Open Access Article
Open Access ArticleA sensitive, rapid and cost-effective RP-HPLC-UV method for detection and quantification of bedaquiline in physiological fluid (pH 7.4)
Simisola
Ayodele
a,
Armorel D.
van Eyk
 b,
Pradeep
Kumar
b,
Pradeep
Kumar
 a and
Yahya E.
Choonara
a and
Yahya E.
Choonara
 *a
*a
aWits Advanced Drug Delivery Platform Research Unit, Department of Pharmacy and Pharmacology, School of Therapeutic Sciences, Faculty of Health Sciences, University of the Witwatersrand, 7 York Road, Parktown, Johannesburg, 2193, South Africa. E-mail: yahya.choonara@wits.ac.za
bDivision of Pharmacology, Department of Pharmacy and Pharmacology, University of the Witwatersrand, 7 York Road, Parktown, Johannesburg, 2193, South Africa
First published on 11th September 2024
Abstract
Bedaquiline, a highly lipophilic molecule, is used in the treatment regimen of multi-drug resistant tuberculosis. A rare complication of pulmonary tuberculosis is tuberculous pericarditis. Ex vivo studies utilising animal pericardium can be used to investigate whether this drug is capable of diffusing across pericardial tissue into simulated pericardial fluid (pH 7.4) to indicate efficacy. For detection of bedaquiline in physiological fluid, a rapid, cost-effective and sensitive method is essential. The aim of this study was thus to develop and validate a simple and sensitive RP-HPLC-UV method for the detection and quantification of bedaquiline, encapsulated in a nanosystem, at pH 7.4 after permeation across excised pericardium. A HPLC Phenomenex Kinetex RPC18 column (150 × 4.6 mm, 5 μm) was utilized for analysis. The mobile phase consisted of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v (A
5 v/v (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B), where (A) methanol
B), where (A) methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (85
acetonitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 v/v)
15 v/v)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (B) triethylamine (1% v/v)
(B) triethylamine (1% v/v)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 mM KH2PO4 buffer (pH 7.4). Running conditions included the following: injection volume 20 μl, flow rate 1.0 ml min−1, detection wavelength 275 nm, 25 °C and running time of 5 min. Bedaquiline eluted as a single symmetrical peak at a retention time of 4.17 min. The method was found to be linear within the range of 1–50 μg ml−1 (R2 = 1). The limit of detection (LOD) and limit of quantification (LOQ) were found to be 0.05 μg ml−1 and 0.15 μg ml−1, respectively (signal-to-noise ratio method). All validation parameters were found to be within acceptable limits (RSD < 2%). The method was fast, reliable, accurate, reproducible, and transient for the detection of bedaquiline in simulated physiological fluid (pH 7.4). This method can thus be applied to easily detect bedaquiline in body fluids (pH 7.4) i.e. blood and pericardial fluid without the accuracy being impacted by ionisation factors of the molecule.
0.15 mM KH2PO4 buffer (pH 7.4). Running conditions included the following: injection volume 20 μl, flow rate 1.0 ml min−1, detection wavelength 275 nm, 25 °C and running time of 5 min. Bedaquiline eluted as a single symmetrical peak at a retention time of 4.17 min. The method was found to be linear within the range of 1–50 μg ml−1 (R2 = 1). The limit of detection (LOD) and limit of quantification (LOQ) were found to be 0.05 μg ml−1 and 0.15 μg ml−1, respectively (signal-to-noise ratio method). All validation parameters were found to be within acceptable limits (RSD < 2%). The method was fast, reliable, accurate, reproducible, and transient for the detection of bedaquiline in simulated physiological fluid (pH 7.4). This method can thus be applied to easily detect bedaquiline in body fluids (pH 7.4) i.e. blood and pericardial fluid without the accuracy being impacted by ionisation factors of the molecule.
Introduction
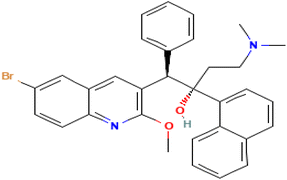
Bedaquiline is chemically identified as (1R,2S)-1-(6-bromo-2-methoxy-3-quinolyl)-4-dimethylamino-2-(1-naphthyl)-1-phenylbutan-2-ol as shown in Fig. 1 below.It is a diarylquinoline with tuberculocidal activity approved for the treatment of multi-drug-resistant tuberculosis (MDR-TB).1 Bedaquiline works by inhibiting the proton pump of M. tuberculosis ATP synthase. It has a MIC of 0.03 mg l−1 against M. tuberculosis H37Rv and 0.12 mg l−1 against a range of M. tuberculosis clinical isolates.2 It also forms two major metabolites through a series N-demethylation by cytochrome p450 isoenzyme 3A4 namely, N-monodesmethyl-bedaquiline (M2) and N-didesmethyl-bedaquiline (M3), with an in vitro potency of about 5- and 187-fold less than that of bedaquiline, respectively.3 Bedaquiline has also been found to be slightly more potent against dormant M.tb strains as compared to rifampicin and isoniazid. It has a half-life of ∼5.5 months.4 TB pericarditis (TBP) is a rare complication of pulmonary tuberculosis caused by lymphatic spread of the TB bacilli from lymph nodes.5 The extent of bedaquiline penetration across the pericardium into the pericardial fluid is not well known, resulting in uncertainty whether this drug reaches high enough levels in the fluid to effectively eradicate the bacilli. Bedaquiline is a lipophilic drug with poor solubility in aqueous fluid but high protein binding (99%), very low MIC and long half-life.6 It is a positively charged molecule with pKa values of 1.57 (imine); 8.91 (amine); and 13.61 (hydroxyl).7 Pericardial permeation seems to favour drugs with low protein binding, thus incorporating drugs with a higher protein binding into nanosystems may result in improved drug penetration and bioavailability in pericardial fluid to effectively treat pericarditis and prevent constrictive pericarditis.8
Ex vivo studies of bedaquiline diffusion characteristics across porcine pericardium may serve as an affective model providing greater information on efficacy. Existing methods have utilized acidic buffers at pH 4.5 and a PDA detector to detect bedaquiline is official dissolution media,9 because bedaquiline is an orally administered drug, such methods are suitable for experiments that involve the oral administration of the drug due to the pH of gastric media. Other methods have employed the use of a gradient method using HPLC connected to a mass spectrometer for the quantification of bedaquiline.10 In addition, some isocratic methods yielded a longer retention time (Rt) of 5.4 min, whilst using a high ratio of acetonitrile.11 Furthermore, use of methanol as an organic component of the mobile phase in the detection of bedaquiline, have shown to result in much longer retention times of more than 10 min, and to up 30 min in some HPLC detection methods.12–14 A HPLC method was thus required that would incorporate a suitable ratio of methanol and acetonitrile that would ensure a fast, reliable and cost-effective method for the detection of bedaquiline, due to methanol's cost-advantage and acetonitrile's elution strength.
Studies that explore the incorporation of bedaquiline into nanoparticle injectables require more sensitive and pH congruent methods for detection in plasma and body fluids with a general pH of 7.4. Due to its pKa of 8.91 (amine), bedaquiline is expected to act as a weak base and thus be slightly ionised in plasma and body fluids. To prevent further dilution of samples with acid after collection, a method with a pH that allows direct analyses of body fluids without the need to change pH of samples containing bedaquiline to fit existing methods, is required. This will maintain the charge of bedaquiline after sample collection and during elution to prevent tailing and splitting of peaks.15
The aim of this study was therefore to develop a sensitive, rapid and cost-effective RP-HPLC method with UV detection to quantify bedaquiline, incorporated into nanoparticles, for the quantification in release and ex vivo diffusion studies.
The method was validated based on the recommendations of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; ICH guidelines Q2(R1) (“ICH Guideline Q2(R1): Validation of Analytical Procedures: Text and Methodology”, 2005).16
Materials and methods
Materials
The materials employed in this study were of the highest purity grade and were purchased from Sigma-Aldrich (Pty) Ltd (St. Louis, Missouri, United States). This included the following: bedaquiline (CAS 843663-66-1, 95% purity), methanol (CAS 67-56-1, 99.9% purity HPLC grade), acetonitrile (CAS 75-05-8, 99.9% HPLC grade), potassium dihydrogen orthophosphate (CAS 7778-77-0, 99.0% purity), triethylamine (CAS 121-44-8, 99.5% purity), orthophosphoric acid (CAS 7664-38-2, 85% purity).Preparation of standards and phosphate buffer for mobile phase
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (85
acetonitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15)
15)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (B) phosphate buffer in a ratio of 90
(B) phosphate buffer in a ratio of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (A
10 (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B) were conducted and no peaks were obtained. Therefore, phosphate buffer containing triethylamine and adjusted with orthophosphoric acid to physiological pericardial pH of 7.4, was prepared and utilized instead of water. The lambda max was determined by dissolving bedaquiline in the proposed mobile phase and obtaining its wavelength on a UV/Vis spectrometer. Running conditions using ratios of (A) methanol
B) were conducted and no peaks were obtained. Therefore, phosphate buffer containing triethylamine and adjusted with orthophosphoric acid to physiological pericardial pH of 7.4, was prepared and utilized instead of water. The lambda max was determined by dissolving bedaquiline in the proposed mobile phase and obtaining its wavelength on a UV/Vis spectrometer. Running conditions using ratios of (A) methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (85
acetonitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15)
15)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (B) phosphate buffer at 90
(B) phosphate buffer at 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 85
10, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and 95
15 and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (A
5 (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B) in a run time of 5 min were tested and the chosen condition was based on system suitability, characteristics of the peak, and retention time. The chromatographic apparatus and conditions employed are displayed below (Table 1).
B) in a run time of 5 min were tested and the chosen condition was based on system suitability, characteristics of the peak, and retention time. The chromatographic apparatus and conditions employed are displayed below (Table 1).
| Parameter | Conditions |
|---|---|
| Instrument | A PerkinElmer Flexar HPLC system, consisting of a binary pump, autosampler, column oven and a UV/Vis detector. The system was equipped with Chromera® software for data acquisition and analysis (PerkinElmer Inc., Waltham, Massachusetts, USA) |
| Column | Phenomenex Kinetex C18 RP LC column with dimensions of 150 × 4.6 mm and particle size of 5 μm |
| Mobile phase | An organic phase of (A): (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (85 acetonitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15)) and an aqueous phase of (B): (triethylamine (10 ml) and potassium phosphate buffer (0.15 mM) adjusted with orthophosphoric acid to a pH of 7.4). Ratio of 95 15)) and an aqueous phase of (B): (triethylamine (10 ml) and potassium phosphate buffer (0.15 mM) adjusted with orthophosphoric acid to a pH of 7.4). Ratio of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (A 5 (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B). The phosphate buffer was filtered using a vacuum pump through a 0.45 μm membrane B). The phosphate buffer was filtered using a vacuum pump through a 0.45 μm membrane |
| Flow rate | 1 ml min−1 |
| Detection wavelength | 275 nm |
| Injection volume | 20 μl |
| Run time | 5 min |
| Column temperature | 25 °C |
| Elution technique | Isocratic |
Validation parameters
| y = mx + c |
Intraday precision. Three samples (1 μg ml−1, 10 μg ml−1, and 50 μg ml−1) were used to determine intraday precision. Samples were injected in triplicate in the morning and afternoon (8 hours apart) on the same day. The acceptance criteria for intra-day precision are set to be a % relative standard deviation (RSD) ≤ 2.0.
Interday precision. Three, low, medium and high-quality control (QC) samples from points on the line of regression (1 μg ml−1, 10 μg ml−1, and 50 μg ml−1) were used to determine inter-day precision. Samples were injected in triplicate for three consecutive days. The acceptance criteria for intra-day precision are set to be a % relative standard deviation (RSD) ≤ 2.0.
Results
RP-HPLC method development and validation for bedaquiline
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 85
10, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and 95
15 and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (A
5 (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B) containing (A) methanol/acetonitrile (85
B) containing (A) methanol/acetonitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15)
15)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (B) phosphate buffer pH 7.4 were all tested. A mobile phase ratio of 95
(B) phosphate buffer pH 7.4 were all tested. A mobile phase ratio of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (A
5 (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B) was the only ratio to show a peak within a run time of 5 min, and was therefore selected for method validation. The lack of a chromatogram within the 5 min run time was found to be due to a retention time shift of up to 0.55 min per 1% change of mobile phase from organic to aqueous phase, as shown in the robustness section. The mobile phase ratio of 95
B) was the only ratio to show a peak within a run time of 5 min, and was therefore selected for method validation. The lack of a chromatogram within the 5 min run time was found to be due to a retention time shift of up to 0.55 min per 1% change of mobile phase from organic to aqueous phase, as shown in the robustness section. The mobile phase ratio of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (A
5 (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
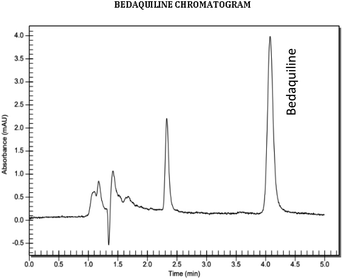
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B) was chosen since it produced a narrow and symmetrical peak with a retention time of 4.17 minutes that allowed a run time of less than 5 minutes (Fig. 2), low pressure, and a lesser chance of crystallization. As the buffer ratio increased in the system, crystallization was observed. Organic solvents were predominantly utilised due to the hydrophobic nature of bedaquiline. The lipophilicity of the molecule caused it to precipitate in mobile phases with a high aqueous content. To prevent crystallization in the column, the column was first equilibrated with 95
B) was chosen since it produced a narrow and symmetrical peak with a retention time of 4.17 minutes that allowed a run time of less than 5 minutes (Fig. 2), low pressure, and a lesser chance of crystallization. As the buffer ratio increased in the system, crystallization was observed. Organic solvents were predominantly utilised due to the hydrophobic nature of bedaquiline. The lipophilicity of the molecule caused it to precipitate in mobile phases with a high aqueous content. To prevent crystallization in the column, the column was first equilibrated with 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (A
5 (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B) (A) methanol/acetonitrile
B) (A) methanol/acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (B) water for 10 minutes. Thereafter, the water was replaced with the prepared phosphate buffer and equilibrated for 30 minutes at 95
(B) water for 10 minutes. Thereafter, the water was replaced with the prepared phosphate buffer and equilibrated for 30 minutes at 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (A
5 (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B).
B).
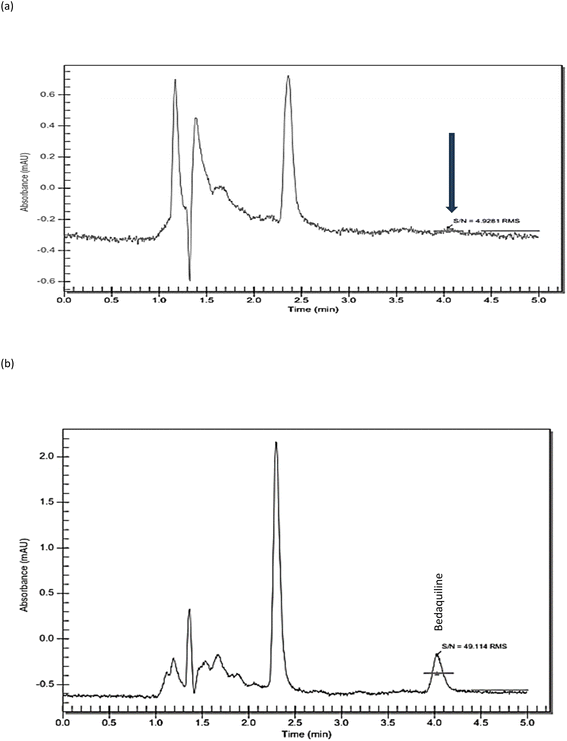
Linearity. Linear regression of the standard curves displayed the suitability of the method within the prescribed concentration range (1–50 μg ml−1) for bedaquiline (R2 = 1) (Fig. 3a and b, Table 2). The LOD and LOQ were determined to be 0.05 μg ml−1 and 0.15 μg ml−1 (Fig. 4a and b), respectively using signal-to-noise ratio (S/N)17 and 0.62 μg ml−1 and 1.90 μg ml−1 using the standard deviation of response and slope calculation method.18
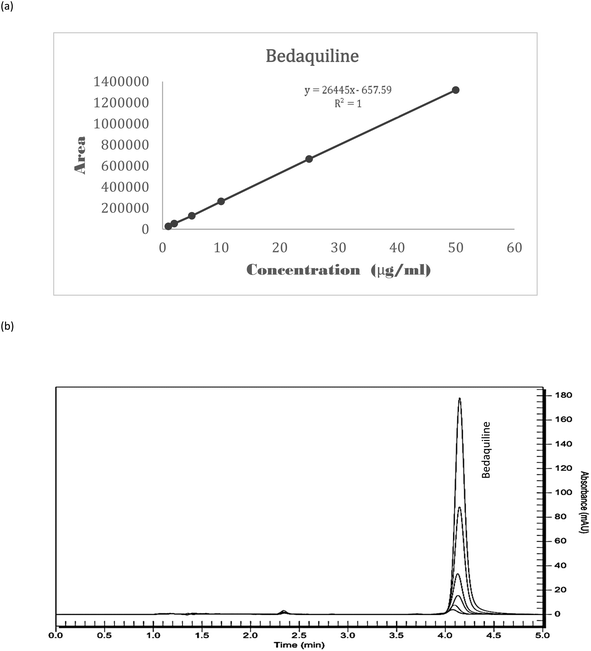 | ||
| Fig. 3 Linearity of bedaquiline (a) a standard curve of bedaquiline (n = 3) (each standard was injected 3 times), (b) overlay of bedaquiline standard curve chromatograms (Rt = 4.17 minutes). | ||
| Concentration (μg ml−1) | Std curve 1 | Std curve 2 | Std curve 3 | Overall mean | % RSD |
|---|---|---|---|---|---|
| Mean peak area ± SD | Mean peak area ± SD | Mean peak area ± SD | Peak area ± SD | ||
| 1 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 972.74 ± 722.63 972.74 ± 722.63 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370.32 ± 205.15 370.32 ± 205.15 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550.40 ± 147.99 550.40 ± 147.99 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 964.49 ± 1499.55 964.49 ± 1499.55 |
5.56 |
| 2 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 405.93 ± 709.35 405.93 ± 709.35 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550.11 ± 233.54 550.11 ± 233.54 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 765.03 ± 157.07 765.03 ± 157.07 |
53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240.35 ± 2986.97 240.35 ± 2986.97 |
5.61 |
| 5 | 126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 958.76 ± 999.31 958.76 ± 999.31 |
124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 289.06 ± 1010.07 289.06 ± 1010.07 |
129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325.74 ± 279.57 325.74 ± 279.57 |
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 857.85 ± 2057.45 857.85 ± 2057.45 |
1.62 |
| 10 | 253![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 987.42 ± 405.98 987.42 ± 405.98 |
267![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 336.73 ± 1456.12 336.73 ± 1456.12 |
268![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280.89 ± 432.23 280.89 ± 432.23 |
263![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 201.68 ± 6526.85 201.68 ± 6526.85 |
2.48 |
| 25 | 669![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440.35 ± 5174.52 440.35 ± 5174.52 |
676![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 459.28 ± 290.18 459.28 ± 290.18 |
651![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 068.14 ± 384.35 068.14 ± 384.35 |
665![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 655.92 ± 10 655.92 ± 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 705.73 705.73 |
1.61 |
| 50 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634.92 ± 10 634.92 ± 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 707.34 707.34 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 344 344![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 631.17 ± 5026.61 631.17 ± 5026.61 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 313 313![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333.77 ± 1835.96 333.77 ± 1835.96 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319 319![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533.29 ± 18 533.29 ± 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 488.61 488.61 |
1.40 |
Precision. The inter-day and intra-day precision showed reproducibility over 3 days in the detection of bedaquiline, with a % RSD of <2% for all selected standard concentrations, which is within the acceptance criteria (Table 3).
| Drug | Concentration (μg ml−1) | Intraday precision | Interday precision | ||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |||||
| Mean peak | % RSD | Mean peak | % RSD | Mean peak | % RSD | ||
| Area ± SD | Area ± SD | Area ± SD | |||||
| a BDQ: bedaquiline; % RSD: % relative standard deviation. | |||||||
| BDQ | 1 | 362![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608.88 ± 250.47 608.88 ± 250.47 |
0.095 | 362![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 272.36 ± 235.70 272.36 ± 235.70 |
0.142 | 362![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 473.30 ± 183.72 473.30 ± 183.72 |
0.114 |
| 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732 732![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 359.35 ± 686.47 359.35 ± 686.47 |
0.197 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732 732![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700.69 ± 1352.44 700.69 ± 1352.44 |
0.206 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730 730![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 004.29 ± 3884.79 004.29 ± 3884.79 |
0.128 | |
| 50 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 416![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 987.88 ± 2095.96 987.88 ± 2095.96 |
0.043 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 411 411![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 933.39 ± 2309.15 933.39 ± 2309.15 |
0.031 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 411 411![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 276.29 ± 1137.87 276.29 ± 1137.87 |
0.041 | |
Accuracy. The method was efficient, with an overall recovery of 99.69% with an RSD of 0.47% (n = 6) after a spike of the PBS, containing 0.2% SLS, with 25 μg ml−1 bedaquiline, as presented in Table 4.
| Drug | Level, % | Concentration (μg ml−1) | Amount recovered ± SD (μg ml−1) | Recovery ± SD% | RSD (%) |
|---|---|---|---|---|---|
| a BDQ: bedaquiline; SD: standard deviation; RSD: relative standard deviation. | |||||
| BDQ | 80 | 8.00 | 7.96 ± 0.015 | 99.45 ± 0.186 | 0.55 |
| 100 | 10.00 | 10.02 ± 0.027 | 100.24 ± 0.267 | 0.24 | |
| 120 | 12.00 | 11.92 ± 0.005 | 99.37 ± 0.039 | 0.63 | |
Robustness. The developed method is consistent, showing repeatability and specificity in the established running conditions. The robustness of the HPLC method was investigated by small variations in the following conditions: flow rate, temperature, and the organic mobile phase component (Table 5). A slight increase in temperature did not alter the retention time (% RSD < 2%) and did not alter the peak area detected for bedaquiline significantly, with a recovery range of 96.0–97.8%. However, the retention time decreased by 3.2% and the area detected was slightly less stable with a slight temperature decrease, with a recovery range of 92.0–96.8%. As the flow rate decreased (by 0.1 ml min−1), elution occurred later by a difference of 8.51%. The % recovery increased to a range of 104.3–114.1%. As the flow rate increased (by 0.1 ml min−1), elution occurred earlier by a difference of 10.86%. The % recovery decreased to a range of 89.7–96.2%. As the organic component in the mobile phase decreased, the peak eluted later by a difference of 13.55%. The % recovery decreased slightly to a range of 97.2–98.4%. As the organic component in the mobile phase increased, the peak eluted earlier by a difference of 9.66%. The % recovery was found to be 95.1–101.1%, where the change favoured higher concentrations of 10 μg ml−1 and 50 μg ml−1 (% RSD < 2%).
| Condition | Bedaquiline | ||||
|---|---|---|---|---|---|
| Mean peak area ± SD | Amount tested/recovered (μg ml−1) | % recovery | Mean Rt ± SD | Mean Rt % RSD | |
| a FR: flow rate; T: temperature; MP: mobile phase; Rt: retention time; % RSD: % relative standard deviation from normal conditions. | |||||
| FR: 1 ml min−1 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149.03 ± 357.53 149.03 ± 357.53 |
0.86 ± 0.04 | — | 4.16 ± 0.014 | — |
MP: 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
224![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 328.14 ± 3339.72 328.14 ± 3339.72 |
8.51 ± 0.15 | — | ||
| T: 25 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126 126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410.03 ± 3203.44 410.03 ± 3203.44 |
42.62 ± 0.15 | — | ||
| FR change: 0.9 ml min−1 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 132.39 ± 282.05 132.39 ± 282.05 |
0.90 ± 0.04 | 104.3 ± 4.44 | 4.52 ± 0.039 | 8.51 |
246![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750.78 ± 278.72 750.78 ± 278.72 |
9.36 ± 0.04 | 110.0 ± 0.43 | |||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 285![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 761.89 ± 4065.01 761.89 ± 4065.01 |
48.65 ± 0.18 | 114.1 ± 0.37 | |||
| FR change: 1.1 ml min−1 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 794.46 ± 394.04 794.46 ± 394.04 |
0.77 ± 0.04 | 89.7 ± 5.19 | 3.71 ± 0.024 | 10.86 |
210![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510.93 ± 2006.28 510.93 ± 2006.28 |
7.99 ± 0.10 | 93.9 ± 1.25 | |||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 084 084![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 096.45 ± 2869.08 096.45 ± 2869.08 |
41.02 ± 0.13 | 96.2 ± 0.32 | |||
| T change: 24 °C | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318.04 ± 131.28 318.04 ± 131.28 |
0.79 ± 0.03 | 92.0 ± 3.80 | 4.13 ± 0.014 | 1.07 |
213![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138.20 ± 1753.37 138.20 ± 1753.37 |
8.08 ± 0.09 | 95.0 ± 1.11 | |||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 081.38 ± 1627.26 081.38 ± 1627.26 |
41.25 ± 0.09 | 96.8 ± 0.22 | |||
| T change 1: 26 °C | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 477.79 ± 116.04 477.79 ± 116.04 |
0.84 ± 0.03 | 97.1 ± 3.57 | 4.03 ± 0.011 | 3.20 |
215![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 379.26 ± 248.68 379.26 ± 248.68 |
8.17 ± 0.03 | 96.0 ± 0.37 | |||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 101 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 493.03 ± 963.25 493.03 ± 963.25 |
41.68 ± 0.06 | 97.8 ± 0.14 | |||
MP change: 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (A 6 (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B) B) |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520.981 ± 227.78 520.981 ± 227.78 |
0.84 ± 0.03 | 97.2 ± 3.57 | 4.72 ± 0.034 | 13.55 |
219![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 065.3141 ± 352.46 065.3141 ± 352.46 |
8.31 ± 0.04 | 97.7 ± 0.48 | |||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 108 108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 243.451 ± 1460.23 243.451 ± 1460.23 |
41.93 ± 0.08 | 98.4 ± 0.19 | |||
MP change: 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (A 4 (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B) B) |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 022.98616 ± 68.23 022.98616 ± 68.23 |
0.82 ± 0.03 | 95.1 ± 3.66 | 3.76 ± 0.011 | 9.66 |
224![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 131.9622 ± 197.77 131.9622 ± 197.77 |
8.50 ± 0.03 | 99.9 ± 0.35 | |||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138 138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 549.174 ± 2307.75 549.174 ± 2307.75 |
43.08 ± 0.11 | 101.1 ± 0.26 | |||
Stability. Bedaquiline showed good stability when stored at 4 °C and room temperature (25 °C) in the first week of storage. From week 2 onwards, an increase in concentration was observed due to the high percentage of methanol's low vapour pressure causing it to volatilize into air (Table 6). Bedaquiline demonstrated better stability in the fridge (4 °C) as compared to at room temperature. Both compounds may be stored for at least one week before analyses at either room temperature (25 °C) or fridge (4 °C).
| Concentration (μg ml−1) | Bedaquiline | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 10 | 50 | |||||
| Temperature °C | 25 | 4 | 25 | 4 | 25 | 4 | |
| a W0: initial concentration; W1: week 1; W2: week 2; W3: week 3; Rec: recovery; % RV: % recovery. Error bars = SD. | |||||||
| W0 | Rec (μg ml−1) | 0.98 | 0.78 | 10.13 | 8.76 | 50.87 | 42.74 |
| % RV | — | — | — | ||||
| W1 | Rec (μg ml−1) ± SD | 1.06 ± 0.08 | 0.79 ± 0.01 | 10.55 ± 0.42 | 8.78 ± 0.02 | 52.85 ± 1.98 | 42.23 ± 0.51 |
| % RV | 107.80 | 100.97 | 104.12 | 100.28 | 103.89 | 98.81 | |
| W2 | Rec (μg ml−1) ± SD | 1.07 ± 0.09 | 0.86 ± 0.08 | 11.60 ± 1.05 | 9.47 ± 0.71 | 55.84 ± 4.97 | 44.77 ± 2.03 |
| % RV | 108.82 | 110.68 | 114.44 | 108.20 | 109.77 | 104.75 | |
| W3 | Rec (μg ml−1) ± SD | 1.27 ± 0.29 | 0.88 ± 0.10 | 14.48 ± 4.35 | 10.24 ± 1.48 | 66.14 ± 15.27 | 49.04 ± 6.30 |
| % RV | 128.54 | 113.09 | 142.92 | 116.98 | 130.01 | 114.74 | |
Application of the validated method. After method validation, the method was tested for suitability of quantification of bedaquiline, which was loaded into a nanosystem during ex vivo animal studies. These studies involved the investigation of the diffusion kinetics of a bedaquiline-loaded nanosystem across porcine pericardium. Porcine pericardial tissue was collected from the University of the Witwatersrand Central Animal Unit (ethics waiver numbers 04-06-2020-O and 02-04-2024-O), snap frozen in liquid nitrogen and stored at −70 °C. The nanosystem was developed through a one-step ionic gelation probe sonication method.19 After the pericardial tissue was thawed in PBS (pH 7.4), the tissue was sectioned into 7 pieces (1 cm2 each) and mounted in the flow-through cells of a PermeGear 7-In-Line Flow-through Diffusion system (PermeGear Inc, Hellertown, USA). The bedaquiline-loaded nanosystem (1 ml
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mg ml−1) was added to the donor compartments of each flow-through diffusion cell. PBS containing 0.2% (w/v) sodium lauryl sulphate (SLS) was pumped through the receptor compartments at a rate of 1.5 ml h−1 at 37 °C and samples collected in a fraction collector 2-hourly for 24 hours. The collected samples were filtered and subsequently diluted 50
1 mg ml−1) was added to the donor compartments of each flow-through diffusion cell. PBS containing 0.2% (w/v) sodium lauryl sulphate (SLS) was pumped through the receptor compartments at a rate of 1.5 ml h−1 at 37 °C and samples collected in a fraction collector 2-hourly for 24 hours. The collected samples were filtered and subsequently diluted 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 with mobile phase and analysed using the developed HPLC method, without the need for pre-treatment. Fig. 5, 6 and Table 7 show the chromatogram and cumulative amounts (μgcm−2) versus time (min) graph and values determined from a preliminary diffusion study across excised porcine pericardium over a period of 24 hours. The method proved that it could successfully detect and quantify the amount of bedaquiline that diffused across the pericardium without any precipitation of the drug occurring in the receptor compartments after diffusion. Subsequent to this preliminary study showing the suitability of the method, a full study was performed on bedaquiline-loaded nanosystems and the diffusion characteristics across both excised porcine and human pericardium, which was submitted for publication.19 This study indicated that injected bedaquiline-loaded nanosystems may thus be considered for further studies in the treatment of tuberculous pericarditis.19
50 with mobile phase and analysed using the developed HPLC method, without the need for pre-treatment. Fig. 5, 6 and Table 7 show the chromatogram and cumulative amounts (μgcm−2) versus time (min) graph and values determined from a preliminary diffusion study across excised porcine pericardium over a period of 24 hours. The method proved that it could successfully detect and quantify the amount of bedaquiline that diffused across the pericardium without any precipitation of the drug occurring in the receptor compartments after diffusion. Subsequent to this preliminary study showing the suitability of the method, a full study was performed on bedaquiline-loaded nanosystems and the diffusion characteristics across both excised porcine and human pericardium, which was submitted for publication.19 This study indicated that injected bedaquiline-loaded nanosystems may thus be considered for further studies in the treatment of tuberculous pericarditis.19
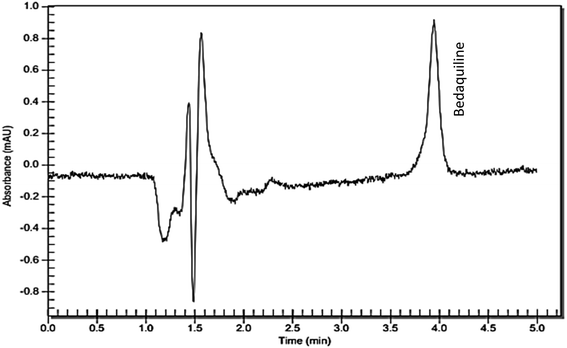 | ||
| Fig. 5 RP-C18 HPLC chromatogram showing bedaquiline detection (Rt = 4.01 min) from bedaquiline-loaded nanosystem samples that diffused across excised porcine pericardium. | ||
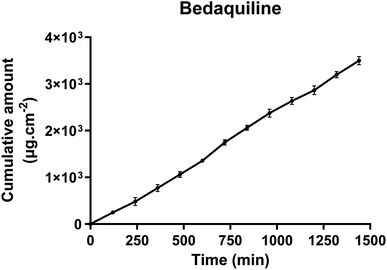 | ||
| Fig. 6 The diffusion kinetics of bedaquiline from bedaquiline-loaded nanoparticles across excised porcine pericardium (n = 3). | ||
| Time (min) | Cumulative amount μg cm−2 |
|---|---|
| 0 | 0 |
| 120 | 249.10 ± 19.23 |
| 240 | 481.21 ± 83.39 |
| 360 | 770.01 ± 75.06 |
| 480 | 1062.59 ± 61.05 |
| 600 | 1353.63 ± 18.56 |
| 720 | 1746.78 ± 59.46 |
| 840 | 2061.49 ± 55.14 |
| 960 | 2376.51 ± 85.39 |
| 1080 | 2635.83 ± 73.74 |
| 1200 | 2864.26 ± 95.30 |
| 1320 | 3199.81 ± 68.99 |
| 1440 | 3499.49 ± 87.15 |
Discussion
The results from this study indicated that a simple and cost-effective HPLC method with UV detection was developed and validated for the detection and quantification of bedaquiline present in simulated pericardial fluid (pH 7.4). The method was precise, accurate, rapid and sensitive, with bedaquiline eluting at a retention time of 4.17 min. All validation parameters were found to be within the acceptable limits as specified by the regulatory guidelines.16 A search of the literature indicated very few HPLC methods were available for the detection of bedaquiline. Most methods employ the use of a high organic solvent in the presence of an acidic buffer.20,21 A pH of 7.4 was selected to suit the pH of the simulated pericardial fluid samples to be collected. The buffer was initially made with triethylamine, which decreased retention time and strengthened peaks by reducing tailing.22 The buffer was then adjusted to pH 7.4 using orthophosphoric acid.A pH of 7.4 was suitable due to its proximity with the pKa value of 8.91 of bedaquiline.23 Although bedaquiline is slightly more soluble in an acidic pH,24 the use of a high ratio of organic phase in the method neutralises this solubility limitation. In ex vivo and in vitro studies, the use of SLS prevents precipitation of bedaquiline from samples. Body fluids are known to contain surfactants therefore, mimicking the presence of surfactants in ex vivo and in vitro studies further demonstrates the practicality of the method.25
Bedaquiline is a water-insoluble drug that can only be eluted in a highly organic mobile phase; therefore a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 organic solvent
5 organic solvent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer ratio was furthermore required to elute the drug. Methanol was initially used as the primary solvent at a ratio of 95
buffer ratio was furthermore required to elute the drug. Methanol was initially used as the primary solvent at a ratio of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 methanol
5 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer. The run time was set at 5 min and no elution of bedaquiline was observed in that time frame. Acetonitrile was gradually added to the methanol to make a 95
buffer. The run time was set at 5 min and no elution of bedaquiline was observed in that time frame. Acetonitrile was gradually added to the methanol to make a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 methanol
5 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile organic phase and slowly, the ratio of acetonitrile was increased by 5 (to make 90
acetonitrile organic phase and slowly, the ratio of acetonitrile was increased by 5 (to make 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 85
10 and 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 methanol
15 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile organic phase). A bedaquiline peak was observed at a ratio of 85
acetonitrile organic phase). A bedaquiline peak was observed at a ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 methanol
15 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile organic phase, with the mobile phase 95
acetonitrile organic phase, with the mobile phase 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (organic phase
5 (organic phase![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer) and the retention time of bedaquiline was observed within 5 min.
buffer) and the retention time of bedaquiline was observed within 5 min.
The peak was found to be symmetrical and consistent, thereafter the method validation was conducted according to the ICH guidelines. The developed method is comparable to established methods with a rapid elution time, very low LOD (0.05 μg ml−1) and LOQ (0.15 μg ml−1) as compared to the already established methods, with high sensitivity. It is unique in its ability to quantify bedaquiline is media with pH 7.4, allowing the easy quantification of bedaquiline in body fluids.
Flow rate variation was found to significantly affect the recovery of bedaquiline as compared to the effects of temperature and mobile phase variation for the validated method. The developed method in this study is acceptable and sensitive considering no other method could be found in the literature to detect bedaquiline at a physiological pH of 7.4.
Conclusion
A method was successfully developed and validated to detect bedaquiline in physiological fluid (pH 7.4) e.g. pericardial fluid using an isocratic HPLC-UV method. It can be used to detect the drug when studies are performed utilizing bedaquiline-loaded nanosystems as compared to the current methods available, which are more suitable for the detection of bedaquiline in oral formulations and acidic (gastric pH) conditions. The method was found to be sensitive, accurate, precise, and reliable. It was successfully employed in the detection of bedaquiline using simulated pericardial fluid in an ex vivo pericardial diffusion study of bedaquiline-loaded nanoparticles across the pericardium. This study further provides the prospective applicability of the method for the qualitative and quantitative analysis of bedaquiline in physiological fluids with a pH of 7.4 i.e., blood samples.Abbreviations
| M2 | 2 major metabolites N-monodesmethyl |
| M3 | N-Didesmethyl-bedaquiline |
| pKa | Acid dissociation constant |
| TB | Tuberculosis |
| MDR | Multi-drug resistant |
| XRD | Extensively drug resistant |
| ICH | International Conference on Harmonisation |
| HPLC | High performance liquid chromatography |
| BDQ | Bedaquiline |
| RSD | Relative standard deviation |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| SD | Standard deviation |
| MIC | Minimum inhibitory concentration |
Data availability
Ayodele et al., RP-C18-HPLC-UV method development and validation for the detection and quantification of bedaquiline at physiological pH 7.4. The authors declare that the data supporting the findings of this study are available within the paper. Should any data files be needed in any other format, they are available from the corresponding author upon reasonable request.Author contributions
Simisola Ayodele: writing – original draft; writing – review & editing, methodology; investigation, Armorel D. van Eyk: investigation; methodology; supervision; writing – original draft, Pradeep Kumar: conceptualization; investigation; supervision; writing – review & editing, Yahya E. Choonara: conceptualization; funding acquisition; project administration; supervision; writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Research Foundation (NRF) of South Africa, and the South African Medical Research Council (SAMRC).References
- G. M. Raj, Introduction to Basics of Pharmacology and Toxicology: Volume 2: Essentials of Systemic Pharmacology: From Principles to Practice, 2021, pp. 869–904 Search PubMed.
- M. M. Alajlani, Molecules, 2022, 27, 753 CrossRef CAS PubMed.
- S. Esposito, S. Bianchini and F. Blasi, Expet Opin. Pharmacother., 2015, 16, 2319–2330 CrossRef CAS PubMed.
- E. B. Chahine, L. R. Karaoui and H. Mansour, Ann. Pharmacother., 2014, 48, 107–115 CrossRef PubMed.
- J. González, O. Deirdre, A. González, M. Heres and G. Yusti, Rev. Am. Med. Respir., 2023, 23, 333–337 Search PubMed.
- A. Negi, S. Perveen, R. Gupta, P. P. Singh and R. Sharma, J. Med. Chem., 2024, 67, 2264–2286 CrossRef CAS PubMed.
- Committee for Medicinal Products for Human Use (CHMP) Assessment Report SIRTURO, International Non-proprietary Name: Bedaquiline Procedure No. EMEA/H/C/002614/0000, EMA, 2013, vol. 107, p. 2013 Search PubMed.
- X. Liu, B. Testa and A. Fahr, Pharmaceut. Res., 2011, 28, 962–977 CrossRef CAS PubMed.
- V. Pardhi, G. Pant and S. J. S. Flora, Futur. J. Pharm. Sci., 2020, 6, 42 CrossRef.
- N. Köhler, H. Karaköse, H.-P. Grobbel, D. Hillemann, S. Andres, C. König, B. Kalsdorf, T. T. Brehm, L. Böttcher and I. Friesen, Pharmaceutics, 2023, 15, 2543 CrossRef PubMed.
- A. K. H. Kumar, V. Sudha, A. Vijayakumar and C. Padmapriyadarsini, Int. J. Pharm. Pharm. Sci., 2021, 36–40 CrossRef CAS.
- M. A. M. Momin, B. Rangnekar and S. C. Das, J. Liq. Chromatogr. Relat. Technol., 2018, 41, 415–421 CrossRef CAS.
- V. Pardhi, G. Pant and S. J. S. Flora, Futur. J. Pharm. Sci., 2020, 6, 42 CrossRef.
- V. K. Baksam, S. Nimmakayala, V. R. Pocha, B. Gouri, S. Shandilya and P. Kumar, J. Chromatogr. Sci., 2022, 60, 848–858 CAS.
- D. V. McCalley, J. Chromatogr. A, 2015, 1411, 41–49 CrossRef CAS PubMed.
- ICH-Guidelines Q2(R1), Validation of Analytical Procedures: Text and Methodology, 2005 Search PubMed.
- C. Meyer, P. Seiler, C. Bies, C. Cianciulli, H. Wätzig and V. R. Meyer, Electrophoresis, 2012, 33, 1509–1516 CrossRef CAS PubMed.
- D. P. Lokhande, Int. J. Trend Sci. Res. Dev., 2019, 3, 2456–6470 Search PubMed.
- S. Ayodele, A. van Eyk, P. Kumar and Y. Choonara, Discover Nano, 2024 Search PubMed , submitted.
- V. Pardhi, G. Pant and S. J. S. Flora, Futur. J. Pharm. Sci., 2020, 6, 1–10 CrossRef.
- M. A. M. Momin, B. Rangnekar and S. C. Das, J. Liq. Chromatogr. Relat. Technol., 2018, 41, 415–421 CrossRef CAS.
- D. P. Thomas and J. P. Foley, J. Chromatogr. A, 2008, 1205, 36–45 CrossRef CAS PubMed.
- M. A. Okezue and S. J. Byrn, J. Infect. Dis. Diagn. Ther., 2022, 3(1), 004 Search PubMed.
- S. E. Maloney, I. E. Stewart, B. K. Podell, H. E. Gary, J. B. Mecham, B. J. Berube, S. L. Baldwin, R. N. Coler and A. J. Hickey, Pharmaceuticals, 2023, 16, 729 CrossRef CAS PubMed.
- A. Fathi-Azarbayjani and A. Jouyban, Bioimpacts, 2015, 5, 29 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |