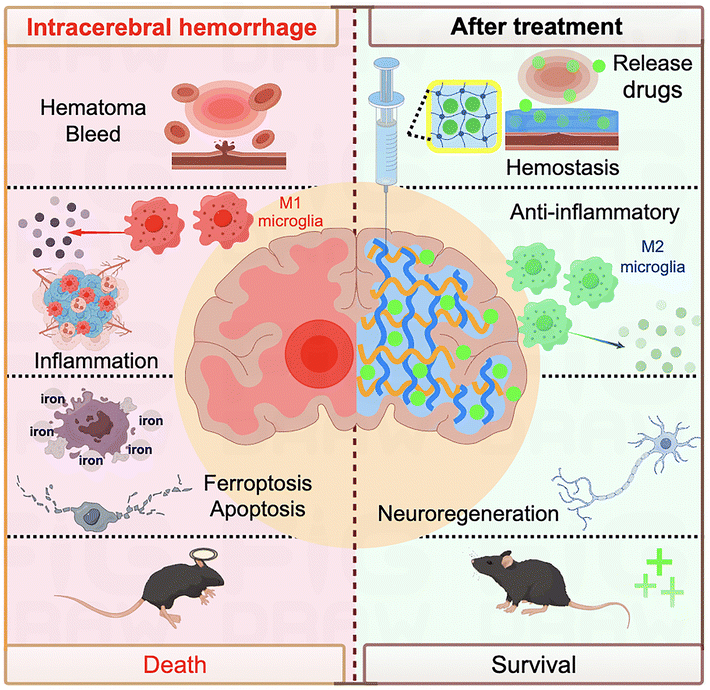 | ||
| Fig. 7 Schematic diagram of the hydrogel used to treat intracerebral hemorrhage. The figure was edited using Figdraw. | ||
3.1 Self-assembling peptide-based hydrogels
Self-assembling peptides (SAPs), usually composed of 20 naturally occurring amino acids, undergo self-assembly upon formation. This process results in the creation of material structures with specific functions from the designed peptide units.RADA16-I is an example of SAPs, characterized by its regular sequence of both ionophilic and hydrophobic amino acids. This peptide can form stable beta-sheet structures that eventually turn into hydrogel. In the treatment of ICH, RADA16-I scaffold replaced cerebral hematomas, reduced acute brain injury and cerebral cavernous formation, and improved functional recovery, and the scaffolds attenuated the concentration of these factors in the area surrounding the hematoma and limited the inflammatory response.86 As we know, hydrogels formed by the SAP RADA16-I (Ac-(RADA)4-CONH2) suffer from major disadvantages associated with low pH, which damages cells and tissues upon direct contact. So, Sun et al. designed two SAP hydrogels carrying IKVAV and RGD, respectively, which are designed to carry opposite charges in aqueous solutions at physiological pH, and when these SAPs are combined together, they form a 3D nanofiber hydrogel. They maintain a constant neutral pH value during self-assembly and can be delivered directly into tissues by simple injection without causing acid corrosion and tissue damage. In the ICH model, regenerated nerve fibers were observed in -IKVAV/-RGD grafts. Studies on three models of nerve injury including sciatic nerve defect, ICH, and spinal cord transection demonstrated that the designed IKVAV/-RGD nanofiber hydrogel provided a more forgiving environment for nerve regeneration than the RADA 16-I hydrogel.87 Another study showed that self-assembly peptide nanofibrous scaffold (SAPNS) was modified to be acidic compared to RADA16-I scaffolds, resulting in a neutral pH value, and confirmed that there were fewer microglia and apoptotic cells in ICH mice, while there were more surviving cells in this group. In addition, new nerve fibers have grown into this new SAPNS, suggesting that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mix of RADA16-RGD and RADA16-IKVAV (RADA16 mix) could serve as a bridge for nerve fiber growth.88
1 mix of RADA16-RGD and RADA16-IKVAV (RADA16 mix) could serve as a bridge for nerve fiber growth.88
RGD-containing elastin-like polypeptide (REP) is a modified elastin-like polypeptide (ELP) with higher functionality in promoting cell adhesion and tissue regeneration compared to ELP due to its RGD-containing sequences. REP is capable of self-assembling into a hydrogel at body temperature. The results showed that the application of REP was able to reduce the number of activated microglial cells, attenuate the expression of von Willebrand factor (vWF), and prevent the leakage of immunoglobulin G (IgG) into the brain parenchyma, which enhanced vascular integrity and provided a new therapeutic strategy for the treatment of ICH.89
The novel self-assembling peptide hydrogel, Alpha2 was also investigated and confirmed to be safe, well tolerated and retained in ICH lesions for several weeks, allowing for host cell infiltration and an increase in the number of value-added cells.90
3.2 Other hydrogels
Luo et al. found that a human hair keratinizing enzyme hydrogel (KG) loaded with minocycline hydrochloride (MH) efficiently absorbed non-heme iron and released iron chelators over time in the region of cerebral hematomas following ICH surgery. Localized hydrogel injection of MH are associated with lower doses and better recovery than systemic medications. However, higher concentration of MH decrease survival, suggesting the need for dose adjustment and consideration of the slow-release properties of the hydrogel when determining dosing.91 In addition, human hair keratin protein hydrogels (K-gels) with hemostatic ability have been reported in previous studies.92 So He et al. explored the therapeutic effect of K-gels on rebleeding in ICH. The results showed that K-gels have good hemostatic ability and biocompatibility, can significantly reduce hematoma volume after ICH, and improve the survival rate of rats, which has a great potential in clinical practice.93 Although in situ keratin hydrogels offer a promising strategy for ICH treatment, the injection performance of conventional keratin hydrogels is less than satisfactory to provide adaptive filling for irregularly shaped lesion defects. Therefore, Zhu et al. synthesized thermo-sensitive keratin hydrogels (TKGs) by grafting keratin with poly(N-isopropylacrylamide) (PNIPAM) to enhance the injectability and iron removal efficiency of keratin hydrogels. They administered TKGs loaded with deferoxamine mesylate (DFO) into the hematoma region for treatment. The lower critical solution temperature of these TKGs can be adjusted between 28.5 and 31.8 °C. Owing to the sol–gel transition property, TKGs can seamlessly fill the intricate contours of the lesion cavity. In comparison with keratin gels, TKGs absorb iron more rapidly and more effectively mitigate iron overload and subsequent brain damage post-ICH.94In the aforementioned study, Gong et al. employed hydrogel-encapsulated iron chelators to design a keratin-based core–shell hydrogel system aimed at enhancing the therapeutic effectiveness of stem cells, particularly by enhancing the viability of MSCs. To achieve this, they enveloped the iron chelator MH within a shell hydrogel, and encapsulated nanoparticles carrying epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) along with BMSCs within the core hydrogel. By utilizing the MH contained in the shell hydrogel to eliminate excess iron and simultaneously safeguard the BMSCs within the core hydrogel for proliferation and secretion of trophic factors, the study successfully alleviated the damage caused by iron overload post-ICH and facilitated neurological recovery.95
In addition to keratin hydrogels, gelatin hydrogels have also been used in the treatment of ICH. In a study by Lim et al., they filled irregularly shaped cavities with EGF-containing gelatin hydrogels, which promoted the migration of a variety of CNS cells as well as the differentiation of neural precursor cells to neurons, and was able to improve the recovery of neurological function in a rat model of ICH. Notably, in their experiments, it could be seen that high-dose injection of EGF could not have the same effect as the hydrogel-loaded EGF group. This suggests a critical role for hydrogels in providing structure for cell migration and maintaining EGF release.96 Xu et al. also prepared gelatin hydrogels for the treatment of ICH mice and found that gelatin hydrogels with RGD sequences may modulate microglia/macrophages polarization toward an anti-inflammatory phenotype by regulating the expression of integrin β, reduce the release of inflammatory cytokines, and promote the recovery of neurological function by inhibiting neurological inflammation to promote neurological recovery and improve the outcome of ICH.97
Semi-permeable polymer network (SIPN) hydrogels have been reported to promote cell migration, proliferation, and differentiation through the use of bioactive polymers and the provision of appropriate mechanical properties.98,99 Hyaluronic acid (HA) is an important component of the animal extracellular matrix (ECM) and a common choice for SIPN formation in hydrogel networks.100 Thus, in their study, Liu et al. added HA to chitosan hydrogels to make them hydrogels with the SIPN structure. Compared with CS hydrogel without HA, CH hydrogel induced reduction in both inflammatory response and glial scar formation after implantation into rat brain. Subsequently, they evaluated the efficacy of the CH hydrogel by injecting it into the brain cavity of a collagenase-induced ICH rat model. The CH hydrogel was confirmed to reduce brain tissue atrophy with the promotion of neurobehavioral recovery, and enhanced NSC neurogenesis recruitment and functionalization. The in vivo and in vitro results also demonstrated that HA-containing CH hydrogels provide more favorable conditions for stem cells to survive, and that the modified CH hydrogels also provide a relaxed microenvironment for axonal growth.101
However, most of the previous studies were limited to hydrogels loaded with a single drug, and such hydrogels could not release the appropriate drugs for treatment according to the different pathological stages of ICH. In a recent study, Lin et al. successfully developed a chitosan micellar dual-loaded hydrogel (CMD) with asynchronous release kinetics. This innovation allowed the hydrogel to be loaded with both the hydrophilic drug minocycline and the hydrophobic drug edaravone. Through the asynchronous release kinetics, the anti-inflammatory and neural regenerative drugs were released sequentially. They were released according to the subacute and chronic pathological stages of the rats with ICH stroke, respectively. This is clinically important in the rescue and subsequent treatment of ICH stroke. The results showed that rats treated with CMD hydrogel exhibited behavioral improvement and reduced brain atrophy. Although angiogenesis is an important aspect of brain tissue repair, the effect of angiogenesis has not been reported in any therapeutic studies of hydrogels for ICH before. In this study, the researchers attributed the angiogenesis-promoting effect of CMD hydrogels to the neuroprotective agent edaravone.102 Their first use of asynchronous dual-drug delivery system for ICH treatment may open a new window for the future application of hydrogels in ICH (Table 5).
| Hydrogel types | Encapsulating agents | Animal model | Method of administration | Ref. |
|---|---|---|---|---|
| Chitosan micellar self-healing hydrogel (CM hydrogel) | Minocycline hydrochloride/edaravone | Collagenase, rat | In situ injection | 102 |
| Chitosan hydrogel with semi-permeable polymer network (SIPN) containing hyaluronic acid | — | Collagenase, rat | In situ injection | 101 |
| Gelatin hydrogel | — | Collagenase, mouse | In situ injection | 97 |
| Gelatin hydrogel | EGF | Collagenase, rat | In situ injection | 96 |
| Keratin-based core-shell hydrogel | MH, EGF, bFGF, BMSCs | FeCl2, rat | In situ injection | 95 |
| Thermosensitive keratin hydrogels (TKGs) synthesized by grafting keratin with poly(N-isopropylpropylamide) (PNIPAM) | Deferoxamine mesylate (DFO) | Autologous blood, rat | In situ injection | 94 |
| Human hair keratin hydrogels (K-gels) | — | Collagenase, rat | In situ injection | 93 |
| Human hair keratose hydrogel (KG) | Minocycline hydrochloride (MH) | Autologous blood, rat | In situ injection | 91 |
| Self-assembled peptide hydrogels formed by RADA16-RGD and RADA16-IKVAV (RADA16-IKVAV/-RGD) | — | Collagenase, mouse | In situ injection | 87 |
| RGD-containing elastin-like polypeptide (REP) | — | Collagenase, rat | Right internal carotid artery Injection | 89 |
| Self-assembled peptide hydrogels formed by RADA16-I | Collagenase, rat | In situ injection | 86 | |
Self-assembled peptide hydrogels formed by RADA16-RGD and RADA16-IKVAV (RADA16 mix, mixed at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) 1 ratio) |
Collagenase, mouse | In situ injection | 88 |
4. Future perspectives
Stroke is the second leading cause of death worldwide and a major cause of disability.1 As the most severe stroke subtype,103 ICH has much higher rates of disability and death than ischemic stroke, which has a much higher prevalence. Unlike other diseases, ICH lacks specific and effective treatments and medications, and surgical treatment can only be used to save the patient's life in critical moments, and has no significant benefit in treating secondary injury caused by blood toxicity and improving neurological recovery after ICH.5 Anti-inflammatory treatments and neuroprotection after stroke are not part of the clinical guidelines for the time being and are limited to animal experiments; clinical translation is currently one of the biggest challenges. Moreover, due to the specificity of the anatomical structure of the nervous system, it is difficult for drugs to reach the lesion to exert therapeutic effects. In recent years, the application of biomaterials and nanomedicines in the nervous system have gradually increased. They have improved the stability, bioavailability, targeting, and ability to penetrate the BBB of drugs in the CNS, which plays a great role in depression,104 Parkinson's disease,105 Alzheimer's disease106 and other central nervous system diseases. Compared with ICH, we can find that biomaterials are more commonly and intensively studied in ischemic stroke.47 For instance, edaravone (EDA), used for stroke management, faces challenges like high toxicity and the need for frequent dosing. Zhang et al.'s study introduced a pH/glutathione-responsive EDA-loaded nanogel (NG/EDA), designed for controlled delivery through the BBB to the ischemic brain. This system showed potential in reducing neuronal damage and restoring glutathione levels, with a stable structure that prevents early EDA release, minimizing toxicity risks. Notably, this nanogel delivery system has not yet been explored in the context of ICH.107 Although the pathogenesis of ICH and ischemic stroke are not exactly the same, there are similar pathophysiological processes, such as neuroinflammation after stroke.108 Compared with other diseases, the application of biomaterials in ICH has more room for improvement, and we can learn from ischemic stroke or other neurological diseases and incorporate some recent advances in drug research to develop more biomaterials and nanomedicines suitable for ICH.109–111To investigate ICH, various animal models have been developed, including microballoon insertion, autologous whole blood injection, collagenase injection, and thrombin injection models. These models aim to replicate different pathophysiological mechanisms, enabling researchers to explore potential therapeutic approaches. Particularly prevalent are the collagenase and autologous blood injection models. However, these animal models have limitations due to their singular modeling factors. For instance, while microballoon insertion models can mimic the space-occupying effect of a hematoma, they do not simulate the impact of blood on the brain tissue or the effects of substances released from the hematoma post-bleeding. Conversely, the autologous whole blood injection model, despite introducing blood, lacks reproducibility and does not involve actual vessel rupture, leading to variable hematoma volumes, a lower success rate, and potential issues such as hemorrhage and backflow of injected blood between the ventricle and subdural space. The collagenase model, offering higher success rates and better reproducibility, more accurately represents spontaneous hemorrhage and subsequent secondary damage in ICH. However, collagenase's cytotoxicity may induce severe inflammatory reactions in the brain tissue, potentially skewing experimental outcomes. Hypertension, the most common precursor to spontaneous ICH, and many patients’ comorbid chronic conditions such as atherosclerosis, diabetes mellitus, and hyperlipidemia are not adequately replicated in these models. Although studies have been conducted on hypertension-based ICH models, they too face issues such as poor reproducibility, high costs, and unpredictable hemorrhagic lesion sizes and locations.10,112 Before developing animal models that are closer to clinical diseases, we can conduct tests in different animal models to validate the efficacy of drugs through different pathophysiological mechanisms, which will make our studies more complete and clinically relevant.
In this review, we summarize many biomaterials and nanomaterials applied to ICH treatment, such as lipid nanoparticles, exosomes, polymer nanoparticles, inorganic nanoparticles and hydrogels. All of them have unique roles and all of them show surprising effects in ICH. However, while we observe their efficacy, we also need to consider some of their safety issues, for example, the biocompatibility and biodegradability of the nanomaterials, i.e., whether the nanomaterials will cause toxic reactions or immune reactions in brain tissues, and whether the nanomaterials can be efficiently cleared or degraded in the body. Because the toxicology and degradation processes of many materials are not known, nanomedicines may accumulate in the body and become potentially toxic.113 Some specific nanomaterials also have their own unique problems. For example, liposomes encounter several barriers during transportation. Unmodified liposomes, for instance, are rapidly identified and eliminated by the mononuclear phagocytic system (MPS). However, modifying liposomes with polyethylene glycol (PEG) can mitigate this rapid clearance by MPS, thereby extending their circulatory half-life and enhancing therapeutic efficacy. Optimizing the design and functionality of liposomes is crucial to augment their clinical effectiveness and safety.34 As mentioned earlier, among exosomes, exosomes derived from inflammatory cells promote neuronal necrosis and thus aggravate the loss after ICH, which requires us to have a clear understanding of the cargoes carried by exosomes when using exosomes for treatment, as well as the requirements for their extraction, purification, and identification.65
Hydrogels, as emerging biomaterials, represent a promising avenue in the treatment of ICH, have garnered extensive research interest and have been applied across diverse fields. However, their application in the context of ICH remains nascent. The current research trajectory and therapeutic strategies concerning ICH are primarily focused on three objectives: hemostasis, targeted drug delivery, and fostering a regenerative milieu for neuronal cells. Hydrogels have the potential to modulate the pathophysiological dynamics of ICH via distinct mechanisms. Clinically, post-hematoma evacuation, hydrogels laden with therapeutic agents can be administered into the resultant cavity through stereotactic injection. This technique enables the hydrogel to be prepared and administered in a liquid state, subsequently undergoing an autogelation process post-injection to form an appropriate gel matrix. Hydrogels hold promise for enhancing patient prognosis and quality of life through multifaceted treatment approaches. Despite their potential, the deployment of hydrogels in treating ICH is not devoid of challenges. Primarily, hydrogels must exhibit excellent biocompatibility, necessitating the selection of materials that are compatible with central nervous system tissues to avoid immunogenic reactions or inflammation. In addition, hydrogels should maintain stability within the body and be capable of safe degradation post-treatment, obviating the need for invasive removal procedures. This entails the utilization of biodegradable gel materials with non-toxic degradation by-products. Furthermore, the drug release kinetics within the CNS must be meticulously calibrated to sustain therapeutic concentrations at the lesion site. Consequently, the sustained release rates of the encapsulated drugs warrant rigorous evaluation. Concurrently, inherent limitations of the gel materials, such as their propensity to swell and potentially exacerbate intracranial hypertension, require careful assessment of their swelling rates. The gelation time also emerges as a critical parameter, and an overly brief gelation period risks occluding the injection apparatus, compromising precise targeting. Conversely, protracted gelation may perturb drug release kinetics and therapeutic efficacy, underscoring the imperative to optimize gelation timing. These considerations underscore the necessity for continued research to ensure that hydrogels can successfully transition to clinical applications. In addition to ensuring personal safety, it is imperative to address the socio-ethical implications of using biomaterials, particularly their potential to pollute or compromise the environment during the manufacturing process. Thus, the clinical application of biomaterials necessitates a comprehensive focus not only on their efficacy but also on a thorough analysis and exploration of a multitude of concerns.
Although numerous studies have showcased the potential of various nanomaterials and hydrogels in enhancing cognitive recovery and reducing inflammation post-cerebral hemorrhage, the transition from animal models to clinical practice remains fraught with challenges. Key among these is the need to establish the biocompatibility and toxicity profiles of biomaterials within the human body. While safety data derived from animal studies provide a foundation, they cannot be extrapolated to humans without further toxicological research and biocompatibility assessments. Moreover, dosages optimized for animal models do not translate directly to human medicine, necessitating rigorous determination of effective doses and strategies for controlling nanomaterial distribution and metabolism within the human body. Some biomaterials, such as hydrogels, require in situ injection at the lesion site due to their material properties, potentially limiting their clinical application to invasive procedures like craniotomies unless alternative administration routes are developed. Consequently, clinical trial designs must meticulously consider the unique characteristics of biomaterials, including dosage, administration methods, and therapeutic timing. Furthermore, as the field of biomaterials is evolving, the ongoing development of regulatory policies and standards could impede the swift clinical adoption of biomaterials. Timely updates and refinements to legal frameworks are imperative to ensure that clinical trials are ethically sound and receive the necessary regulatory approvals. The scalability of products from laboratory to industrial production presents another hurdle, compounded by the high costs of advanced therapeutic modalities, which may restrict their broader implementation. Given the heterogeneity of patient responses in clinical settings, extensive and prolonged trials are essential to verify the safety and efficacy of biomaterials. Addressing these challenges that necessitates interdisciplinary approaches promoting collaboration among various disciplines is crucial for the swift translation of biomaterials into clinical applications.
In conclusion, we provide an overview of the current state of ICH and the biomaterials commonly employed in its treatment. We discuss the limitations encountered by nanomaterials and hydrogels in animal models and the issues faced during their clinical application. Nevertheless, with the rapid progression of science and technology, they demonstrate immense potential and vast opportunities for advancing the therapeutic outcomes of ICH medications. We are confident that biomaterials will resolve the present challenges in ICH treatment, introduce novel therapeutic approaches, and ultimately deliver hopeful news to patients afflicted with ICH.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Lukui Chen and Dongfang Zhou jointly conceptualized the focus of this review and reviewed the final manuscript. Wei Wang and Xiaowen Liu collected the literature and wrote the manuscript. Wei Wang, Xiaowen Liu and Yupeng Wang conducted statistical analyses of the data presented in the review and created data visualizations. Lukui Chen, Yupeng Wang and Dongfang Zhou revised the manuscript. All authors have read and approved the final version of the manuscript, contributing collaboratively to this research.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by grants from the National Key Research and Development Program of China (2021YFC2400600/2021YFC2400602 and 2022YFC3601900/2022YFC3601902), the National Natural Science Foundation of China (22275081 and 82372117), the Innovation Team Project (2023KCXTD007) and the Special Project in Key Areas of Guangdong Province (2021ZDZX2011). We thank Fig.Draw (https://www.figdraw.com/) for editing Fig. 1, 3 and 7.References
- G. B. D. S. Collaborators, Lancet Neurol., 2021, 20, 795–820 CrossRef PubMed.
- C. J. van Asch, M. J. Luitse, G. J. Rinkel, I. van der Tweel, A. Algra and C. J. Klijn, Lancet Neurol., 2010, 9, 167–176 CrossRef PubMed.
- D. A. Wilkinson, A. S. Pandey, B. G. Thompson, R. F. Keep, Y. Hua and G. Xi, Neuropharmacology, 2018, 134, 240–248 CrossRef CAS PubMed.
- A. Shao, Z. Zhu, L. Li, S. Zhang and J. Zhang, EBioMedicine, 2019, 45, 615–623 CrossRef PubMed.
- L. Puy, A. R. Parry-Jones, E. C. Sandset, D. Dowlatshahi, W. Ziai and C. Cordonnier, Nat. Rev. Dis. Primers, 2023, 9, 14 CrossRef PubMed.
- S. Urday, W. T. Kimberly, L. A. Beslow, A. O. Vortmeyer, M. H. Selim, J. Rosand, J. M. Simard and K. N. Sheth, Nat. Rev. Neurol., 2015, 11, 111–122 CrossRef PubMed.
- G. Xi, R. F. Keep and J. T. Hoff, Lancet Neurol., 2006, 5, 53–63 CrossRef PubMed.
- M. A. Kirkman, S. M. Allan and A. R. Parry-Jones, J. Cereb. Blood Flow Metab., 2011, 31, 2135–2151 CrossRef PubMed.
- J. Wan, H. Ren and J. Wang, Stroke Vasc. Neurol., 2019, 4, 93–95 CrossRef PubMed.
- M. Zille, T. D. Farr, R. F. Keep, C. Romer, G. Xi and J. Boltze, EBioMedicine, 2022, 76, 103880 CrossRef CAS PubMed.
- J. C. Hemphill, 3rd, S. M. Greenberg, C. S. Anderson, K. Becker, B. R. Bendok, M. Cushman, G. L. Fung, J. N. Goldstein, R. L. Macdonald, P. H. Mitchell, P. A. Scott, M. H. Selim, D. Woo, American Heart Association Stroke, Council on Cardiovascular and Stroke Nursing and Council on Clinical Cardiology, Stroke, 2015, 46, 2032–2060 CrossRef PubMed.
- M. L. Flaherty and J. Beck, Stroke, 2013, 44, 2953–2954 CrossRef PubMed.
- Y. Jin, Y. Zhuang, M. Liu, J. Che and X. Dong, Drug Discovery Today, 2021, 26, 916–930 CrossRef CAS PubMed.
- K. N. Sheth, N. Engl. J. Med., 2022, 387, 1589–1596 CrossRef CAS PubMed.
- C. Tschoe, C. D. Bushnell, P. W. Duncan, M. A. Alexander-Miller and S. Q. Wolfe, J. Stroke, 2020, 22, 29–46 CrossRef PubMed.
- P. Huang, Y. Zhu, H. Zhong, P. Chen, Q. Shi, J. Chen, J. Lai, Y. F. Tu, S. Liu and L. Liu, Biomater. Sci., 2022, 10, 1267–1280 RSC.
- Y. Wang, L. Wang, T. Li, M. Ouyang, H. Xiong and D. Zhou, Acta Pharm. Sin. B, 2024, 14, 1787–1800 CrossRef CAS PubMed.
- Y. Wang, J. Yu, Q. Shi, Z. Luo, G. Liu, F. Wu, Z. Wang, Y. Huang and D. Zhou, Adv. Mater., 2021, 2103497 CrossRef CAS PubMed.
- F. Dilnawaz, S. Acharya and S. K. Sahoo, Int. J. Pharm., 2018, 538, 263–278 CrossRef CAS PubMed.
- B. Li, W. Wang, L. Zhao, Y. Wu, X. Li, D. Yan, Q. Gao, Y. Yan, J. Zhang, Y. Feng, J. Zheng, B. Shu, J. Wang, H. Wang, L. He, Y. Zhang, M. Pan, D. Wang, B. Z. Tang and Y. Liao, Nat. Nanotechnol., 2024, 19, 834–845 CrossRef CAS PubMed.
- B. Yu, W. Sun, J. Lin, C. Fan, C. Wang, Z. Zhang, Y. Wang, Y. Tang, Y. Lin and D. Zhou, Adv. Sci., 2024, 11, e2307798 CrossRef PubMed.
- H. F. Liang, Y. R. Yan, W. Sun, X. G. Ma, Z. W. Su, Z. X. Liu, Y. Chen and B. Yu, Int. J. Mol. Sci., 2023, 24, 8740 CrossRef CAS PubMed.
- W. Wang, W. Mo, X. Xiao, M. Cai, F. Feng, Y. Wang and D. Zhou, Asian J. Pharm. Sci., 2024, 100926, DOI:10.1016/j.ajps.2024.100926.
- W. Wang, Z. Hu, W. Mo, M. Ouyang, S. Lin, X. Li, C. Wang, F. Yu, Y. Wang and D. Zhou, Eng. Regen., 2024, 5, 111–123 Search PubMed.
- Y. Fu, J. Hao, N. Zhang, L. Ren, N. Sun, Y. J. Li, Y. Yan, D. Huang, C. Yu and F. D. Shi, JAMA Neurol., 2014, 71, 1092–1101 CrossRef PubMed.
- J. J. Chang, M. Kim-Tenser, B. A. Emanuel, G. M. Jones, K. Chapple, A. Alikhani, N. Sanossian, W. J. Mack, G. Tsivgoulis, A. V. Alexandrov and T. Pourmotabbed, Eur. J. Neurol., 2017, 24, 1384–1391 CrossRef CAS PubMed.
- A. Y. Fouda, A. S. Newsome, S. Spellicy, J. L. Waller, W. Zhi, D. C. Hess, A. Ergul, D. J. Edwards, S. C. Fagan and J. A. Switzer, Stroke, 2017, 48, 2885–2887 CrossRef CAS PubMed.
- M. Selim, L. D. Foster, C. S. Moy, G. Xi, M. D. Hill, L. B. Morgenstern, S. M. Greenberg, M. L. James, V. Singh, W. M. Clark, C. Norton, Y. Y. Palesch, S. D. Yeatts and i-DEF Investigators, Lancet Neurol., 2019, 18, 428–438 CrossRef CAS PubMed.
- O. A. Sobowale, A. R. Parry-Jones, C. J. Smith, P. J. Tyrrell, N. J. Rothwell and S. M. Allan, Stroke, 2016, 47, 2160–2167 CrossRef PubMed.
- S. D. Yeatts, Y. Y. Palesch, C. S. Moy and M. Selim, Neurocrit. Care, 2013, 19, 257–266 CrossRef CAS PubMed.
- M. D. Sweeney, Z. Zhao, A. Montagne, A. R. Nelson and B. V. Zlokovic, Physiol. Rev., 2019, 99, 21–78 CrossRef CAS PubMed.
- F. Zhu, L. Zi, P. Yang, Y. Wei, R. Zhong, Y. Wang, C. You, Y. Li, M. Tian and Z. Gu, ACS Appl. Mater. Interfaces, 2021, 13, 9729–9738 CrossRef CAS PubMed.
- P. Szwargulski, M. Wilmes, E. Javidi, F. Thieben, M. Graeser, M. Koch, C. Gruettner, G. Adam, C. Gerloff, T. Magnus, T. Knopp and P. Ludewig, ACS Nano, 2020, 14, 13913–13923 CrossRef CAS PubMed.
- T. M. Allen and P. R. Cullis, Adv. Drug Delivery Rev., 2013, 65, 36–48 CrossRef CAS PubMed.
- R. Tenchov, R. Bird, A. E. Curtze and Q. Zhou, ACS Nano, 2021, 15, 16982–17015 CrossRef CAS PubMed.
- J. Xu, Z. Chen, F. Yu, H. Liu, C. Ma, D. Xie, X. Hu, R. K. Leak, S. H. Y. Chou, R. A. Stetler, Y. Shi, J. Chen, M. V. L. Bennett and G. Chen, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 32679–32690 CrossRef CAS PubMed.
- Q. Li, X. Lan, X. Han, F. Durham, J. Wan, A. Weiland, R. C. Koehler and J. Wang, Brain, Behav., Immun., 2021, 94, 437–457 CrossRef CAS PubMed.
- A. Liesz, A. Bauer, J. D. Hoheisel and R. Veltkamp, Neurosci. Lett., 2014, 579, 18–23 CrossRef CAS PubMed.
- R. N. Fedorak, A. Gangl, C. O. Elson, P. Rutgeerts, S. Schreiber, G. Wild, S. B. Hanauer, A. Kilian, M. Cohard, A. LeBeaut and B. Feagan, Gastroenterology, 2000, 119, 1473–1482 CrossRef CAS PubMed.
- E. Radwanski, A. Chakraborty, S. Van Wart, R. D. Huhn, D. L. Cutler, M. B. Affrime and W. J. Jusko, Pharm. Res., 1998, 15, 1895–1901 CrossRef CAS PubMed.
- I. Shlomovitz, M. Speir and M. Gerlic, Cell Commun. Signaling, 2019, 17, 139 CrossRef PubMed.
- V. A. Fadok, D. L. Bratton, S. C. Frasch, M. L. Warner and P. M. Henson, Cell Death Differ., 1998, 5, 551–562 CrossRef CAS PubMed.
- R. Toita, T. Kawano, M. Murata and J. H. Kang, Biomaterials, 2016, 110, 81–88 CrossRef CAS PubMed.
- R. R. Han, X. Lan, Z. Han, H. L. Ren, S. Aafreen, W. S. Wang, Z. P. Hou, T. Y. Zhu, A. Qian, X. N. Han, R. C. Koehler and G. S. Liu, Biomaterials, 2023, 301, 122277 CrossRef CAS PubMed.
- T. P. Crowe, M. H. W. Greenlee, A. G. Kanthasamy and W. H. Hsu, Life Sci., 2018, 195, 44–52 CrossRef CAS PubMed.
- L. Fan, L. Jin, T. Tang, Y. Zheng, Z. Chen, H. Lin, C. Ding, T. Wang, H. Chen, Y. Guo, C. Xu, H. Zhou, X. Wu, X. Fu, F. Yan, Z. Mao and G. Chen, Theranostics, 2024, 14, 283–303 CrossRef CAS PubMed.
- X. Tian, T. Fan, W. Zhao, G. Abbas, B. Han, K. Zhang, N. Li, N. Liu, W. Liang, H. Huang, W. Chen, B. Wang and Z. Xie, Bioact. Mater., 2021, 6, 2854–2869 CAS.
- R. Tenchov, J. M. Sasso, X. M. Wang, W. S. Liaw, C. A. Chen and Q. A. Zhou, ACS Nano, 2022, 16, 17802–17846 CrossRef CAS PubMed.
- G. Raposo and W. Stoorvogel, J. Cell Biol., 2013, 200, 373–383 CrossRef CAS PubMed.
- J. Saint-Pol, F. Gosselet, S. Duban-Deweer, G. Pottiez and Y. Karamanos, Cells, 2020, 9, 851 CrossRef CAS PubMed.
- S. Mathivanan, H. Ji and R. J. Simpson, J. Proteomics, 2010, 73, 1907–1920 CrossRef CAS PubMed.
- H. Xin, Y. Li, Y. Cui, J. J. Yang, Z. G. Zhang and M. Chopp, J. Cereb. Blood Flow Metab., 2013, 33, 1711–1715 CrossRef CAS PubMed.
- Y. Zhang, M. Chopp, Y. Meng, M. Katakowski, H. Xin, A. Mahmood and Y. Xiong, J. Neurosurg., 2015, 122, 856–867 Search PubMed.
- M. Li, X. Li, D. Wang, X. Gao, S. Li, X. Cheng, Y. Shen, S. Li, Q. Jia and Q. Liu, FASEB J., 2021, 35, e21617 CAS.
- Y. Han, D. Seyfried, Y. Meng, D. Yang, L. Schultz, M. Chopp and D. Seyfried, J. Neurosurg., 2018, 131, 290–300 Search PubMed.
- H. Shen, X. Yao, H. Li, X. Li, T. Zhang, Q. Sun, C. Ji and G. Chen, J. Mol. Neurosci., 2018, 64, 421–430 CrossRef CAS PubMed.
- J. Sun and G. Xu, Stem Cell Rev. Rep., 2023, 19, 1907–1921 CrossRef CAS PubMed.
- H. Zhang, Y. Wang, Q. Lv, J. Gao, L. Hu and Z. He, Front. Neurol., 2018, 9, 931 CrossRef PubMed.
- S. Duan, F. Wang, J. Cao and C. Wang, Drug Des., Dev. Ther., 2020, 14, 3143–3158 CrossRef CAS PubMed.
- M. Guo, X. Ge, C. Wang, Z. Yin, Z. Jia, T. Hu, M. Li, D. Wang, Z. Han, L. Wang, X. Xiong, F. Chen and P. Lei, Brain Sci., 2023, 13, 639 CrossRef CAS PubMed.
- B. Tang, M. Song, X. Xie, D. Le, Q. Tu, X. Wu and M. Chen, Neurochem. Res., 2021, 46, 2387–2402 CrossRef CAS PubMed.
- X. Gao, H. Yang, W. Xiao, J. Su, Y. Zhang, H. Wang, W. Ni and Y. Gu, Biomater. Res., 2022, 26, 67 CrossRef CAS PubMed.
- H. Ding, Y. Jia, H. Lv, W. Chang, F. Liu and D. Wang, J. Endocrinol. Invest., 2021, 44, 2685–2698 CrossRef CAS PubMed.
- W. Yang, N. Ding, R. Luo, Q. Zhang, Z. Li, F. Zhao, S. Zhang, X. Zhang, T. Zhou, H. Wang, L. Wang, S. Hu, G. Wang, H. Feng and R. Hu, Bioact. Mater., 2023, 27, 1–14 CAS.
- M. Wei, C. Li, Z. C. Yan, Z. W. Hu, L. Dong, J. Zhang, X. D. Wang, Y. P. Li and H. Z. Zhang, Neurochem. Res., 2021, 46, 1337–1349 CrossRef CAS PubMed.
- S. Rashki, K. Asgarpour, H. Tarrahimofrad, M. Hashemipour, M. S. Ebrahimi, H. Fathizadeh, A. Khorshidi, H. Khan, Z. Marzhoseyni, M. Salavati-Niasari and H. Mirzaei, Carbohydr. Polym., 2021, 251, 117108 CrossRef CAS PubMed.
- T. Guo, Y. Guo, Y. Gong, J. Ji, S. Hao, J. Deng and B. Wang, Int. J. Pharm., 2019, 566, 46–56 CrossRef CAS PubMed.
- C. Yang, M. Han, R. Li, L. Zhou, Y. Zhang, L. Duan, S. Su, M. Li, Q. Wang, T. Chen and Y. Mo, Int. J. Nanomed., 2021, 16, 8049–8065 CrossRef CAS PubMed.
- Y. Mo, L. Duan, Y. Yang, W. Liu, Y. Zhang, L. Zhou, S. Su, P. C. Lo, J. Cai, L. Gao, Q. Liu, X. Chen, C. Yang, Q. Wang and T. Chen, Nanoscale, 2021, 13, 3827–3840 RSC.
- B. Wilson, Nanomedicine, 2009, 4, 499–502 CrossRef CAS PubMed.
- C. Y. Chung, J. T. Yang and Y. C. Kuo, Biomaterials, 2013, 34, 9717–9727 CrossRef CAS PubMed.
- C. Xu, Y. Pan, H. Zhang, Y. Sun, Y. Cao, P. Qi, M. Li, O. U. Akakuru, L. He, C. Xiao, B. Sun, L. Bian, J. Li and A. Wu, Adv. Healthc. Mater., 2023, 12, e2300797 CrossRef PubMed.
- M. Li, G. Liu, K. Wang, L. Wang, X. Fu, L. Y. Lim, W. Chen and J. Mo, J. Nanobiotechnol., 2020, 18, 61 CrossRef CAS PubMed.
- P. Dharmalingam, G. Talakatta, J. Mitra, H. Wang, P. J. Derry, L. G. Nilewski, E. A. McHugh, R. H. Fabian, K. Mendoza, V. Vasquez, P. M. Hegde, E. Kakadiaris, T. Roy, I. Boldogh, V. L. Hegde, S. Mitra, J. M. Tour, T. A. Kent and M. L. Hegde, ACS Nano, 2020, 14, 2827–2846 CrossRef CAS PubMed.
- L. Zi, W. Zhou, J. Xu, J. Li, N. Li, J. Xu, C. You, C. Wang and M. Tian, Int. J. Nanomed., 2021, 16, 2933–2947 CrossRef PubMed.
- F. Tang, L. Li and D. Chen, Adv. Mater., 2012, 24, 1504–1534 CrossRef CAS PubMed.
- Y. Yang, G. Deng, P. Wang, G. Lv, R. Mao, Y. Sun, B. Wang, X. Liu, L. Bian and D. Zhou, Int. J. Nanomed., 2021, 16, 775–788 CrossRef PubMed.
- J. Zheng, J. Lu, S. Mei, H. Wu, Z. Sun, Y. Fang, S. Xu, X. Wang, L. Shi, W. Xu, S. Chen, J. Yu, F. Liang and J. Zhang, J. Neuroinflammation, 2021, 18, 43 CrossRef CAS PubMed.
- X. Xu, Z. H. Han, D. Li, X. S. Xu, Y. B. Liu, C. Cao, J. Tao, J. Cheng, J. H. Zhang, L. Cheng and G. Chen, Adv. Funct. Mater., 2024, 2313198 CrossRef.
- M. K. Kang, T. J. Kim, Y. J. Kim, L. Kang, J. Kim, N. Lee, T. Hyeon, M. S. Lim, H. J. Mo, J. H. Shin, S. B. Ko and B. W. Yoon, Int. J. Mol. Sci., 2020, 21, 3658 CrossRef CAS PubMed.
- T. Wang, H. Lei, X. Li, N. Yang, C. Ma, G. Li, X. Gao, J. Ge, Z. Liu, L. Cheng and G. Chen, Small, 2023, 19, e2206982 CrossRef PubMed.
- M. Graeser, F. Thieben, P. Szwargulski, F. Werner, N. Gdaniec, M. Boberg, F. Griese, M. Moddel, P. Ludewig, D. van de Ven, O. M. Weber, O. Woywode, B. Gleich and T. Knopp, Nat. Commun., 2019, 10, 1936 CrossRef CAS PubMed.
- S. Liu, Q. Zhang, J. Yu, N. Shao, H. Lu, J. Guo, X. Qiu, D. Zhou and Y. Huang, Adv. Healthcare Mater., 2020, 9, e2000198 CrossRef PubMed.
- H. Cao, L. Duan, Y. Zhang, J. Cao and K. Zhang, Signal Transduction Targeted Ther., 2021, 6, 426 CrossRef CAS PubMed.
- L. R. Nih, S. T. Carmichael and T. Segura, Curr. Opin. Biotechnol, 2016, 40, 155–163 CrossRef CAS PubMed.
- L. Y. Sang, Y. X. Liang, Y. Li, W. M. Wong, D. K. Tay, K. F. So, R. G. Ellis-Behnke, W. Wu and R. T. Cheung, Nanomedicine, 2015, 11, 611–620 CrossRef CAS PubMed.
- Y. Sun, W. Li, X. Wu, N. Zhang, Y. Zhang, S. Ouyang, X. Song, X. Fang, R. Seeram, W. Xue, L. He and W. Wu, ACS Appl. Mater. Interfaces, 2016, 8, 2348–2359 CrossRef CAS PubMed.
- N. Zhang, Y. Luo, L. He, L. Zhou and W. Wu, Nanomedicine, 2016, 12, 1205–1217 CrossRef CAS PubMed.
- J. Park, J. Y. Kim, S. K. Choi, J. Y. Kim, J. H. Kim, W. B. Jeon and J. E. Lee, Nanomedicine, 2017, 13, 1853–1862 CrossRef CAS PubMed.
- F. Bolan, B. Dickie, J. R. Cook, J. M. Thomas, E. Pinteaux, S. M. Allan, A. Saiani and C. B. Lawrence, Transl. Stroke Res., 2023 DOI:10.1007/s12975-023-01189-7.
- T. Luo, T. Guo, Q. Yang, S. Hao, J. Wang, Z. Cheng, Q. Qu, Y. He, Y. Gong, F. Gao, W. Li, H. Xia and B. Wang, Int. J. Pharm., 2017, 534, 179–189 CrossRef CAS PubMed.
- L. R. Burnett, M. B. Rahmany, J. R. Richter, T. A. Aboushwareb, D. Eberli, C. L. Ward, G. Orlando, R. R. Hantgan and M. E. Van Dyke, Biomaterials, 2013, 34, 2632–2640 CrossRef CAS PubMed.
- Y. He, Q. Qu, T. Luo, Y. Gong, Z. Hou, J. Deng, Y. Xu, B. Wang and S. Hao, ACS Biomater. Sci. Eng., 2019, 5, 1113–1122 CrossRef CAS PubMed.
- Q. Zhu, Y. Gong, T. Guo, J. Deng, J. Ji, B. Wang and S. Hao, Int. J. Pharm., 2019, 566, 342–351 CrossRef CAS PubMed.
- Y. Gong, Y. Wang, Q. Qu, Z. Hou, T. Guo, Y. Xu, R. Qing, J. Deng, B. Wang and S. Hao, J. Controlled Release, 2020, 320, 381–391 CrossRef CAS PubMed.
- T. C. Lim, E. Mandeville, D. Weng, L. S. Wang, M. Kurisawa, K. Leite-Morris, M. H. Selim, E. H. Lo and M. Spector, Transl. Stroke Res., 2020, 11, 412–417 CrossRef PubMed.
- J. Xu, Z. Duan, X. Qi, Y. Ou, X. Guo, L. Zi, Y. Wei, H. Liu, L. Ma, H. Li, C. You and M. Tian, Front. Bioeng. Biotechnol., 2020, 8, 785 CrossRef PubMed.
- H. J. Lee, A. Sen, S. Bae, J. S. Lee and K. Webb, Acta Biomater., 2015, 14, 43–52 CrossRef CAS PubMed.
- J. K. Kutty, E. Cho, J. S. Lee, N. R. Vyavahare and K. Webb, Biomaterials, 2007, 28, 4928–4938 CrossRef CAS PubMed.
- S. C. Skaalure, S. O. Dimson, A. M. Pennington and S. J. Bryant, Acta Biomater., 2014, 10, 3409–3420 CrossRef CAS PubMed.
- Y. Liu, Y. H. Hsu, A. P. Huang and S. H. Hsu, ACS Appl. Mater. Interfaces, 2020, 12, 40108–40120 CrossRef CAS PubMed.
- S. H. Lin, A. P. H. Huang and S. H. Hsu, Adv. Funct. Mater., 2023, 33, 2303853 CrossRef CAS.
- C. Cordonnier, A. Demchuk, W. Ziai and C. S. Anderson, Lancet, 2018, 392, 1257–1268 CrossRef PubMed.
- T. T. Zhu, H. Wang, H. W. Gu, L. S. Ju, X. M. Wu, W. T. Pan, M. M. Zhao, J. J. Yang and P. M. Liu, J. Nanobiotechnol., 2023, 21, 52 CrossRef CAS PubMed.
- M. Sela, M. Poley, P. Mora-Raimundo, S. Kagan, A. Avital, M. Kaduri, G. Chen, O. Adir, A. Rozencweig, Y. Weiss, O. Sade, Y. Leichtmann-Bardoogo, L. Simchi, S. Aga-Mizrachi, B. Bell, Y. Yeretz-Peretz, A. Z. Or, A. Choudhary, I. Rosh, D. Cordeiro, S. Cohen-Adiv, Y. Berdichevsky, A. Odeh, J. Shklover, J. Shainsky-Roitman, J. E. Schroeder, D. Hershkovitz, P. Hasson, A. Ashkenazi, S. Stern, T. Laviv, A. Ben-Zvi, A. Avital, U. Ashery, B. M. Maoz and A. Schroeder, Adv. Mater., 2023, 35, e2304654 CrossRef PubMed.
- G. Zhong, H. Long, T. Zhou, Y. Liu, J. Zhao, J. Han, X. Yang, Y. Yu, F. Chen and S. Shi, Biomaterials, 2022, 288, 121690 CrossRef CAS PubMed.
- Y. Zhang, Z. Zou, S. Liu, F. Chen, M. Li, H. Zou, H. Liu and J. Ding, Asian J. Pharm. Sci., 2024, 19, 100886 CrossRef PubMed.
- D. L. Alsbrook, M. Di Napoli, K. Bhatia, J. Biller, S. Andalib, A. Hinduja, R. Rodrigues, M. Rodriguez, S. Y. Sabbagh, M. Selim, M. H. Farahabadi, A. Jafarli and A. A. Divani, Curr. Neurol. Neurosci. Rep., 2023, 23, 407–431 CrossRef CAS PubMed.
- G. Shao, Eur. Rev. Med. Pharmacol. Sci., 2022, 26, 4574–4582 CAS.
- G. Xu, J. Guo and C. Sun, Pharm. Biol., 2021, 59, 114–120 CAS.
- Y. C. Wang, Y. Q. Wang, J. B. Shen and X. Chen, Lat. Am. J. Pharm., 2021, 40, 2324–2329 CAS.
- Q. Bai, Z. Sheng, Y. Liu, R. Zhang, V. W. Yong and M. Xue, Stroke Vasc. Neurol., 2020, 5, 388–395 CrossRef PubMed.
- D. Wu, Y. Ma, Y. Cao and T. Zhang, Sci. Total Environ., 2020, 702, 134994 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |