4D hydrogels: fabrication strategies, stimulation mechanisms, and biomedical applications
Amit
Nain
 *a,
Srishti
Chakraborty
a,
Nipun
Jain
a,
Saswat
Choudhury
b,
Suravi
Chattopadhyay
a,
Kaushik
Chatterjee
*a,
Srishti
Chakraborty
a,
Nipun
Jain
a,
Saswat
Choudhury
b,
Suravi
Chattopadhyay
a,
Kaushik
Chatterjee
 ab and
Souvik
Debnath
ab and
Souvik
Debnath
 *a
*a
aDepartment of Materials Engineering, Indian Institute of Science, Bangalore, Karnataka 560012, India. E-mail: amitnain@iisc.ac.in; souvikd@iisc.ac.in
bDepartment of Bioengineering, Indian Institute of Science, Bangalore, Karnataka 560012, India
First published on 25th April 2024
Abstract
Shape-morphing hydrogels have emerged as a promising biomaterial due to their ability to mimic the anisotropic tissue composition by creating a gradient in local swelling behavior. In this case, shape deformations occur due to the non-uniform distribution of internal stresses, asymmetrical swelling, and shrinking of different parts of the same hydrogel. Herein, we discuss the four-dimensional (4D) fabrication techniques (extrusion-based printing, dynamic light processing, and solvent casting) employed to prepare shape-shifting hydrogels. The important distinction between mono- and dual-component hydrogel systems, the capabilities of 3D constructs to undergo uni- and bi-directional shape changes, and the advantages of composite hydrogels compared to their pristine counterparts are presented. Subsequently, various types of actuators such as moisture, light, temperature, pH, and magnetic field and their role in achieving the desired and pre-determined shapes are discussed. These 4D gels have shown remarkable potential as programmable scaffolds for tissue regeneration and drug-delivery systems. Finally, we present futuristic insights into integrating piezoelectric biopolymers and sensors to harvest mechanical energy from motions during shape transformations to develop self-powered biodevices.
1. Introduction
Shape morphing is a nature-inspired critical process in biological systems for the survival of organisms.1 For instance, several plants such as mimosa, Venus flytrap, and pinecones undergo a shape change in response to environmental stimuli (e.g., humidity, light, and touch) to adapt to ever-changing complex environments.2 Dynamic shapes can be observed due to the anisotropic tissue composition and random orientation of micro- and nanostructures within the cell. Deformations typically arise from the out-of-plane and in-plane gradient created due to differences in local swelling behavior, amplifying the internal stresses under external stimuli.3 Therefore, special attention is provided to developing man-made nonliving materials capable of mimicking the behaviors perfected by nature over centuries.169 In the past decades, shape-morphing materials such as liquid crystal elastomers, shape-memory polymers, and hydrogels have been widely exploited.4,5 Among these, hydrogels have emerged as one of the most promising candidates.6 Hydrogels are a class of three-dimensional (3D) interconnected networks comprised of hydrophilic polymer chains.7 They swell and shrink in response to external stimuli, including pH, light, temperature, ions, and electric and magnetic fields.8,9However, despite these advantages, dynamic behavior is difficult to realize in hydrogels due to their fragile nature. The deployment and implantation of static 3D hydrogels are inconvenient in surgical sutures, posing a high risk of additional complications and severe infection and inflammation at/around the injured tissue site.10,11 4D hydrogels offer significant advantages compared to traditional 3D hydrogels. For example, their dynamic adaptability allows them to mimic living tissue, which can support cell growth, guide tissue development, and adjust their shape, structure, and properties to constantly evolving environmental conditions more effectively than static materials.12 Further, engineered 4D hydrogels pave the way for designing smart materials and devices with tailored responses to different stimuli (pH, moisture, light, etc.).Four-dimensional (4D) hydrogels offer precise control of the timing, magnitude, and duration of their responses to external stimuli. This level of control allows researchers to fine-tune the properties of materials and manipulate the behavior of hydrogels with high precision, facilitating their applications in biomedical research and clinical therapies. The versatile nature of 4D gels allows customization to meet the specific requirements of different applications, ranging from tissue engineering and drug delivery to soft robotics. Overall, 4D hydrogels represent a significant step forward in biomaterials science, offering unprecedented control and versatility for various applications. Therefore, efforts have been devoted to developing multiple pathways to prepare shape-morphing hydrogels and achieve dynamic architectures.
4D fabrication refers to 3D structures that undergo shape changes in response to certain stimuli with time.13 These shape transformations are typically governed by a non-uniform distribution of internal stresses, resulting from asymmetrical swelling/shrinking of different parts of the same hydrogel system.14 3D printing is the most utilized technique for manufacturing 4D structures,15 which can be broadly categorized into two types, i.e., direct ink-writing and light-based printing. In this review article, one of each category, i.e., extrusion printing and dynamic light processing (DLP), will be meticulously discussed as an example to shed light on the unique process of developing 4D biomaterials.15 Extrusion-based 3D printing utilizes a stereolithography file to create G-Code, providing precise control over the structure and predetermined final shape.16 Alternatively, DLP printers employ projected light sources to cure the complete layer at once.17 DLP printing was chosen over other light-assisted additive manufacturing techniques (stereolithography, two-photon polymerization, direct-laser writing, etc.) because it offers advantages such as high speed, resolution, scalability, material compatibility, customization, and printing capability.17 Both printing technologies have been reported to produce multidimensional dynamic constructs using two or more component systems to create a gradient between the layers.18 To date, only a few recent reports have highlighted that the solvent casting method is also capable of producing these dynamic structures by employing a single material.19 Therefore, we also chose the solvent casting fabrication technique as one of the key highlights of this article. Air drying at an ambient temperature is reported to create sparsely and densely cross-linked regions, leading to spontaneous anisotropic structures.20 Owing to this nature-inspired route, these 3D constructs show reversibility, making them ideal candidates to be explored in soft robotics.
Shape-morphing hydrogels are in high demand due to their dynamic nature. For example, 3D-printed constructs capable of forming tube-like structures have been explored as nerve conduits to repair peripheral nerve injuries.21 Compared to the rigid 3D design, a shape-shifting construct exhibited more significant neuronal development due to its conductive and optoelectronic properties.22 Particularly, by incorporating stimuli-responsive components into the hydrogel matrix, such as proteins or peptides that mimic the extracellular matrix, 4D hydrogels can provide dynamic guidance cues for regenerating nerve cells.22 In addition, dynamic but controlled degradation prevents risks of inflammation and fibrosus and promotes seamless integration with the surrounding tissues. Moreover, the use of 4D hydrogels facilitates precise control over the spatiotemporal presentation of bioactive molecules, growth factors, and cell adhesion peptides within the nerve conduit. In another study, light-induced gradient cross-linking in a 3D printed polymer network yielded a biomimetic patch, encouraging cardiomyocyte maturation and vascularization to promote cardiac regeneration.23 Similar to the nervous system, the heart is also a dynamic organ that experiences continuous mechanical forces. 4D hydrogels are designed to dynamically adjust their mechanical properties in response to variations in pressure or stretching. This dynamic behavior helps mimic the natural mechanical cues experienced by cardiac cells, promoting their alignment, maturation, and functionality within the engineered tissue. Moreover, an advanced drug delivery platform can be created using a 4D fabrication approach, allowing exact control over administered medicines, biomolecules, and cells in a programmed manner. Drug release can be actuated by various stimuli such as temperature, selective enzymes, pH, and humidity/moisture.24 However, it is challenging to achieve and maintain the ideal temperature for drug release, and dramatic changes in pH often contribute to the rapid degradation of the scaffold.25 Furthermore, although moisture is a cell-friendly stimulation, achieving complete deformation at the superficial tissue site, such as skin, is difficult due to its higher dehydration rate.26 Nevertheless, despite these challenges, shape-shifting biomimetic hydrogels have gained attention from the scientific community, which is evident from the literature published in the past seven years.27
Several years ago, Furukawa and group summarized the literature on shape-memory hydrogels and their potential application in soft robotics.28 Similarly, a review article on shape-morphing hydrogels was published last year by Liu et al.,29 highlighting the utility of shape-morphing characteristics in grippers, sensors, valves, soft robotics, and other mechanical applications.29 Therefore, an article highlighting the potential of programmable and dynamic hydrogels in the biomedical field was lacking. In this case, Dong et al. published an article on 4D printed hydrogels, discussing their fabrication strategies, materials, and applications in actuators and drug delivery.30 In the same year, Champeau et al. provided a comprehensive review on the state-of-the-art of 4D printing technology for the fabrication of shape-morphing hydrogels.31 However, although these articles were well drafted from a materials perspective, there have been several new developments in the past five years. Furthermore, these papers only focused on 3D printing technology, while other methods such as solvent casting have been employed to prepare 4D hydrogels. In addition, an in depth discussion on the utility of these hydrogels in bioengineering is still missing. Thus, in this review, we aim to provide a compressive discussion on various 4D fabrication techniques, including extrusion-based 3D printing, DLP, and solvent casting, and their application in stimuli-triggered complex tissue engineering and drug delivery. While discussing these innovative methods, an important distinction between mono- and dual-component, uni- and bi-directional shape change, and pristine and composite hydrogel systems is made. Herein, we also highlight the distinctive features of shape-morphing hydrogels that help produce intricate scaffold designs such as tubes that can serve as potential grafts, stents, or conduits. Furthermore, we present comparative arguments that will provide better readability and understanding of shape-morphing systems in tissue engineering and drug delivery applications. Subsequently, we summarize the importance of this emerging class of biomaterials and identify opportunities for the future. Finally, we discuss the potential of integrating electrical components and sensors within the dynamic structures that can revolutionize regenerative engineering. Rapid growth in the biomaterial domain and the capabilities of data science to create imaginative tools and techniques based on artificial intelligence and deep-learning algorithms can widen the field of biorobotics.
2. Types of 4D fabrication techniques
Hydrogels are gel-like substances and belong to a class of soft materials, which are capable of absorbing and retaining large amounts of water. These networks of gels can be prepared via physical or chemical crosslinking of polymer chains to form a stable matrix, which can be tailored to control mechanical strength, porosity, and other physicochemical properties. Hydrogels exhibit stimuli responsiveness depending on the formulation strategy and choice of polymer. Additionally, 3D hydrogels undergo shape morphism to produce more complex designs and shapes, also known as four-dimensional (4D) fabrication.32,33 There are multiple actuators that can drive shape morphism within hydrogels, such as pH, temperature, light, and ions.34–36 In the case of 4D fabrication, hydrogel structures can change their shape or properties over time in response to environmental cues, making them attractive for tissue engineering, drug delivery, and soft robotics applications. Below, we discuss the various strategies for preparing stimuli-responsive hydrogels. We also highlight the influence of composition, structural anisotropy, and formulation strategies on the shape-morphism of the fabricated hydrogels.2.1. Extrusion-based 3D printing
One of the fabrication techniques to achieve shape-morphing hydrogels is 3D printing. 3D printing has emerged as transformative technology in shape morphing, revolutionizing the design and creation of objects that can change their physical form. 3D printing is pivotal in advancing the capabilities of shape-morphing technology, ushering in a new era of design and engineering possibilities in the development of self-assembling structures for aerospace, adaptable medical implants, and shape-shifting architectural elements.37–39 3D printing enables the production of intricate structures with embedded materials that respond to external stimuli, such as pH, ions, and light, to alter their shape.40 3D printing enables the inhomogeneous incorporation of multiple components within the hydrogel, leading to different properties that contribute to variable swelling and attractive shape-morphing patterns, making it a desirable technique for 4D fabrication.41 Some of the most widely used forms of 3D printing include direct-ink writing (DIW), digital light processing (DLP), stereolithography (SLA), fused deposition modeling, two-photon polymerization (TPP), and ion-inkjet-printing (IIP).42 In the typical 4D fabrication of hydrogels, one or more components are employed and designed to generate gradient cross-linking, which enables shape change in the printed structures. Firstly, we discuss dual-component hydrogel systems. In another report, Joshi et al. created a dual-component hydrogel system comprised of alginate and methyl cellulose through extrusion-based 3D printing to develop a self-rolling nerve conduit.43 Two different gels of high (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) and low (4
9) and low (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) porosity were synthesized by adjusting the composition of alginate and methylcellulose (Fig. 1A). Two consecutive layers of 4
6) porosity were synthesized by adjusting the composition of alginate and methylcellulose (Fig. 1A). Two consecutive layers of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 were extruded onto a 3
6 were extruded onto a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 gel (bottom layer) in the form of strips and placed at 0°, 45°, and 45° angles with respect to the base layer to have precise control of the shape-deformation. Shape change occurred when the air-dried structures were immersed in calcium chloride solution due to chemical cross-linking.21 The direction of bending or twisting in the gel could be precisely anticipated and corroborated through computational simulations. Together with a pre-determined CAD design, the infill angle and differential cross-linking between the bottom and top layers were crucial for observing shape-morphism in a dual-component hydrogel system. In another dual-component system, Mao et al. developed a chitin-polydimethylsiloxane (PDMS) hydrogel capable of producing complicated multidimensional architectures.44 PDMS ink containing 90% pre-polymer and 10% curing agent was printed onto chitin film prepared by treating chitin (4 g) with sodium hydroxide (11%) and urea (4%). PDMS strips were3D printed on the chitin film (Fig. 1B) and the printed constructs were cured at 150 °C. Various structures were created by programming twelve components with different printing angles and interlayer spacing, followed by heat treatment and cutting. The actuation speed depended on the thickness of the structures and the shape reversibility was controlled by ethanol. The rapid response in water/ethanol solutions resulted from the significant modulus difference between the chitin film and PDMS, minimizing the swelling, and the hydrophobic nature of the PDMS layer, ensuring stable force and outperforming other dual-component hydrogel systems.
9 gel (bottom layer) in the form of strips and placed at 0°, 45°, and 45° angles with respect to the base layer to have precise control of the shape-deformation. Shape change occurred when the air-dried structures were immersed in calcium chloride solution due to chemical cross-linking.21 The direction of bending or twisting in the gel could be precisely anticipated and corroborated through computational simulations. Together with a pre-determined CAD design, the infill angle and differential cross-linking between the bottom and top layers were crucial for observing shape-morphism in a dual-component hydrogel system. In another dual-component system, Mao et al. developed a chitin-polydimethylsiloxane (PDMS) hydrogel capable of producing complicated multidimensional architectures.44 PDMS ink containing 90% pre-polymer and 10% curing agent was printed onto chitin film prepared by treating chitin (4 g) with sodium hydroxide (11%) and urea (4%). PDMS strips were3D printed on the chitin film (Fig. 1B) and the printed constructs were cured at 150 °C. Various structures were created by programming twelve components with different printing angles and interlayer spacing, followed by heat treatment and cutting. The actuation speed depended on the thickness of the structures and the shape reversibility was controlled by ethanol. The rapid response in water/ethanol solutions resulted from the significant modulus difference between the chitin film and PDMS, minimizing the swelling, and the hydrophobic nature of the PDMS layer, ensuring stable force and outperforming other dual-component hydrogel systems.
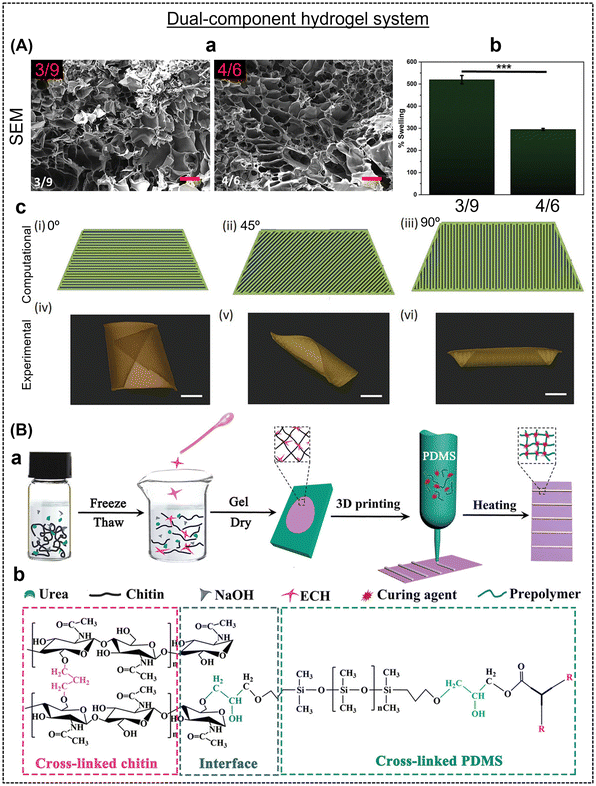 | ||
| Fig. 1 (A) (a) SEM images and (b) % swelling of two different types of gels. Computational prediction and experimental validation of various modeled G-codes and real-time photographs of the deformed gels in the presence of Ca2+. Scale bars are 12 mm. Reproduced with permission.21 Copyright 2023, Wiley Online Library. (B) Fabrication and chemical cross-linking process of shape-shifting actuators developed using chitin/PDMS composite. Reproduced with permission.44 Copyright 2020, Wiley Online Library. | ||
Alternatively, mono-component hydrogel systems have also been demonstrated. However, unlike dual component hydrogel systems, shape-change due to swelling gradient, infill pattern, and infill angle between the two layers are difficult to achieve employing only one material. Lai et al. printed multiple layers of alginate-based ink, which, apparently on-air drying, formed varying intra-structure network densities (dense and sparse region), leading to anisotropic swelling and controlled shape morphing upon chemical cross-linking. Similar to a dual-component system, a solid bottom and patterned top layers were printed using the same hydrogel ink instead of two.45 The gel was printed at ∼2.5–3 bar, with a layer thickness of 0.2 mm, at the rate of 8 mm s−1 through a 22G nozzle. Recently, Ghosh and group demonstrated the development of a shape-morphing mono-component system using a customized DIW printer.46 The chitosan-based ink could print at 5 bars through a nozzle (diameter: 500 μm) at the rate of 5 mm s−1, and the printed structures were cross-liked via thermal curing (150 °C, 10 min). The authors observed shape-morphism in the printed structure only when one side of the chitosan film was exposed to the solvent. This dynamic behavior of the chitosan structure was not demonstrated upon complete immersion, which is mainly due to the net-zero concentration gradient across the film. The reported hydrogel was employed as a gripper to lift objects seven times its weight.
Shape-morphing is an attractive feature of a material; however, to date, most of the published reports showed unidirectional shape-morphism. It is a challenge to create multidirectional or reversible actuators. In this case, our group reported a the fabrication of a hydrogel with a humidity-responsive interpenetrating network, which was capable of changing its shape in more than one direction due to gradient photo-crosslinking and anisotropic water absorption (Fig. 2A).43 The hydrogel ink was comprised of methacrylated carboxymethyl cellulose, a photoinitiator (lithium phenyl-2,4,6-trimethylbenzoylphosphinate), and methylcellulose. During the process of photocrosslinking, the gradual reduction in light intensity as it penetrates deeper into the material results in a spatial gradient of cross-linking density throughout the thickness of the hydrogel, leading to the upper portion receiving more intense exposure to the incoming irradiation, and consequently forming a higher degree of cross-links compared to the lower portion. After the hydrogel beam was submerged in deionized water, this gradient in photo cross-linking led to uneven swelling within the beam, resulting in rapid bending into a ring shape. As discussed above, Ghosh and group also highlighted the solvent-actuated reversibility in chitosan-based hydrogels, which is probably due to the limited solubility of chitosan in ethanol, which led to the contraction of the polymer chains.46 Moreover, Mao et al. commented on the reversibility of the printed structures in a water/ethanol system. The bidirectional shape morphism is truly a unique phenomenon.44 In another report, Naficy et al. demonstrated temperature-induced reversible or bidirectional shape-morphism in a photo cross-linked poly(NIPAM) and poly(HEMA) bilayer hydrogel.47 The bilayer hydrogel flat sheets underwent shape morphism when they became fully swollen at temperatures below 32 °C and reverted to their flat state when the temperature exceeded 32 °C (Fig. 2B). The thickness and length of the printed materials were two crucial parameters to achieve reversibility.
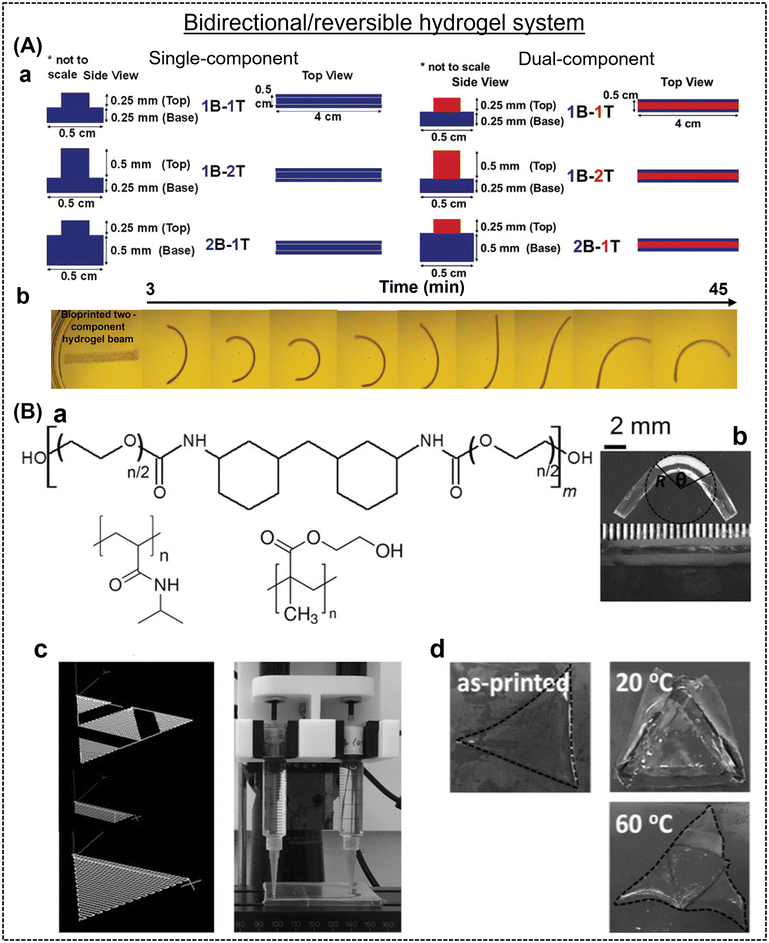 | ||
| Fig. 2 (A) (a) 4D hydrogel fabrication process and (b) bi-directional shape-morphing features of single- and dual-component hydrogel systems. Reproduced with permission.43 Copyright 2023, Wiley Online Library. (B) (a) Extrudable inks prepared using polyether-based linear polyurethane, poly(NIPAM), and poly(HEMA), (b) bilayer hinges in a deformed state, (c) hydrogel printing process and its design, and (d) real-time images of printed hydrogels at different temperatures. Reproduced with permission.47 Copyright 2016, Wiley Online Library. | ||
2.2. Dynamic-light processing
DLP is an alternative printing method to create shape-morphing hydrogels; however, there have been few reports on this topic thus far. The first report by Zhao et al. explored the feasibility of designing intricate wettability behavior-induced shape-shifting structures.48 An innovative composite comprised of a hydrophilic layer (polyethylene glycol diacrylate; PEGDA) that swells in water and a hydrophobic coating (poly(propylene glycol) dimethacrylate; PPGDMA) providing structural support was fabricated with sequential actuation behavior using a DLP projector (Fig. 3A). Instead of relying on intricate photomasks, the polymer structures were generated by illuminating predefined grayscale images, either in a single-step or multi-step manner. A mismatch in strain was induced by fixing hydrophilic PEGDA rubber on both sides of the hydrophobic PPGDMA sheet to achieve shape change. Eventually, the DLP-processed flat sheets were immersed in water, leading to equilibrium swelling and attaining their final shapes. For example, non-uniform bending and subsequent out-of-plane buckling of a ring-shaped plate transformed into a wavy ring. DLP technology offered the advantage of seamlessly introducing a light intensity gradient into the illumination patterns, facilitating complex shape transformations. Based on a similar technique, our group formulated a humidity-responsive photopolymerizable bio-ink to print shape-morphing adult human tissue.49 The bioink featured cell-adhesive Arg-Gly-Asp (RGD) peptide sequences rich in methacrylated gelatin (GelMA) and PEGDM to provide cell support and mechanical integrity to the printed structures, respectively. DLP processing involved the use of visible light (405 nm)-responsive photoinitiators, specifically lithium phenyl-2,4,6-trimethylbenzoylphosphinate, and a photoabsorber, tartrazine (Fig. 3B). The first two layers of the bio-ink, each having a thickness of 100 μm, were exposed to a consistent curing time of 56 s, while the subsequent layers received a shorter curing time of 14 s. Subsequently, these sheets were extracted from the build plate and immersed in water for 2 h to observe their shape change. Tartrazine governed the depth of curing in the layer-by-layer printed structures, thereby imparting a gradient in cross-linking across the construct.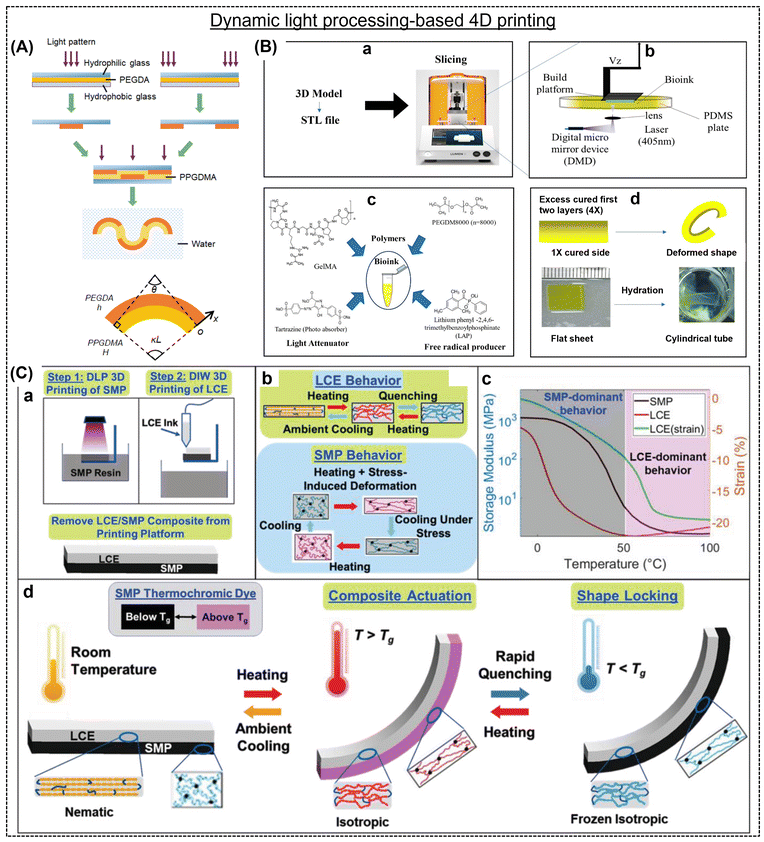 | ||
| Fig. 3 (A) Schematic representation of the hydrophilic/hydrophobic composite hydrogel preparation and shape-morphing feature. Two independent PEGDA patterns were cross-linked using different light, and subsequently the hydrophilic glass was removed from the hydrophobic glass. Two PEGDA patterns were assembled and injected with PPGDMA liquid resin to cure the whole structure. Reproduced with permission.48 Copyright 2018, ACS. (B) 4D Bioprinting of P2G12.5 Bioink in Cell Culture Media and structural deformations after hydration. Reproduced with permission.49 Copyright 2023, ACS. (C) Schematic representation of (a) two-step 3D printing (DLP&DIW), (b) thermomechanical behavior, (c) storage modulus curves, and (d) shape-transformation of the LCE and SMP composites. Reproduced with permission.51 Copyright 2022, Wiley Online Library. | ||
In recent times, the realm of grayscale curing has been extended to DLP-based printing by Kuang et al.50 They developed a hybrid ink containing bisphenol A ethoxylatediacrylate (BPADA), glycidyl methacrylate (GMA), n-butyl acrylate (BA), a diamine cross-linker [poly(propylene glycol) bis(2-aminopropyl ether); D230], photoinitiators (Irgacure 819) and photo-absorbers (Sudan I). In single-vat g-DLP 3D printing, grayscale images, representing the light intensity in monochrome mode, were employed to solidify the resin layer-by-layer selectively. The desired structure was initially divided into images corresponding to individual printing layers. These images were processed using a MATLAB code to create grayscale distributions based on the desired properties. Subsequently, a UV projector was employed to print each layer with grayscale patterns. Initially, the acrylates underwent radical-induced photopolymerization, forming a polymer network that defines the shape of the printed part. The cross-linking density and modulus of a material decrease as the grayscale percentage increases. Following a desolvation treatment in a water/acetone solution (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), the compact 3D-printed flower transformed into a fully blossomed state, triggered by the contraction of the outer layer of each petal. Subsequently, when treated with acetone, the blossomed flower reverted to its original un-blossomed form, driven by the swelling disparity between the outer layer with a lower cross-linking density and the inner layer with a higher cross-linking density. This reversible shape-shifting process continued to occur iteratively as acetone was absorbed, and subsequently evaporated. In another study by Roach et al., a 4D-printed composite material composed of LCE and SMP was developed.51 The printed material showed rapid and reversible shape changes, while retaining a mechanical stiffness of approximately 1 GPa in its actuated state (Fig. 3C). Shape changes were triggered by the thermo-mechanical properties of LCE and SMP, which depended on the cooling rate. Specifically, varying cooling rates allowed the composite to assume different shapes, while maintaining high stiffness at lower temperatures (Fig. 3C-d). A multi-material 4D printing approach was utilized to construct a bilayer composite, where SMP and LCE were printed using the DLP and DIW techniques, respectively. This novel approach opens the door to potential applications in various fields, including space exploration, biomedicine, soft robotics, and energy technologies.
1 v/v), the compact 3D-printed flower transformed into a fully blossomed state, triggered by the contraction of the outer layer of each petal. Subsequently, when treated with acetone, the blossomed flower reverted to its original un-blossomed form, driven by the swelling disparity between the outer layer with a lower cross-linking density and the inner layer with a higher cross-linking density. This reversible shape-shifting process continued to occur iteratively as acetone was absorbed, and subsequently evaporated. In another study by Roach et al., a 4D-printed composite material composed of LCE and SMP was developed.51 The printed material showed rapid and reversible shape changes, while retaining a mechanical stiffness of approximately 1 GPa in its actuated state (Fig. 3C). Shape changes were triggered by the thermo-mechanical properties of LCE and SMP, which depended on the cooling rate. Specifically, varying cooling rates allowed the composite to assume different shapes, while maintaining high stiffness at lower temperatures (Fig. 3C-d). A multi-material 4D printing approach was utilized to construct a bilayer composite, where SMP and LCE were printed using the DLP and DIW techniques, respectively. This novel approach opens the door to potential applications in various fields, including space exploration, biomedicine, soft robotics, and energy technologies.
2.3. Solvent casting
Solvent casting is a facile alternative to the abovementioned additive manufacturing techniques; however, it is rarely explored for 4D fabrication due to the lack of control over the internal structures. Briefly, a dissolved polymer solution solidifies via the evaporation of the solvent in a mold of the desired shape and size.52 Solvent casting can offer a unique approach to creating dynamic materials. Unlike 3D printers, where the fidelity of the ink is critical to the process, solvent casting is versatile and suitable for most polymers. In addition, the cost of a 3D printer and harboring skilled manpower are significant, while solvent casting only requires a container and precursor. As discussed above, shape transformation is often a result of gradient crosslinking, which is generated carefully by printing one or more materials in a way that their different swelling behaviors and porosity can be exploited. On the contrary, solvent casting involves the drying of the solvent. If the reaction occurs at room temperature, a natural intra-structural gradient is created due to the depth of the solution, subsequently triggering shape-morphism in response to moisture. For example, Hu et al. reported a solvent casting method to produce a chitosan hydrogel film (mono-component) capable of shape-transformation due to the gradient crosslinking and geometry effect created by the top-down diffusion of glutaraldehyde molecules within a chitosan pre-gel solution (Fig. 4A).36 The chitosan film forms tight networks (densely cross-linked) at the top surface owing to complete exposure to the glutaraldehyde solution.53 In contrast, the bottom surface was only sparsely cross-linked due to the inability of glutaraldehyde to penetrate the deeper sites. The formation of dense-sparse networks within the structure was validated by the variation in pore size and Young's modulus value (Fig. 4A-b). In the report by Guo et al.,54 they showed that even air-dried 3D printed structures can undergo shape-morphing due to the naturally created anisotropy within the films. Poly(stearyl acrylate-co-acrylic acid) (P(SA-co-AAc)) dissolved in ethanol was extruded onto a substrate, then allowed to partially solidify through solvent evaporation in the air, and subsequently subjected to solvent exchange in water to create gel constructs with altered morphologies. The differences in swelling properties induced a transformation in the gel structures. Solvent casting fabrication techniques, though rare, have still been found in the literature to develop shape-shifting structures, mainly using air-drying techniques. By carefully controlling this crosslinking gradient, engineers and researchers can manipulate the response of a material to external stimuli.55 This innovative approach involves selectively varying the degree of crosslinking within a material, creating a gradient in its stiffness or rigidity. Shape-morphing materials designed by air drying typically rely on moisture absorption or desorption as their triggering mechanism. Therefore, their structure may gradually return to its original state upon dehydration.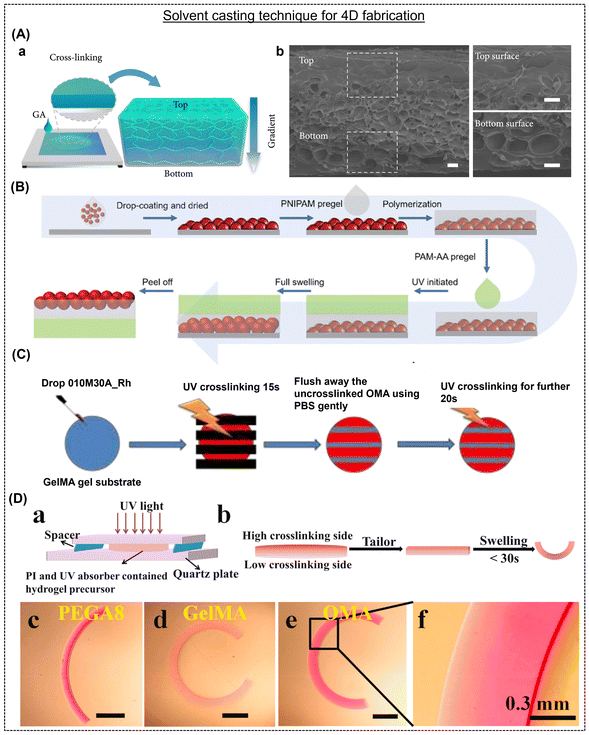 | ||
| Fig. 4 (A) Schematic representation indicating gradient cross-linking density within the chitosan hydrogel films. Reproduced with permission.36 Copyright 2021, Science. (B) Fabrication process of a hydrogel with structural and shape change properties. Reproduced with permission.56 Copyright 2022, Wiley Online Library. (C) Schematic illustration of patterning OMAs onto a single GelMA. Reproduced with permission.57 Copyright 2021, Wiley Online Library. (D) (a and b) Typical experimental setup to prepare hydrogel with differential gradients and (c–f) microscopic images of various hydrogels after swelling. Reproduced with permission.58 Copyright 2022, Elsevier. | ||
Yu et al. employed a process to fabricate reversible grippers involving the fabrication of a bi-layered structure comprised of poly(N-isopropylacrylamide) (PNIPAM) and inert poly(acrylamide–acrylic acid).56 The multi-step fabrication technique involved the synthesis of a thermos-responsive poly(N-isopropylacrylamide-co-styrene) (PNIPAMST) microgel emulsion through surfactant-free precipitation polymerization, followed by the preparation of the PNIPAMST microgel film by coating it onto a glass coverslip and obtaining a dry film, and then the fabrication of a PNIPAM pregel by dissolving its precursors in deionized water (Fig. 4B). A dried PNIPAMST microgel film was used as the base, onto which NIPAM solution was casted, followed by AM-AA/multi-walled carbon nanotube (MWCNT) dispersion, resulting in a bilayer hydrogel that was shaped into a flower and tested for its flexibility using varying thickness ratios and lengths in the absence of the PNIPAMST microgel layer. The structure could successfully replicate the function of an octopus, together with having the properties of tunable color and shape under the influence of a temperature stimulus. The near infrared (NIR)-active MWCNTs also contributed the added benefit of the flower structure blooming or opening up upon irradiation.
In another report, Ding et al. demonstrated a natural polymer-based hydrogel system capable of “on-demand”, multiple, and reversible shape-morphing due to multilayered oxidized methacrylated alginate (OMA) and GelMA hydrogels (Fig. 4C).57 The facile fabrication method involved initially UV-crosslinking the OMA hydrogel, followed by crosslinking the GelMA solution between the OMA layers, thereby offering simplicity, convenience, and adaptability, and consequently facilitating the production of hydrogel actuators with varying levels of complexity. Alsberg's group showed 4D biofabrication using a mono or dual component strategy. A mixture containing polymer (OMA 6% w/v, GelMA 14% w/v, or PEGA8 20% w/v), PI (0.05% w/v), and UV absorber, fluorescein isothiocyanate derivatives, and/or 4′-hydroxy-3′-methylacetophenone in a cell medium in the absence and presence of mammalian cell lines was placed between two quartz plates (with a 0.6 mm spacer) and photo-cross-linked with UV light (20 mW cm−2) for 30 s (OMA), 180 s (GelMA), and 30 s (PEGA8) to fabricate a hydrogel bar (13 mm × 2 mm × 0.6 mm) (Fig. 4D).58 OMA gel precursors were placed underneath a pre-gelled GelMA layer (30 s UV) and cut into bilayer bars to form bilayered constructs. The hydrogel bars were immersed in an aqueous solution to observe shape-morphing and reached maximal bending in 30 min. This method facilitated the synthesis of large-scale microstructures in a substantially simpler, economical, and quicker way. Utilizing the gradient crosslinking principle, it becomes feasible to intentionally create multiple gradients featuring more than one direction within a single hydrogel framework. Consequently, this method simplifies the generation of intricate structures possessing asymmetric geometries within a monolayer hydrogel.
3. Types of stimulation and their mechanism
Reflecting on the recent advancements in the processing of hydrogels, they are considered a class of “smart” materials by demonstrating programmable shape-morphing behavior upon exposure to various chemical and physical stimuli. Various stimulation strategies exist to drive the shape change in hydrogels, including chemical, physical and biochemical signals.29 Hydrogels are intrinsically isotropic materials that experience uniform volumetric expansion and contraction in response to suitable stimuli.1 However, upon careful fabrication, hydrogels can be tuned to exhibit differential swelling by restricting and/or increasing swelling in certain positions or directions in the same structure.59 Careful generation of anisotropy in the structure of hydrogels will allow them to transform into different shapes immediately after swelling. In this section, we discuss the various stimulation mechanisms that can yield shape morphing in hydrogels.3.1. Solvent/humidity
The natural phenomenon in hydrogels, i.e., swelling and shrinking, has been leveraged to realize anisotropic swelling-mediated shape morphing.6 These gels can be smartly sandwiched between passive layers (unresponsive to moisture) to drive out-of-plane motions. For instance, an active layer comprised of a copolymer of PEG and polytetramethylene glycol (PTMG) and an inactive layer of beeswax were stacked on each other, which underwent fast-hygroscopic expansion of the copolymer, leading to the structure bending towards the inactive layer side (Fig. 5A).60 This phenomenon is due to the swelling differences between the two layers. Another way of generating anisotropy in a structure is to fabricate composites of a soft gel and a stiffer reinforcement. For example, Gladman et al. 3D printed a humidity-responsive N,N-dimethylacrylamide-based matrix incorporated with stiff nanofibrillated cellulose, which caused alignment of the fibrils along the printing path, leading to anisotropic stiffness in the printed part and a curved shape after swelling (Fig. 5A-b).61 The shape changes were programmable based on the print path, and the final curvature was predicted using modeling. Besides reinforcement-induced anisotropy, different compositions of the same material, which have significant differences in their swelling rates, can be stacked upon each other during 3D printing to drive anisotropic swelling and out-of-plane movements. Our group prepared a dual-component hydrogel system with shape-shifting capabilities.21 Two gels with profoundly different swelling behaviors in water were prepared by varying the composition of alginate and methylcellulose. Both gels were stacked on each other by extrusion-3D printing, which also controlled the infill angles and density of the top (lower swelling rate) and bottom layer (higher swelling rate). This led to programmable shape changes in a single structure by varying the printing parameters, and theoretical simulations predicted the same. Based on a similar principle, Ji et al. showed a one-step 3D printing process to prepare a moisture shape-morphing hydrogel system comprised of polyethylene glycol diacrylate (PEG400DA) and 2-hydroxyethyl methacrylate (HEMA) (Fig. 5A-c).62 Anisotropy was introduced by the secondary grooves on one side of the structure, which led to either bending or twisting due to local curvatures and asymmetrical swelling. Water is an inert and biocompatible stimulus for triggering the shape morphing of constructs embedded with cells; therefore, it can be leveraged for fabricating complex tissue constructs.30,63–66 For example, Kirillova et al. employed a mixture of methacrylated alginate and methacrylated hyaluronic acid, 3D printed with bone marrow stromal cells, photo cross-linked, and mildly dried.67 The crosslinking gradient induced by the top layer by absorbing more light than the bottom, bioprinted constructs exhibited instant self-folding into tubes with the cells distributed evenly along the walls of the formed tube Although. However, although moisture/solvent is a natural and benign actuation method, a key bottleneck remains to be resolved, i.e., the long-term stability of the formed constructs in vivo and their performance in tissue regeneration.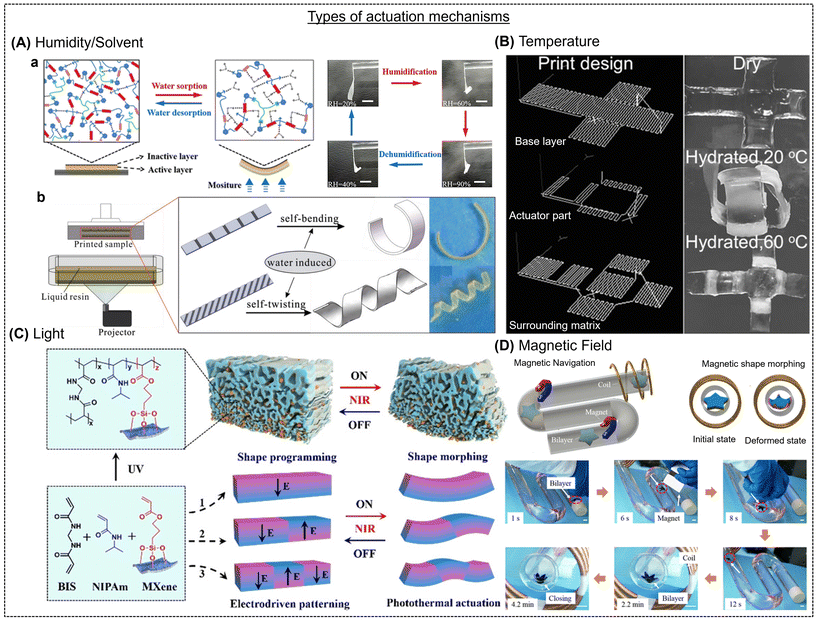 | ||
| Fig. 5 (A) Humidity/solvent-responsive shape-morphing hydrogels. (A-a) Structural changes in the copolymer comprised of an active (yellow) and inactive (black) layer involving chitosan bonds upon moisture sorption and desorption. Reproduced with permission.60 Copyright 2019, Wiley Online Library. (A-b) Experimental printing setup (left) and shape-morphing of the 3D-printed construct with pre-programed secondary grooves. Reproduced with permission.62 Copyright 2019, Wiley Online Library. (B) Three layers of a 3D printed construct, base layer, thermos-responsive part, and a surrounding matrix and their reversible shape-shifting phenomenon in response to temperature. The scale bar is 1 cm. Reproduced with permission.47 Copyright 2016, Wiley Online Library. (C) Chemical structures, 3D printed materials, electro-driven shape-programming, and light-induced shape-changes in MXene-containing hydrogels. Reproduced with permission.76 Copyright 2020, Wiley Online Library. (D) Navigation and shape morphing of a star-like bilayer in response to an alternating magnetic field. The scale bar is 10 mm. Reproduced with permission.84 Copyright 2019, ACS. | ||
3.2. Temperature
Changes in temperature, especially in a range compatible with human tissues of ≈36–38 °C, can be an attractive trigger to achieve shape deformations in hydrogels. The temperature-dependent molecular interactions between the polymer chains in a hydrogel and the solvent lead to profound swelling or shrinkage. Thermally responsive hydrogels can exhibit a lower critical solution temperature (LCST) or an upper critical solution temperature (UCST). The LCST hydrogels with negative thermosensitivity are shrunk above their critical temperature, while UCST hydrogels with positive thermosensitivity remain swollen above their critical temperature.68 Thermoresponsive gel systems are largely dominated by reversible LCST-based hydrogels, and most notably poly(N-isopropyl acrylamide) (pNIPAM) and its derivatives.69 This is due to the large volume changes in pNIPAM at a relatively low LCST temperature of ≈32 °C.70 For example, Naficy et al. developed bilayered hydrogels from thermosensitive pNIPAM and non-active polyHEMA, which could self-morph from a flat shape to controllable 3D structures when the temperature was above 32 °C and back to the flat state when the temperature was below 32 °C (Fig. 5B).47 This temperature-dependent reversible shape-morphing phenomenon was explained by a simple model that correlates the material and printing parameters with the final 3D structure of the hydrogels.47 In another report, Xu et al. prepared Fe3+ ions cross-linked hydrogels from pNIPAM and sodium methyl acrylate monomers to enable complex shape changes, including origami designs.55 Through the local release of Fe3+ ions into the gel network and further periodic crosslinking, the gel exhibited shape deformations into a helix below 50 °C, and the helix deformed above 50 °C, leading to reversible out-of-plane deformations. However, going further, more rapid and complex shape changes need to be designed in hydrogels at temperatures around the physiological range to fulfill a range of biomedical applications.3.3. Light
Light stimulation has attracted significant interest due to the ability to precisely control its properties, such as wavelength and intensity, and the capability of contactless remote manipulation.71,72 In contrast to other actuation modes that enable the gel to respond globally, light can trigger specific and localized reconfigurations by irradiating specific regions of the sample.73 Photoresponsive gels exhibit large volume changes via swelling or shrinkage due to water uptake or release, respectively, upon light radiation.74 The swelling rate of a gel can be tuned by the polymer hydrophilicity and crosslinking density, which can also be manipulated using light. NIR light is preferred for biomedical applications over ultraviolet and visible light due to its higher tissue penetration depth. NIR-triggered shape morphing was explored in alginate-polydopamine cell-laden gels, wherein the initial lamellar structure transformed into a saddle-like shape by gradual dehydration under NIR.75 In another report, Xue et al. employed a photopolymerizable MXene nanomonomer in conjunction with thermosensitive PNIPAm hydrogels to induce a concentration gradient of MXene nanosheets across the thickness of the gel, resulting in a shape change (Fig. 5C).76 These gels exhibited fast light-driven directional shape morphing due to the photothermal effect of MXene. Other strategies include inserting regions of a light-active composite, i.e., photothermal reduced graphene oxide (rGO) fillers in a thermoresponsive gel, PNIPAm, into a non-responsive matrix, which generates swelling mismatch, and consequently shape change.77,78 The shape change could be global by immersing the structure in water at 50 °C (above the LCST of PNIPAm) or localized by directing light on a specific region of the strips. One of the major limitations of light-driven shape morphing gels is the small extent of shape change, which limits its application prospects.3.4. Magnetic field
A magnetic field is a preferred stimulus for driving remote and local shape changes and is biocompatible even at high field strengths.79,80 Magnetic hydrogels are usually fabricated by reinforcing magnetic fillers, such as iron oxide and cobalt oxide, which can respond to an external magnetic field (MF).81 Three fundamental fabrication strategies exist for developing magnetic 3D hydrogels, as follows: (a) in situ precipitation, (b) blending, and (c) grafting-onto method.82,83 For instance, Wang et al. prepared magnetic hydrogel nanocomposites (also called ferrogels) that can respond to an external MF in a specific way, leading to shape morphing.77 Magnetic poly(N-isopropyl acrylamide) hydrogel/elastomer hydrogel/elastomer hybrids could change shape and move in response to a direct or an alternating magnetic field. For example, Tang et al. designed a magnetic octopus-like hydrogel by stacking two different gels via 3D printing, where the magnetic nanoparticles incorporated acrylamide carbomer and the top part only contained acrylamide-carbomer ink (Fig. 5D).84 The structure could be actuated using a programmed MF. Another approach is to apply a magnetic field to the dispensing nozzle of a 3D printer during the process of printing microparticles of neodymium–iron–boron (NdFeB) alloy and fumed silica nanoparticles in a silicone rubber matrix, leading to the reorientation of the ferromagnetic domains. Remote shape morphing can also be obtained by exploiting the magnetothermal effects of magnetic nanoparticles well dispersed in a gel network. In another report by Tang et al., iron oxide nanoparticles were precipitated in situ on a pNIPAM hydrogel cross-linked with nanoclay, and a bilayer hydrogel was designed with the pNIPAM composite gel and an elastomer.85 The bilayered gel underwent self-folding upon the application of an alternating MF due to the selective heating of the pNIPAM composite gel. In situ heating raised the local temperature of the gel higher than its LCST. Thus, the respective layer shrunk, causing a bend in the structure. However, the utility of MF-actuated hydrogel systems is limited due to the requirement of bulky instrumentation and potential interferences in medical imaging.3.5. Other(s)
There are a few other actuating mechanisms for shape-morphing hydrogels. For example, pH-responsive hydrogels are comprised of ionic (cationic or anionic) pendant groups in their polymeric backbones.29 Upon exposure to the desired pH and ionic strength solution, the pendant groups tend to ionize and form fixed charges on the polymer network, leading to swelling or shrinkage of the gel.86 For instance, Gupta et al. reported the swelling of anionic hydrogels such as polyacrylic acid at a pH value higher than its pKa (acid dissociation constants), owing to the ionization of the pendant groups and large osmotic swelling force.87 Alternatively, the polyacrylamide-based cationic hydrogel shrunk at a pH value higher than its pKa.88 An electric field is an efficient way of driving shape morphing in hydrogels. Hydrogels immersed in an electrolyte solution generate charged ions and counter ions at a particular voltage, which are attracted to opposite directions by electrophoretic forces, leading to the electroosmotic movement of water molecules. The electrically induced diffusion of water molecules from the gel led to swelling/shrinkage.89 The extent of deformation depended on the intrinsic (e.g., stiffness and charge density) and extrinsic (e.g., ionic strength and applied voltage) properties of the gel.90 Han et al. prepared an electroactive gel-based ink comprised of a monomer, i.e., acrylic acid, and a cross-linker, i.e., PEGDA, which was printed using projection microstereolithography.91 The 3D printed structures were subjected to different electric fields and the ionic strength of the electrolyte (PBS in this case) to analyze their bending curvatures. A few reports utilized more than one stimulation strategy to achieve shape-morphism. For example, Li et al. demonstrated an interesting hydrogel-metal hybrid responsive to light and magnetic fields.92 Biological stimuli, such as enzymatic degradation, can also be utilized as a stimulation mechanism to realize shape morphing in gels.934. Tissue engineering and drug delivery applications of dynamic 4D hydrogels
4.1. Tissue engineering
Tissue engineering emerged from the field of biomaterials and refers to the application of an assembly of constructs of cells, scaffolds, and bioactive molecules to repair damaged tissues and restore cellular functionalities.94,95 However, it is associated with four major challenges including material choice, cell source, limited vascularization, and poor drug delivery systems.96 Consequently, shape-morphing hydrogels have emerged as alternative biomaterials possessing multiple, smart, and stimuli-responsive features.23 Shape-morphing hydrogels exhibit a matching tissue stiffness and can mimic anisotropic tissue composition, allowing them to transform into predetermined shapes in response to external stimuli.61 Hydrogel-based scaffolds can direct or induce cells to mature into a structure through a hierarchical differentiation process, resulting in replica tissue. The scaffold is essential for physical and structural support and promotes cell growth and optimum nutrient diffusion.97 Conventional 3D printing technology failed to create tissue-mimicking dynamic matrices.98–100 Therefore, developing 4D fabrication techniques is the key to creating a biological constructs that can substitute a defective tissue and restore organ function by fusing engineering principles with biological sciences.This dynamic nature of tissue constructs printed with cells allows the assembly and disassembly of the printed parts. Cell-laden structures mature through self-regulation, migration, and matrix deposition, gradually developing into an active construct.101 For instance, Garcia et al. co-polymerized acrylic acid with PEGMA to yield a pH-sensitive construct using SLA printing.102 The resultant antimicrobial scaffolds demonstrated improved mammalian cell proliferation after curing with ethanol. In another report, Miao et al. prepared a temperature-responsive shape-shifting soybean oil-epoxidized acrylate-based scaffold.103 The dynamic gels were biocompatible and endowed a proliferation support matrix for multipotent human bone marrow mesenchymal stem cells. Constante et al. developed a dual-component alginate-methylcellulose (extrusion printing) and PCL fiber (melt-electrowetting)-based scaffold that self-rolled in the presence of Ca2+ ions.104 They reported that the C2C12 myoblasts grown on these 4D gel matrices showed good vitality, proliferation, and sequence positioning across the PCL fibers. Gladman et al. used an N,N-dimethyl acrylamide hydrogel to control the hardness and water solubility of the printed constructs.61 The local orientation of the colloidal fibers followed a theoretical model for a three-dimensional structure, opening the door for multiple tissue engineering approaches. More interestingly, the hydrogel constructs printed with mammalian cells also demonstrated shape-deformation (Table 1). Recently, our group developed a bio-ink comprised of GelMA and PEGDM 8000 that could be fabricated with DLP printing with a visible range light source (405 nm). These printed hydrogels folded in response to water, were non-toxic, and showed continuous proliferation of embedded cells.49 Below, we discuss numerous dynamic hydrogels and their importance in drug delivery and tissue regeneration (cardiac and neural tissue).
| Material | Fabrication strategy | Stimuli | Application | Characteristic feature | Ref. |
|---|---|---|---|---|---|
| Alginate and hyaluronic acid | Extrusion | Moisture | Vascular tissue | • Employs two biopolymers | 67 |
| • Fabrication of hollow self-folding tubes | |||||
| • The diameter of the folded tube is ∼20 μm | |||||
| • Enabled 4D bio fabrication (bone marrow cells) | |||||
| Poly(glycerol dodecanoate) acrylate (PEGDA) | Extrusion | Temperature | Vascular stent | • Ideal for room temperature shape programming | 105 |
| • Potential for deployment within the human body | |||||
| • Suitable thermo-rheological properties enabled the printing of multifunctional 3D structures | |||||
| Soybean oil epoxidized acrylate (SOEA) | Photolithographic-stereolithographic-tandem strategy | Temperature | Cardiac tissue | • Rapid development of films (within seconds) having varied thicknesses | 106 |
| • Printed constructs indicated unique surficial micropatterns | |||||
| • Biocompatible and promotes hMSC proliferation | |||||
| GelMA and PEGDA | SLA | Solvent | Cardiac tissue | • A 4D cardiac patch with physiological adaptability | 107 |
| • Designed to improve biomechanical properties and the dynamic integration of the patch with the beating heart | |||||
| • Increased cell engraftment and vascular supply in a murine chronic MI model | |||||
| PEGDA, bisphenol A diglycidyl ether, poly(propylene glycol) bis(2-aminopropyl) ether, and decylamine | DLP and replica molding | Light | Cardiac tissue | • Cardiac construct with highly aligned microstructure and adjustable curvature | 108 |
| • Capacity to actuate remote-controlled spatiotemporal transformation | |||||
| • Efficient method for manufacturing curved tissue architectures | |||||
| Bisphenol A diglycidyl ether, decylamine, and poly (propylene glycol) bis(2-aminopropyl) ether, graphene nanoplatelets | FDM, extrusion, and replica molding | Temperature and light | Neural tissue | • Nanomaterial-engineered stimuli-responsive hydrogel | 109 |
| • Dynamic and on-demand shape transformation | |||||
| • Created 3D patterned biological structures that can spatiotemporally control their shapes | |||||
| SOEA | SLA | Solvent (water/ethanol) | Neural tissue | • Stress-induced shape transformation | 110 |
| • A proof-of-concept of smart nerve guidance nano-engineered conduit | |||||
| • Created multi-responsive smart architectures | |||||
| 4-Hydroxy butyl acrylate, urethane-polyethylene glycol-polypropylene glycol (PU-EO-PO) monomer and fracture | DLP | Magnetoelectric | Neural tissue | • Developed electro-magnetized carbon nanocookies | 111 |
| • Fostered neuron cell differentiation and proliferation in vitro and in vivo | |||||
| • Enhances cell adhesion and allows direct manipulation of electromagnetic stimulation of the cells | |||||
| • Showed magnetic field-guided in vivo neuron regeneration | |||||
| Polyvinyl alcohol (PVA) | FDM | Moisture and temperature | Drug delivery (Allopurinol) | • Shapes were designed in principle to enable gastric retention | 112 |
| • Temporary shapes were compatible with oral administration into capsules | |||||
| • Prototypes caused water-induced shape recovery | |||||
| PEGDA | DLP and Projection micro stereolithography | Hydration/dehydration | Drug delivery (Rhodamine B) | • Development of minimally invasive, pain-free, and easy-to-use microneedle | 113 |
| • Bioinspired backward-facing curved barbs for enhanced tissue adhesion (>18-fold) | |||||
| • Sustained drug release | |||||
| PVA and glycerol | Hot melt extrusion and FDM | Moisture | Drug delivery (Caffeine) | • Shape transformation at body temperature | 114 |
| • Device could be administered via a catheter in a deformed state | |||||
| Sodium alginate and Pluronic F127 diacrylate macromer | Extrusion | Ions | Drug delivery (Methotrexate) | • Dual network structure | 115 |
| • A stable network is a cross-linked and reversible network that undergoes ionic cross-linking | |||||
| • Rapid in vitro drug release |
Several recent studies have reported a 4D fabrication strategy to produce these intricate designs,122,170 also allowing the synthetic cardiovascular stent to be delivered and performed at the site in a minimally invasive manner. In most investigations, the shape-morphing feature is attempted to be achieved under physiological conditions. In this regard, Cui et al. developed an innovative 4D cardiac patch using GelMA and PEGDA via beam-scanning SLA.107 Light-mediated cross-linking created graded internal stress, resulting in shape-morphism to yield a wavy and curved structure. This biomimetic patch was cultured with induced pluripotent stem cells, mesenchymal stem cells, and cardiomyocyte maturation and vascularization were observed (Fig. 6A).107 Three weeks after implantation, the patches developed a strong bond with the epicardium, improving vascular infiltration and cell engraftment and leading to cardiac regeneration. An implantable device that can be readily regulated at room temperature would be beneficial. Toward this, PGDA-based polymers that can inevitably adapt to the physiologically relevant environment and the structural and mechanical adaptability of biomedical implants led to the development of new regenerative therapies for cardiovascular disorders. These are useful methods for addressing issues and enhancing the endurance of the infarcted area.123,124 To adhere to the heart and heal damaged myocardial tissue, the prepared scaffold patches should possess a curvature design that can create medically realistic surfaces for better alignment and incorporate dynamic mechanical stimulation.125
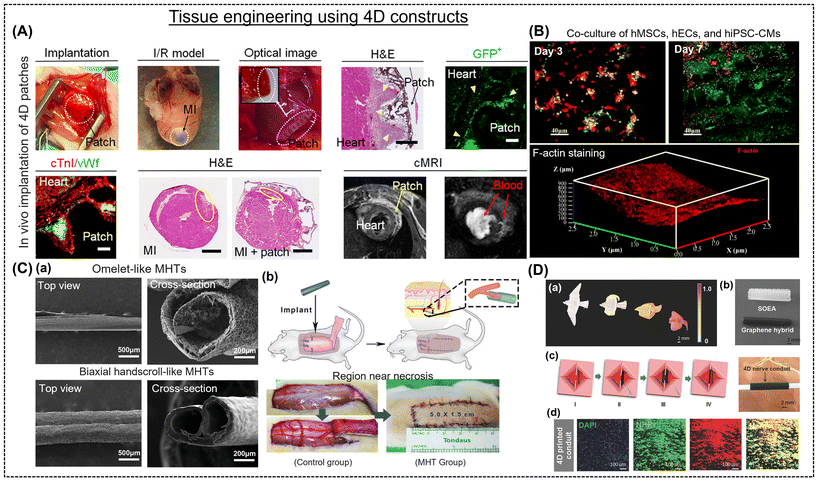 | ||
| Fig. 6 (A) Implantation and long-term evaluation of 4D patches in a mouse model. Reproduced with permission.107 Copyright 2020, Science. (B) Immunofluorescence staining images of a co-culture (hMSCs, hECs, and hiPSC-CMs) on biomimetic 4D cardiac tissue. F-actin staining of cells on the deformed shape. Reproduced with permission.108,128 Copyright 2021, ACS. (C-a) SEM images of the “omelet”- and “biaxial handscroll”-like micro-scaled hollow tubules (MHTs). (C-b) Schematic representation of the MHTs surgically implanted beneath the skin flap. The left and right images in (C-b) are after the placement of GelMA MHTs and the suture of the random flap after surgery. Reproduced with permission.128 Copyright 2020, Wiley Online Library. (D-a) Bird flying architectures designed with varying graphene concentrations (0–0.8%). (D-b) Dynamic nerve conduit prepared without graphene (white) and with 0.8% graphene (0.8 × 1.5 cm). (D-c) Representation of the tubulation of nerve conduit via a “thermomechanical programming” shape transformation. (D-d) Immunofluorescent images of neurogenic differentiation of hMSCs on the nerve conduit and its UV-crosslinked counterpart. Reproduced with permission.110 Copyright 2018, Wiley Online Library. | ||
The application of NIR light has increased due to its non-intrusiveness, higher penetration depth, and ability to regulate shape modification.109,126 Wang et al. showed that printing inks combined with graphene nanoplatelets can be used to develop micropatterned structures for the restoration of heart function.108 Due to NIR-based photothermal effects, the constructs displayed a change, towards a curvature, thus imitating the native architecture of the heart (Fig. 6B).108 This highlights the potential for controlling the building shape remotely and even cell distribution alongside myocardial maturation.127 Toward this, Miao et al. demonstrated that smart SOEA inks can create cardiac patches using an integrated SLA and photolithography process. The hMSCs grown on the patches formed a micro pattern, thereby controlling the alignment and differentiation of the cells.106 Due to the continuous self-folding and regeneration of the4D scaffold, it was employed as a myogenic bioreactor. Similarly, blood arteries can be built using a 4D self-folding polymer that, when moistened, can transform into a tube-like configuration.22 In this regard, Zhang et al. created 50–500 mm diameter microvascular tubules using methacrylated GelMA and hyaluronic acid that mimics microvasculature (Fig. 6C-a).71 Animal testing revealed that these constructs do not block circulation, promote vascularization, and boost the blood supply (Fig. 6C-b).128 The formation of 3D micro-scaled hollow tubules (MHTs) from a 2D-planar geometry in response to environmental cues was realized to mimic small vascularized models. For instance, extrusion-based bioprinting was employed to create bilayer MHTs of molten electro-writing polymer composites and photo cross-linkable hydrogels.19 Similarly, Kirillova et al. fabricated 20-micron size tubes using alginate and hyaluronic acid, and this adaptable 4D bio-fabrication architecture enabled the production of structures with sizes similar to the finest vessels.67 Cui et al. enabled the development of micropatterned constructs using inkjet printing to precisely control the cell–cell arrangement. These structures exhibited distinct swelling rates (the top layer swelled slowly compared to the bottom), forming a curved construct on exposure to water. The sacrificial layer allowed the control of the initiation of the self-folding process of the hydrogel and facilitated easy culturing of HUVECs, thereby mimicking human microvessels.129
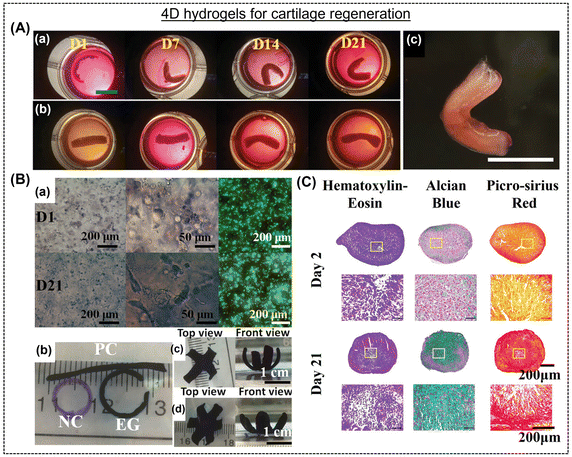 | ||
| Fig. 7 (A) Changes in the shapes of human mesenchymal stem cell condensate-laden bilayer strip ((a) experimental and (b) control groups) grown in chondrogenic media at different times (day 1, 7, 14, and 21). A (c) “C”-shaped cartilage mimicking tissue after three weeks of culture. Scale bar is 10 mm. Reproduced with permission.145 Copyright 2022, Wiley Online Library. (B) (a) Photomicrographs displaying morphology and distribution of cells together with live/dead staining assays after culturing in chondrogenic medium for day 1 and day 21. (B) (b) Printed hydrogels, (e) four- and (f) six-petal flower-shaped hydrogels after chondrogenesis for 21 days in culture medium. Reproduced with permission.146 Copyright 2022, Wiley Online Library. (C) Histological analysis using hematoxylin and eosin (purple), Alcian blue, and picrosirius-red staining of MSC pellets cultured in chondrogenic conditions for different time interval.147 Copyright 2022, Wiley Online Library. | ||
4.2. Drug delivery systems
Drug delivery platforms have been designed to perform at their optimum range when paired with a particular application (such as maximizing drug efficacy, minimizing side effects, enhancing bioavailability, prolonging residence time, and reducing drug administration frequency).148,149 The distribution method should be specifically tailored to the intended use and stimulus response to achieve optimal drug release outcomes. In this case, more advanced and target-specific systems can be made using the 4D printing approach. Advanced drug delivery devices can be created that allow programmed control of administered medicines, biomolecules, and cells. They can facilitate response-mediated, sustained administration and better retention at the targeted site.150 Using 4D printing technology, tablets, dermal skin patches, nano-suspensions, and rectal and vaginal delivery systems can be created with excellent precision.114,151,152 Combined, these factors will significantly lessen the efforts of medical staff, infection risk, operation, and patient recovery time.112 Due to these characteristics, researchers can use a modified structure in response to an external stimulus for a dynamic function. Drug-hydrogel associations are necessary for efficient storage and release under controlled conditions. Numerous chemical and physical reactions have been investigated for their potential and efficient delivery.153 For example, a UV-triggered chitosan-based helical micro-swimmer was fabricated by Bozuyuk et al.154 The micro-swimmer showed good biodegradability and drug release. The core–shell hydrogel system in a 4D-printed capsule fabricated by Gupta et al.155 released medications on demand at precise tissue site. The capsule contained ethylene glycol, poly(vinyl alcohol), and biomolecules in its core and gold nanorods (AuNRs) and poly(lactic-co-glycolic acid) in its shell. The photo-responsive AuNRs burst and released medicines upon to laser stimulation.It is possible to create multiple organ-retentive devices meant to be kept inside organs and gradually release drugs in the interim. In this regard, Malachowski and colleagues fabricated a multi-fingered theragripper (TG) drug-eluting device using photolithography. It facilitated controlled drug release through its layers of pores, and as it entered the body, it spontaneously grabbed hold of the tissue. Consequently, it effectively immobilized at a specific place and delayed drug release, allowing for improved control and decreased adverse effects (Fig. 8A).156 Recently, Zu and colleagues created a capsule using extrusion printing with an exterior made of a hydrogel (Fig. 8B).157 This temperature-sensitive capsule could compress and release most of its contents within 48 h. The amount of MMP-2 increased in a tumor metastatic area, breaking down the collagen proteins in the tissue remodeling process and acting as a biological catalyst that causes the targeted release of anti-inflammatory medicines from negatively charged hydrogels. In this process, enzymes break down the drug-loaded hydrogel to distribute the medications to the appropriate bodily locations as needed.158 In another report, Ceylan et al. developed a GelMA-based microrobot hydrogel that releases drugs by sensing MMP-2 in the microenvironment (Fig. 8C).159 Consequently, the increase in the concentration of these proteases acts as a stimulus for drug release. Similarly, Li et al. prepared a hybrid-actuated soft microrobot with iron oxide particles, PHEMA, and PEGDA, which allowed the targeted delivery of anti-tumor medicines (Fig. 8D).160 The therapeutic drug molecules used in 4D-printed devices are released after exposure to the appropriate stimulation from the designated sites. Osmolarity gradients can release the pertinent drug molecules from complicated multisome system frameworks.161 For example, Wang and colleagues created alginate and diacrylate Pluronic-based 4D-printed drug delivery patches and CaCl2 was used to initiate the brief shape change under external stress, and the ionically cross-linked (reversible) network was recovered using a sodium carbonate solution.115 The authors highlighted the effects of drug release based on the device shape and surface area. Conversely, Zhao et al. printed heparin-loaded bilayered GelMA hydrogels that fold into a tubular fashion in response to water and release about 70% of the drug over 30 h.162 In another report, Melocchi et al. used FDM printing to create a temporary rod-shaped poly(vinyl alcohol) construct for easy insertion into the bladder.114 After that, the hydrogels swelled to a curled shape as they came in contact with water for organ retention and rapidly released all the loaded molecules in 2 h. The same group focused their research in the area of extendable devices for retention in the stomach. The apparatus was anticipated to revert to its initial form as it encountered gastric fluids.112 Han et al. constructed a PEGDA-based hypodermic needle array with curved barbs facing backward to improve tissue adherence while delivering drugs (Fig. 8E).113 The photocurable polymer used in the original structures featured a gradient of cross-linking density, meaning that the tops of the barbs displayed more cross-linking than the bottoms. The barbs were oriented horizontally. When the spikes dried, they curved backward to form a hook-like shape.
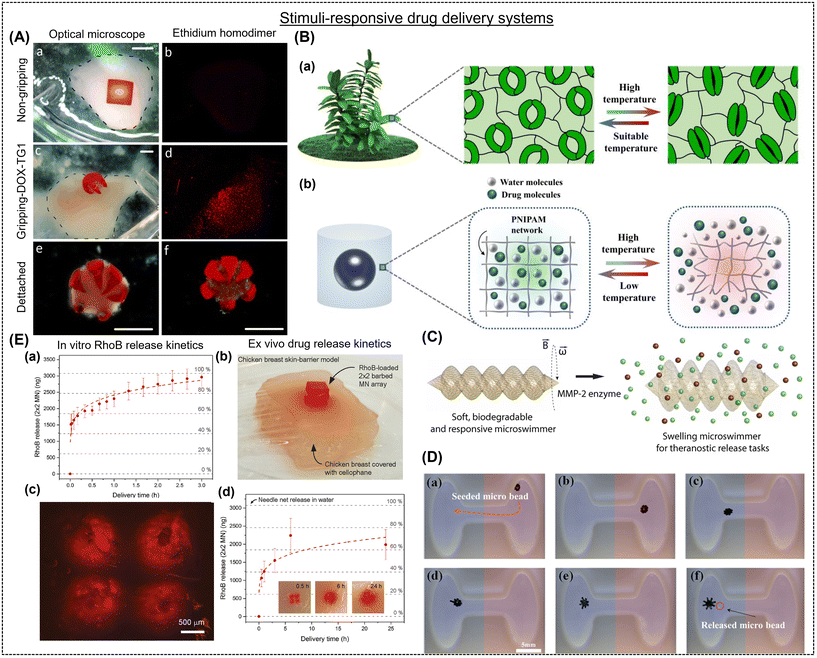 | ||
| Fig. 8 (A) In vitro model of doxorubicin (DOX) elution from (a and b) non-gripping (negative control) and (c–e) theragripper ((b and c) attached and (c and d) detached). The scale bar in (A) is 2 mm. Reproduced with permission.156 Copyright 2014, Wiley Online Library. (B-a) Schematic representation of opening and closing in response to temperature. (B-b) Dynamic water and drug transport channels in the hydrogel capsule microstructures. Reproduced with permission.157 Copyright 2022, Elsevier. (C) Enzymatic drug delivery using a 3D-printed microswimmer (length = 20 μm and diameter = 6 μm) prepared by two-photon polymerization. Reproduced with permission.159 Copyright 2019, ACS. (D) Magnetic field-actuated pH-responsive behavior of hydrogel-based soft microrobot. The red and blue areas represent high and low pH values, respectively. The scale bar in (D) is 5.0 mm. Reproduced with permission.160 Copyright 2016, IOP Science. (E) (a) In vitro and (b) ex vivo drug release test using barbed microneedle array. (E-c) Fluorescence microscope image of a chicken breast skin-barrier model stained with RhoB-loaded microneedle array (0.5 h after insertion). (E-d) Quantification of released RhoB from microneedle array into the chicken breast at different intervals (0.5, 1, 3, 6, and 24 h). Reproduced with permission.113 Copyright 2020, IOP Science. | ||
5. Feasibility of next-generation 4D hydrogels
Although 4D hydrogels show immense potential for biomedical applications, their journey towards widespread clinical use is associated with considerations regarding sustainability, safety, and efficient translation. Sustainability concerns arise from the materials and fabrication processes involved. Traditional hydrogel components such as synthetic polymers often possess poor biodegradability or require energy-intensive synthesis. Moving towards biocompatibility, naturally derived materials such as biopolymers or decellularized matrices offer a more sustainable alternative.163 Fabrication techniques such as 3D/4D printing, while enabling precise control, can be limited by the use of solvents, high energy consumption and high costs, making them a poor choice for large-scale production. Thus, it is inevitable to explore biocompatible inks and optimize the printing parameters for sustainable practices.164 Safety considerations stem from the potential cytotoxicity of the components, degradation products, or leachable unreacted chemicals of hydrogels. Rigorous in vitro and in vivo testing, together with biocompatibility assessments, are essential before clinical implementation.165 Additionally, the dynamic nature of 4D hydrogels necessitates careful evaluation of potential unintended responses or immunogenic reactions triggered by their time-dependent transformations.Clinical translation presents its own set of challenges. Scaling up production for clinical needs, while maintaining intricate design features and functionalities remains an ongoing hurdle. Conventional manufacturing techniques may not be readily adaptable, necessitating innovation in scalable fabrication methods. Furthermore, the inherent instability of some 4D hydrogels, prone to swelling-induced structural collapse or premature degradation, necessitates strategies to enhance their robustness for long-term in vivo applications. The mechanically fragile nature of certain 4D hydrogels also raises concerns about their ability to withstand physiological stresses, and strategies to improve their mechanical strength are actively pursued.165,166
Finally, commercial viability and availability are important hurdles. The high cost of material development, specialized equipment, and stringent regulatory requirements can hinder widespread adoption. Collaborative efforts among academia, industry, and regulatory bodies are crucial to bridge this gap and make 4D hydrogels more commercially accessible.167 Although 4D hydrogels show immense promise for revolutionizing biomedicine, achieving their sustainable and safe clinical translation necessitates addressing sustainability concerns, ensuring rigorous safety evaluations, developing scalable fabrication methods, enhancing their stability and mechanical strength, and fostering collaborations to improve their commercial viability. Overcoming these challenges will pave the way for 4D hydrogels to realize their full potential in advancing human healthcare.
6. Conclusions, challenges, and futuristic vision
Shape morphism is a bioinspired event critical for the survival of living organisms to adapt to ever-changing, complex environments. Now, efforts are being directed toward developing synthetic materials capable of mimicking the behaviors that nature has perfected over centuries. Hydrogels have emerged as one of the most promising candidates among the shape-morphing materials. Shape deformations in hydrogels are observed due to the non-uniform distribution of internal stresses resulting from the asymmetrical swelling/shrinking of different parts of the same hydrogel system. These out-of-plane and in-plane gradients are created due to the differences in the local swelling behavior, amplifying the internal stresses under external stimuli. Actuators, including moisture, light, temperature, pH, and magnetic field, and their role in achieving the desired and pre-determined shape were presented. Herein, we discussed the various 4D fabrication techniques (extrusion printing, light-based 3D printing, and solvent casting) to prepare shape-shifting hydrogels. Differences between mono- and dual-component hydrogel systems, the capabilities of 3D constructs to undergo uni- or bi-directional shape changes, and advantages of composite hydrogels compared to their pristine counterparts were meticulously discussed. These 4D gels have shown remarkable potential as dynamic tissue scaffolds for regenerative engineering and drug-delivery applications due to their shape-morphing properties. This review also highlighted the advantages of 4D printing over the conventional 3D printing to develop live tissue scaffolds with intricate designs.The development of shape-morphing hydrogels involves critical consideration of many factors, such as material choice, stimuli responsiveness, and fabrication process, to achieve predetermined complex shapes and precise control of the shape-change kinetics and reversibility. A mechanistic understanding of the material behavior and its response to external stimuli is crucial. In this regard, theoretical insights using tools such as finite element analysis, molecular dynamics, density functional theory, and other bimolecular simulations can enable the prediction of cell–material interaction to provide advanced architectures. Significant attention should be provided to increase the magnitude of shape change and larger out-of-plane movements by carefully selecting biopolymers and designing 3D constructs. The hydrogel recipe must incorporate multi-stimuli responsive moieties to achieve multi-step shape transformations and complicated designs. Triggers such as temperature and magnetic field should be active in the physiological range to be exploited to their fullest potential. This information is useful for guidance in designing effective and tailored materials for tissue-specific applications. Additionally, pre-clinical investigations should be conducted to assess the safety, strength, and efficacy of these shape-morphing hydrogels to repair damaged tissue. The hydrogel matrix must be able to change shape in situ and promote cell adherence and proliferation for efficient regeneration. The positioning of hydrogels is challenging due to body fluids, making them bend/fold/twist before reaching the target site.168 These critical issues must be resolved for the successful translation of shape-morphing biomaterials.
The development of shape-morphing hydrogels through additive manufacturing, including DIW and DLP, has been presented over the years to achieve precise, programmable, and customizable designs. Unlike solvent casting, which is a natural air-drying process, 3D printing technology enables the development of multi-material-based scaffolds with intricate patterns, offering the possibility of integrating piezoelectric biopolymers and sensors to potentially develop state-of-the-art bioelectronics interfaces. Adopting this idea will enable harvesting mechanical energy from motions such as bending, twisting, and rolling during shape transformations to develop self-powered biodevices. Data science has emerged as a dominant research theme in several engineering disciplines in the past decade. The application of deep-learning algorithms and artificial intelligence in shape-morphing hydrogels can help predict the tissue response by varying the biopolymers, composition, size, and arrangement of stimuli-response and non-responsive layers in hydrogel systems. Further integrating hydrogels with Internet of Things (IoT) technology will enable real-time monitoring of in situ shape transformations, regenerative processes, and surrounding organs. In summary, the futuristic growth in shape-morphing hydrogels needs immense collaboration among biomedical engineers, biomaterial scientists, data scientists, and clinicians. Reliable, quantitative, and rapid tools must be developed for advanced mechanistic insights into the correlation between shape-morphing hydrogels and tissue regeneration. Intelligent biomaterials and the predictive capabilities of data science will profoundly accelerate the discovery of next-generation hydrogel-assisted regenerative therapy.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Department of Science and Technology (DST) sponsored Innovation in Science Pursuit for Inspired Research (INSPIRE) Faculty Programme (DST/INSPIRE/04/2021/001535). The authors acknowledge support from the Science and Engineering Research Board (SERB), Government of India (IPA/2020/000025).References
- S.-J. Jeon, A. W. Hauser and R. C. Hayward, Acc. Chem. Res., 2017, 50, 161–169 CrossRef CAS PubMed.
- H. Le Ferrand, K. S. Riley and A. F. Arrieta, Bioinspiration Biomimetics, 2022, 17, 046002 CrossRef CAS PubMed.
- Z. J. Wang, W. Hong, Z. L. Wu and Q. Zheng, Angew. Chem., Int. Ed., 2017, 56, 15974–15978 CrossRef CAS PubMed.
- H. Kim, S. Ahn, D. M. Mackie, J. Kwon, S. H. Kim, C. Choi, Y. H. Moon, H. B. Lee and S. H. Ko, Mater. Today, 2020, 41, 243–269 CrossRef CAS.
- R. S. Kularatne, H. Kim, M. Ammanamanchi, H. N. Hayenga and T. H. Ware, Chem. Mater., 2016, 28, 8489–8492 CrossRef CAS.
- D. Jiao, Q. L. Zhu, C. Y. Li, Q. Zheng and Z. L. Wu, Acc. Chem. Res., 2022, 55, 1533–1545 CrossRef CAS PubMed.
- R. V. Ulijn, N. Bibi, V. Jayawarna, P. D. Thornton, S. J. Todd, R. J. Mart, A. M. Smith and J. E. Gough, Mater. Today, 2007, 10, 40–48 CrossRef CAS.
- J. M. Rosiak and F. Yoshii, Nucl. Instrum. Methods Phys. Res., Sect. B, 1999, 151, 56–64 CrossRef CAS.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2012, 64, 18–23 CrossRef.
- A. Joshi, S. Choudhury, S. B. Gugulothu, S. S. Visweswariah and K. Chatterjee, Biomacromolecules, 2022, 23, 2730–2751 CrossRef CAS PubMed.
- Z. U. Arif, M. Y. Khalid, R. Noroozi, A. Sadeghianmaryan, M. Jalalvand and M. Hossain, Int. J. Biol. Macromol., 2022, 218, 930–968 CrossRef CAS PubMed.
- E. M. White, J. Yatvin, J. B. Grubbs, J. A. Bilbrey and J. Locklin, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 1084–1099 CrossRef CAS.
- Z. U. Arif, M. Y. Khalid, W. Ahmed and H. Arshad, Bioprinting, 2022, 27, e00203 CrossRef.
- X. He, Y. Sun, J. Wu, Y. Wang, F. Chen, P. Fan, M. Zhong, S. Xiao, D. Zhang, J. Yang and J. Zheng, J. Mater. Chem. C, 2019, 7, 4970–4980 RSC.
- D. Tan, A. Nokhodchi and M. Maniruzzaman, 3D and 4D Printing in Biomedical Applications, Wiley, 2019, pp. 25–52 Search PubMed.
- Z. Jiang, B. Diggle, M. L. Tan, J. Viktorova, C. W. Bennett and L. A. Connal, Adv. Sci., 2020, 7, 17 Search PubMed.
- H. Kadry, S. Wadnap, C. Xu and F. Ahsan, Eur. J. Pharm. Sci., 2019, 135, 60–67 CrossRef CAS PubMed.
- N. Shahrubudin, T. C. Lee and R. Ramlan, Procedia Manuf., 2019, 35, 1286–1296 CrossRef.
- K. J. De France, F. Xu and T. Hoare, Adv. Healthcare Mater., 2017, 7, 1 Search PubMed.
- M. V. Risbud, A. A. Hardikar, S. V. Bhat and R. R. Bhonde, J. Controlled Release, 2000, 68, 23–30 CrossRef CAS PubMed.
- A. Joshi, S. Choudhury, V. S. Baghel, S. Ghosh, S. Gupta, D. Lahiri, G. K. Ananthasuresh and K. Chatterjee, Adv. Healthcare Mater., 2023, 12, 24 Search PubMed.
- D. K. Patel, A. H. Sakhaei, M. Layani, B. Zhang, Q. Ge and S. Magdassi, Adv. Mater., 2017, 29, 1606000 CrossRef PubMed.
- S. S. Imam, A. Hussain, M. A. Altamimi and S. Alshehri, Polymers, 2021, 13, 3858 CrossRef CAS PubMed.
- A. Rath and P. Theato, Adv. Funct. Mater., 2019, 30, 1902959 CrossRef.
- D. Yang, J. Xiao, B. Wang, L. Li, X. Kong and J. Liao, Mater. Sci. Eng., C, 2019, 104, 109927 CrossRef CAS PubMed.
- N. Asadi, A. Mehdipour, M. Ghorbani, M. Mesgari-Abbasi, A. Akbarzadeh and S. Davaran, Int. J. Biol. Macromol., 2021, 193, 734–747 CrossRef CAS PubMed.
- A. Kirillova and L. Ionov, J. Mater. Chem. B, 2019, 7, 1597–1624 RSC.
- M. N. I. Shiblee, K. Ahmed, M. Kawakami and H. Furukawa, Adv. Mater. Technol., 2019, 4, 1–10 Search PubMed.
- X. Liu, M. Gao, J. Chen, S. Guo, W. Zhu, L. Bai, W. Zhai, H. Du, H. Wu, C. Yan, Y. Shi, J. Gu, H. J. Qi and K. Zhou, Adv. Funct. Mater., 2022, 32, 2203323 CrossRef CAS.
- Y. Dong, S. Wang, Y. Ke, L. Ding, X. Zeng, S. Magdassi and Y. Long, Adv. Mater. Technol., 2020, 5, 1–19 Search PubMed.
- M. Champeau, D. A. Heinze, T. N. Viana, E. R. de Souza, A. C. Chinellato and S. Titotto, Adv. Funct. Mater., 2020, 30, 1–22 CrossRef.
- S. Roy Barman, P. Gavit, S. Chowdhury, K. Chatterjee and A. Nain, JACS Au, 2023, 3, 2930–2947 CrossRef CAS PubMed.
- Y. Wang, R. K. Kankala, C. Ou, A. Chen and Z. Yang, Bioact. Mater., 2022, 9, 198–220 CAS.
- Q. Zhao, J. Wang, H. Cui, H. Chen, Y. Wang and X. Du, Adv. Funct. Mater., 2018, 28, 1801027 CrossRef.
- S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991–1003 CrossRef CAS PubMed.
- H. Hu, C. Huang, M. Galluzzi, Q. Ye, R. Xiao, X. Yu and X. Du, Research, 2021, 1–12, 9786128 Search PubMed.
- U. Fasel, D. Keidel, L. Baumann, G. Cavolina, M. Eichenhofer and P. Ermanni, Manuf. Lett., 2020, 23, 85–88 CrossRef.
- M. Zarek, N. Mansour, S. Shapira and D. Cohn, Macromol. Rapid Commun., 2016, 38, 1600628 CrossRef PubMed.
- H. Yi, D. Kim, Y. Kim, D. Kim, J. Koh and M.-J. Kim, Autom. Constr., 2020, 114, 103151 CrossRef.
- L. Chen, M. Weng, F. Huang and W. Zhang, Sens. Actuators, B, 2019, 282, 384–390 CrossRef CAS.
- A. P. Piedade, J. Funct. Biomater., 2019, 10, 9 CrossRef CAS PubMed.
- M. Mao, J. He, X. Li, B. Zhang, Q. Lei, Y. Liu and D. Li, Micromachines, 2017, 8, 113 CrossRef.
- A. Joshi, S. Choudhury, V. S. Baghel, S. Ghosh, S. Gupta, D. Lahiri, G. K. Ananthasuresh and K. Chatterjee, Adv. Healthcare Mater., 2023, 12, e2300701 CrossRef PubMed.
- Z. Mao, K. Zhu, L. Pan, G. Liu, T. Tang, Y. He, J. Huang, J. Hu, K. W. Y. Chan and J. Lu, Adv. Mater. Technol., 2020, 5, 1900974 CrossRef CAS.
- J. Lai, X. Ye, J. Liu, C. Wang, J. Li, X. Wang, M. Ma and M. Wang, Mater. Des., 2021, 205, 109699 CrossRef CAS.
- S. Parimita, A. Kumar, H. Krishnaswamy and P. Ghosh, J. Manuf. Process., 2023, 85, 875–884 CrossRef.
- S. Naficy, R. Gately, R. Gorkin, H. Xin and G. M. Spinks, Macromol. Mater. Eng., 2017, 302, 1600212 CrossRef.
- Z. Zhao, X. Kuang, C. Yuan, H. J. Qi and D. Fang, ACS Appl. Mater. Interfaces, 2018, 10, 19932–19939 CrossRef CAS PubMed.
- S. B. Gugulothu and K. Chatterjee, ACS Macro Lett., 2023, 12, 494–502 CrossRef CAS PubMed.
- X. Kuang, J. Wu, K. Chen, Z. Zhao, Z. Ding, F. Hu, D. Fang and H. J. Qi, Sci. Adv., 2019, 5, 1–9 Search PubMed.
- D. J. Roach, X. Sun, X. Peng, F. Demoly, K. Zhou and H. J. Qi, Adv. Funct. Mater., 2022, 32, 2203236 CrossRef CAS.
- F. V. Borbolla-Jiménez, S. I. Peña-Corona, S. J. Farah, M. T. Jiménez-Valdés, E. Pineda-Pérez, A. Romero-Montero, M. L. Del Prado-Audelo, S. A. Bernal-Chávez, J. J. Magaña and G. Leyva-Gómez, Pharmaceutics, 2023, 15, 1914 CrossRef PubMed.
- S. R. Barman, S. W. Chan, F. C. Kao, H. Y. Ho, I. Khan, A. Pal, C. C. Huang and Z. H. Lin, Sci. Adv., 2023, 9, 1–15 Search PubMed.
- G. Guo, Q. Wu, F. Liu, J. Yin, Z. L. Wu, Q. Zheng and J. Qian, Adv. Funct. Mater., 2021, 32, 2108548 CrossRef.
- Z. Xu and J. Fu, ACS Appl. Mater. Interfaces, 2020, 12, 26476–26484 CrossRef CAS PubMed.
- R. Yu, L. Zhu, Y. Xia, J. Liu, J. Liang, J. Xu, B. Wang and S. Wang, Adv. Mater. Interfaces, 2022, 9, 2200401 CrossRef CAS.
- A. Ding, O. Jeon, R. Tang, Y. Bin Lee, S. J. Lee and E. Alsberg, Adv. Sci., 2021, 8, 2004616 CrossRef CAS PubMed.
- A. Ding, S. J. Lee, S. Ayyagari, R. Tang, C. T. Huynh and E. Alsberg, Bioact. Mater., 2022, 7, 324–332 CAS.
- L. Tang, L. Wang, X. Yang, Y. Feng, Y. Li and W. Feng, Prog. Mater. Sci., 2021, 115, 100702 CrossRef CAS.
- J. Cao, C. Zhou, G. Su, X. Zhang, T. Zhou, Z. Zhou and Y. Yang, Adv. Mater., 2019, 31, 1900042 CrossRef PubMed.
- A. S. Gladman, E. A. Matsumoto, R. G. Nuzzo, L. Mahadevan and J. A. Lewis, Nat. Mater., 2016, 15, 413–418 CrossRef PubMed.
- Z. Ji, C. Yan, B. Yu, X. Zhang, M. Cai, X. Jia, X. Wang and F. Zhou, Adv. Mater. Technol., 2019, 4, 1800713 CrossRef.
- Y. Park and X. Chen, J. Mater. Chem. A, 2020, 8, 15227–15244 RSC.
- P. D. C. Costa, D. C. S. Costa, T. R. Correia, V. M. Gaspar and J. F. Mano, Adv. Mater. Technol., 2021, 6, 1–21 Search PubMed.
- K. Shariati, A. S. Ling, S. Fuchs, B. Dillenburger, W. Liu and M. Ma, Adv. Funct. Mater., 2022, 32, 1–45 CrossRef.
- S. Tawfick, M. De Volder, D. Copic, S. J. Park, C. R. Oliver, E. S. Polsen, M. J. Roberts and A. J. Hart, Adv. Mater., 2012, 24, 1628–1674 CrossRef CAS PubMed.
- A. Kirillova, R. Maxson, G. Stoychev, C. T. Gomillion and L. Ionov, Adv. Mater., 2017, 29, 1703443 CrossRef PubMed.
- V. P. Anju, R. Pratoori, D. K. Gupta, R. Joshi, R. K. Annabattula and P. Ghosh, Soft Matter, 2020, 16, 4162–4172 RSC.
- G. Pasparakis and C. Tsitsilianis, Polymer, 2020, 211, 123146 CrossRef CAS.
- I. Bischofberger and V. Trappe, Sci. Rep., 2015, 5, 15520 CrossRef PubMed.
- L. Zhang, Y. Xiang, H. Zhang, L. Cheng, X. Mao, N. An, L. Zhang, J. Zhou, L. Deng, Y. Zhang, X. Sun, H. A. Santos and W. Cui, Adv. Sci., 2020, 7, 1903553 CrossRef CAS PubMed.
- T. Manouras and M. Vamvakaki, Polym. Chem., 2017, 8, 74–96 RSC.
- H. Kim, J. Kang, Y. Zhou, A. S. Kuenstler, Y. Kim, C. Chen, T. Emrick and R. C. Hayward, Adv. Mater., 2019, 31, 1900932 CrossRef PubMed.
- M. Czugala, C. O'Connell, C. Blin, P. Fischer, K. J. Fraser, F. Benito-Lopez and D. Diamond, Sens. Actuators, B, 2014, 194, 105–113 CrossRef CAS.
- Y. Luo, X. Lin, B. Chen and X. Wei, Biofabrication, 2019, 11, 045019 CrossRef CAS PubMed.
- P. Xue, H. K. Bisoyi, Y. Chen, H. Zeng, J. Yang, X. Yang, P. Lv, X. Zhang, A. Priimagi, L. Wang, X. Xu and Q. Li, Angew. Chem., Int. Ed., 2021, 60, 3390–3396 CrossRef CAS PubMed.
- Z. J. Wang, C. Y. Li, X. Y. Zhao, Z. L. Wu and Q. Zheng, J. Mater. Chem. B, 2019, 7, 1674–1678 RSC.
- Y. H. Lai, S. Roy Barman, A. Ganguly, A. Pal, J. H. Yu, S. H. Chou, E. W. Huang, Z. H. Lin and S. Y. Chen, Chem. Eng. J., 2023, 476, 146744 CrossRef CAS.
- A. Chan, R. P. Orme, R. A. Fricker and P. Roach, Adv. Drug Delivery Rev., 2013, 65, 497–514 CrossRef CAS PubMed.
- J. K. Wychowaniec and D. F. Brougham, Adv. Sci., 2022, 9, 2202278 CrossRef CAS PubMed.
- X. Ni, X. Xing, Y. Deng and Z. Li, Pharmaceutics, 2023, 15, 982 CrossRef CAS PubMed.
- A. Pardo, M. Gómez-Florit, S. Barbosa, P. Taboada, R. M. A. Domingues and M. E. Gomes, ACS Nano, 2021, 15, 175–209 CrossRef CAS PubMed.
- Z. Liu, J. Liu, X. Cui, X. Wang, L. Zhang and P. Tang, Front. Chem., 2020, 8, 1–17 CrossRef PubMed.
- J. Tang, Q. Yin, Y. Qiao and T. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 21194–21200 CrossRef CAS PubMed.
- J. Tang, Z. Tong, Y. Xia, M. Liu, Z. Lv, Y. Gao, T. Lu, S. Xie, Y. Pei, D. Fang and T. J. Wang, J. Mater. Chem. B, 2018, 6, 2713–2722 RSC.
- J. Fu, F. Yang and Z. Guo, New J. Chem., 2018, 42, 17162–17180 RSC.
- P. Gupta, K. Vermani and S. Garg, Drug Discovery Today, 2002, 7, 569–579 CrossRef CAS PubMed.
- P. Yuan, J. M. McCracken, D. E. Gross, P. V. Braun, J. S. Moore and R. G. Nuzzo, Soft Matter, 2017, 13, 7312–7317 RSC.
- T. Shiga and T. Kurauchi, J. Appl. Polym. Sci., 1990, 39, 2305–2320 CrossRef CAS.
- J. Lin, Q. Tang, D. Hu, X. Sun, Q. Li and J. Wu, Colloids Surf., A, 2009, 346, 177–183 CrossRef CAS.
- D. Han, C. Farino, C. Yang, T. Scott, D. Browe, W. Choi, J. W. Freeman and H. Lee, ACS Appl. Mater. Interfaces, 2018, 10, 17512–17518 CrossRef CAS PubMed.
- C. Li, G. C. Lau, H. Yuan, A. Aggarwal, V. L. Dominguez, S. Liu, H. Sai, L. C. Palmer, N. A. Sather, T. J. Pearson, D. E. Freedman, P. K. Amiri, M. O. de la Cruz and S. I. Stupp, Sci. Robot., 2020, 5, 1–12 Search PubMed.
- B. Narupai, P. T. Smith and A. Nelson, Adv. Funct. Mater., 2021, 31, 2011012 CrossRef CAS.
- B. Dhandayuthapani, Y. Yoshida, T. Maekawa and D. S. Kumar, Int. J. Polym. Sci., 2011, 2011, 1–19 CrossRef.
- N. Nguyen, Z. H. Lin, S. R. Barman, C. Korupalli, J. Y. Cheng, N. X. Song, Y. Chang, F. L. Mi, H. L. Song, H. W. Sung and Y. J. Lin, Nano Energy, 2022, 99, 107393 CrossRef CAS.
- H. Bramfeld, G. Sabra, V. Centis and P. Vermette, Curr. Med. Chem., 2010, 17, 3944–3967 CrossRef PubMed.
- S. Chung and M. W. King, Biotechnol. Appl. Biochem., 2011, 58, 423–438 CrossRef CAS PubMed.
- J. Jang, H.-G. Yi and D.-W. Cho, ACS Biomater. Sci. Eng., 2016, 2, 1722–1731 CrossRef CAS PubMed.
- T. Li, J. Chang, Y. Zhu and C. Wu, Adv. Healthcare Mater., 2020, 9, 2000208 CrossRef CAS PubMed.
- A. Do, B. Khorsand, S. M. Geary and A. K. Salem, Adv. Healthcare Mater., 2015, 4, 1742–1762 CrossRef CAS PubMed.
- S. Ghosh, S. Chaudhuri, P. Roy and D. Lahiri, Regener. Eng. Transl. Med., 2023, 9, 339–365 CrossRef CAS.
- C. Garcia, A. Gallardo, D. López, C. Elvira, A. Azzahti, E. Lopez-Martinez, A. L. Cortajarena, C. M. González-Henríquez, M. A. Sarabia-Vallejos and J. Rodríguez-Hernández, ACS Appl. Bio Mater., 2018, 1, 1337–1347 CrossRef CAS PubMed.
- S. Miao, W. Zhu, N. J. Castro, M. Nowicki, X. Zhou, H. Cui, J. P. Fisher and L. G. Zhang, Sci. Rep., 2016, 6, 27226 CrossRef CAS PubMed.
- G. Constante, I. Apsite, H. Alkhamis, M. Dulle, M. Schwarzer, A. Caspari, A. Synytska, S. Salehi and L. Ionov, ACS Appl. Mater. Interfaces, 2021, 13, 12767–12776 CrossRef CAS PubMed.
- C. Zhang, D. Cai, P. Liao, J.-W. Su, H. Deng, B. Vardhanabhuti, B. D. Ulery, S.-Y. Chen and J. Lin, Acta Biomater., 2021, 122, 101–110 CrossRef CAS PubMed.
- S. Miao, H. Cui, M. Nowicki, S. Lee, J. Almeida, X. Zhou, W. Zhu, X. Yao, F. Masood, M. W. Plesniak, M. Mohiuddin and L. G. Zhang, Biofabrication, 2018, 10, 035007 CrossRef PubMed.
- H. Cui, C. Liu, T. Esworthy, Y. Huang, Z. Yu, X. Zhou, H. San, S. Lee, S. Y. Hann, M. Boehm, M. Mohiuddin, J. P. Fisher and L. G. Zhang, Sci. Adv., 2020, 6, 1–12 Search PubMed.
- Y. Wang, H. Cui, Y. Wang, C. Xu, T. J. Esworthy, S. Y. Hann, M. Boehm, Y.-L. Shen, D. Mei and L. G. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 12746–12758 CrossRef CAS PubMed.
- H. Cui, S. Miao, T. Esworthy, S. Lee, X. Zhou, S. Y. Hann, T. J. Webster, B. T. Harris and L. G. Zhang, Nano Res., 2019, 12, 1381–1388 CrossRef PubMed.
- S. Miao, H. Cui, M. Nowicki, L. Xia, X. Zhou, S. Lee, W. Zhu, K. Sarkar, Z. Zhang and L. G. Zhang, Adv. Biosyst., 2018, 2, 1800101 CrossRef PubMed.
- J.-H. Fang, H.-H. Hsu, R.-S. Hsu, C.-K. Peng, Y.-J. Lu, Y.-Y. Chen, S.-Y. Chen and S.-H. Hu, NPG Asia Mater., 2020, 12, 61 CrossRef CAS.
- A. Melocchi, M. Uboldi, N. Inverardi, F. Briatico-Vangosa, F. Baldi, S. Pandini, G. Scalet, F. Auricchio, M. Cerea, A. Foppoli, A. Maroni, L. Zema and A. Gazzaniga, Int. J. Pharm., 2019, 571, 118700 CrossRef CAS PubMed.
- D. Han, R. S. Morde, S. Mariani, A. A. La Mattina, E. Vignali, C. Yang, G. Barillaro and H. Lee, Adv. Funct. Mater., 2020, 30, 1909197 CrossRef CAS.
- A. Melocchi, N. Inverardi, M. Uboldi, F. Baldi, A. Maroni, S. Pandini, F. Briatico-Vangosa, L. Zema and A. Gazzaniga, Int. J. Pharm., 2019, 559, 299–311 CrossRef CAS PubMed.
- Y. Wang, Y. Miao, J. Zhang, J. P. Wu, T. B. Kirk, J. Xu, D. Ma and W. Xue, Mater. Sci. Eng., C, 2018, 84, 44–51 CrossRef CAS PubMed.
- S. Xu, M. Bendeck and A. I. Gotlieb, Cardiovascular Pathology, Elsevier, 2016, pp. 85–124 Search PubMed.
- H. Cui, S. Miao, T. Esworthy, X. Zhou, S. Lee, C. Liu, Z. Yu, J. P. Fisher, M. Mohiuddin and L. G. Zhang, Adv. Drug Delivery Rev., 2018, 132, 252–269 CrossRef CAS PubMed.
- D. Xue, Y. Wang, J. Zhang, D. Mei, Y. Wang and S. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 19428–19435 CrossRef CAS PubMed.
- W. Hua, W. Shi, K. Mitchell, L. Raymond, R. Coulter, D. Zhao and Y. Jin, Chinese J. Mech. Eng. Addit. Manuf. Front., 2022, 1, 100020 Search PubMed.
- A. Bandyopadhyay, Y. Zhang and B. Onuike, Virtual Phys. Prototyping, 2022, 17, 256–294 CrossRef.
- D. G. Tamay, T. Dursun Usal, A. S. Alagoz, D. Yucel, N. Hasirci and V. Hasirci, Front. Bioeng. Biotechnol., 2019, 7, 1–22 CrossRef PubMed.
- T. van Manen, S. Janbaz, K. M. B. Jansen and A. A. Zadpoor, Commun. Mater., 2021, 2, 56 CrossRef.
- Z. Azhar, N. Haque, S. Ali, M. Mozafari and F. Sefat, in Handbook of Tissue Engineering Scaffolds: Volume One, Elsevier, 2019, pp. 705–728 Search PubMed.
- A. Khan, R. Joshi, M. K. Sharma, A. Ganguly, P. Parashar, T. W. Wang, S. Lee, F. C. Kao and Z. H. Lin, Nano Energy, 2024, 119, 109051 CrossRef CAS.
- Y. Wang, H. Cui, T. Esworthy, D. Mei, Y. Wang and L. G. Zhang, Adv. Mater., 2022, 34, 2109198 CrossRef CAS PubMed.
- F. D. Jochum and P. Theato, Chem. Soc. Rev., 2013, 42, 7468–7483 RSC.
- M. S. Spach, J. F. Heidlage, R. C. Barr and P. C. Dolber, Heart Rhythm, 2004, 1, 500–515 CrossRef PubMed.
- L. Zhang, Y. Xiang, H. Zhang, L. Cheng, X. Mao, N. An, L. Zhang, J. Zhou, L. Deng, Y. Zhang, X. Sun, H. A. Santos and W. Cui, Adv. Sci., 2020, 7, 1903553 Search PubMed.
- C. Cui, D.-O. Kim, M. Y. Pack, B. Han, L. Han, Y. Sun and L.-H. Han, Biofabrication, 2020, 12, 045018 CrossRef CAS PubMed.
- O. A. Carballo-Molina and I. Velasco, Front. Cell. Neurosci., 2015, 9, 1–12 Search PubMed.
- A. R. Nectow, K. G. Marra and D. L. Kaplan, Tissue Eng., Part B, 2012, 18, 40–50 CrossRef CAS PubMed.
- C. H. Fan, H. C. Tsai, Y. S. Tsai, H. C. Wang, Y. C. Lin, P. H. Chiang, N. Wu, M. H. Chou, Y. J. Ho, Z. H. Lin and C. K. Yeh, ACS Nano, 2023, 17, 9140–9154 CrossRef CAS PubMed.
- M. Curcio and F. Bradke, Annu. Rev. Cell Dev. Biol., 2018, 34, 495–521 CrossRef CAS PubMed.
- L. K. Wareham, S. A. Liddelow, S. Temple, L. I. Benowitz, A. Di Polo, C. Wellington, J. L. Goldberg, Z. He, X. Duan, G. Bu, A. A. Davis, K. Shekhar, A. La Torre, D. C. Chan, M. V. Canto-Soler, J. G. Flanagan, P. Subramanian, S. Rossi, T. Brunner, D. E. Bovenkamp and D. J. Calkins, Mol. Neurodegener., 2022, 17, 23 CrossRef CAS PubMed.
- Y. C. Sun, Y. Wan, R. Nam, M. Chu and H. E. Naguib, Sci. Rep., 2019, 9, 1–13 CrossRef PubMed.
- F. Y. Hsieh, H. H. Lin and S. Hui Hsu, Biomaterials, 2015, 71, 48–57 CrossRef CAS PubMed.
- I. Apsite, G. Constante, M. Dulle, L. Vogt, A. Caspari, A. R. Boccaccini, A. Synytska, S. Salehi and L. Ionov, Biofabrication, 2020, 12, 035027 CrossRef CAS PubMed.
- S. Da Wu and S. H. Hsu, Biofabrication, 2021, 13, 045029 CrossRef PubMed.
- Z. Atoufi, P. Zarrintaj, G. H. Motlagh, A. Amiri, Z. Bagher and S. K. Kamrava, J. Biomater. Sci., Polym. Ed., 2017, 28, 1617–1638 CrossRef CAS PubMed.
- G. Li, Y. Kong, Y. Zhao, Y. Zhao, L. Zhang and Y. Yang, J. Biomater. Sci., Polym. Ed., 2015, 26, 899–916 Search PubMed.
- S.-H. Hsiao and S. Hsu, ACS Appl. Mater. Interfaces, 2018, 10, 29273–29287 CrossRef CAS PubMed.
- J. Liu, O. Erol, A. Pantula, W. Liu, Z. Jiang, K. Kobayashi, D. Chatterjee, N. Hibino, L. H. Romer, S. H. Kang, T. D. Nguyen and D. H. Gracias, ACS Appl. Mater. Interfaces, 2019, 11, 8492–8498 CrossRef CAS PubMed.
- D. W. Hutmacher, Biomaterials, 2000, 21, 2529–2543 CrossRef CAS PubMed.
- B. D. Boyan, C. H. Lohmann, J. Romero and Z. Schwartz, Clin. Plast. Surg., 1999, 26, 629–645 CrossRef CAS PubMed.
- A. Ding, S.-J. Lee, R. Tang, K. L. Gasvoda, F. He and E. Alsberg, Small, 2022, 18, 2202196 CrossRef CAS PubMed.
- A. Ding, O. Jeon, D. Cleveland, K. L. Gasvoda, D. Wells, S.-J. Lee and E. Alsberg, Adv. Mater., 2022, 34, 2109394 Search PubMed.
- P. J. Díaz-Payno, M. Kalogeropoulou, I. Muntz, E. Kingma, N. Kops, M. D'Este, G. H. Koenderink, L. E. Fratila-Apachitei, G. J. V. M. van Osch and A. A. Zadpoor, Adv. Healthcare Mater., 2023, 12, 2201891 CrossRef PubMed.
- R. Jamaledin, C. Di Natale, V. Onesto, Z. Taraghdari, E. Zare, P. Makvandi, R. Vecchione and P. Netti, J. Clin. Med., 2020, 9, 542 CrossRef CAS PubMed.
- J. Goole and K. Amighi, Int. J. Pharm., 2016, 499, 376–394 CrossRef PubMed.
- J. Firth, A. W. Basit and S. Gaisford, 3D Printing of Pharmaceuticals, 2018, pp. 133–151 Search PubMed.
- Y. C. Li, Y. S. Zhang, A. Akpek, S. R. Shin and A. Khademhosseini, Biofabrication, 2016, 9, 012001 CrossRef PubMed.
- I. Lukin, S. Musquiz, I. Erezuma, T. H. Al-Tel, N. Golafshan, A. Dolatshahi-Pirouz and G. Orive, Expert Opin. Drug Discovery, 2019, 14, 953–956 CrossRef PubMed.
- J. Li and D. J. Mooney, Nat. Rev. Mater., 2016, 1, 16071 CrossRef CAS PubMed.
- U. Bozuyuk, O. Yasa, I. C. Yasa, H. Ceylan, S. Kizilel and M. Sitti, ACS Nano, 2018, 12, 9617–9625 CrossRef CAS PubMed.
- M. K. Gupta, F. Meng, B. N. Johnson, Y. L. Kong, L. Tian, Y. W. Yeh, N. Masters, S. Singamaneni and M. C. McAlpine, Nano Lett., 2015, 15, 5321–5329 CrossRef CAS PubMed.
- K. Malachowski, J. Breger, H. R. Kwag, M. O. Wang, J. P. Fisher, F. M. Selaru and D. H. Gracias, Angew. Chem., Int. Ed., 2014, 53, 8045–8049 CrossRef CAS PubMed.
- S. Zu, Z. Wang, S. Zhang, Y. Guo, C. Chen, Q. Zhang, Z. Wang, T. Liu, Q. Liu and Z. Zhang, Mater. Today Chem., 2022, 24, 100789 CrossRef CAS.
- Q. Hu, P. S. Katti and Z. Gu, Nanoscale, 2014, 6, 12273–12286 RSC.
- H. Ceylan, I. C. Yasa, O. Yasa, A. F. Tabak, J. Giltinan and M. Sitti, ACS Nano, 2019, 13, 3353–3362 CrossRef CAS PubMed.
- H. Li, G. Go, S. Y. Ko, J.-O. Park and S. Park, Smart Mater. Struct., 2016, 25, 027001 CrossRef.
- G. Villar, A. J. Heron and H. Bayley, Nat. Nanotechnol., 2011, 6, 803–808 CrossRef CAS PubMed.
- Y.-D. Zhao, J.-H. Lai and M. Wang, Nano Life, 2021, 11, 2141001 CrossRef CAS.
- K. Sadtler, A. Singh, M. T. Wolf, X. Wang, D. M. Pardoll and J. H. Elisseeff, Nat. Rev. Mater., 2016, 1, 1–17 Search PubMed.
- K. Osouli-Bostanabad, T. Masalehdan, R. M. I. Kapsa, A. Quigley, A. Lalatsa, K. F. Bruggeman, S. J. Franks, R. J. Williams and D. R. Nisbet, ACS Biomater. Sci. Eng., 2022, 8, 2764–2797 CrossRef CAS PubMed.
- L. Faber, A. Yau and Y. Chen, Biofabrication, 2013, 16, 012001 Search PubMed.
- A. Sheikh, M. A. S. Abourehab and P. Kesharwani, Drug Discovery Today, 2023, 28, 1–15 CrossRef PubMed.
- W. Zhou, Z. Qiao, E. Nazarzadeh Zare, J. Huang, X. Zheng, X. Sun, M. Shao, H. Wang, X. Wang, D. Chen, J. Zheng, S. Fang, Y. M. Li, X. Zhang, L. Yang, P. Makvandi and A. Wu, J. Med. Chem., 2020, 63, 8003–8024 Search PubMed.
- T. R. Hoare and D. S. Kohane, Polymer, 2008, 49, 1993–2007 CrossRef CAS.
- S. Choudhury, A. Joshi, D. Dasgupta, A. Ghosh, S. Asthana and K. Chatterjee, Mater. Adv., 2024, 5, 3345–3356 Search PubMed.
- S. Choudhury, A. Joshi, V. Baghel, G. K. Ananthasuresh, S. Asthana, S. Homer-Vanniasinkam and K. Chatterjee, J. Mater. Chem. B, 2024 10.1039/D4TB00437J.
- A. Joshi, S. Choudhury, S. Asthana, S. Homer-Vanniasinkam, U. Nambiar and K. Chatterjee, Biomater. Sci., 2023, 11, 7703–7708 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |
