 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improved on-line benchtop 31P NMR reaction monitoring via Multi-Resonance SHARPER†
Laura
Tadiello
ab,
Meghan E.
Halse
 *b and
Torsten
Beweries
*b and
Torsten
Beweries
 *a
*a
aLeibniz Institute for Catalysis (LIKAT), Albert-Einstein-Str. 29a, 18059 Rostock, Germany. E-mail: torsten.beweries@catalysis.de
bDepartment of Chemistry, University of York, YO10 5DD, York, UK. E-mail: meghan.halse@york.ac.uk
First published on 15th July 2024
Abstract
On-line reaction monitoring of hydrogenation reactions featuring oxygen-sensitive organometallic complexes is done via a 31P benchtop NMR spectrometer using the Multi-Resonance Sensitive Homogeneous And Resolved PEaks in Real time (MR-SHARPER) sequence. Signal enhancement generated by MR-SHARPER enables monitoring of reactivity on the order of minutes that could not be followed with traditional 31P{1H} NMR detection.
Transition metal complexes are frequently used in homogeneous catalysis and often rely on phosphorous-based ligands. These can be readily tuned sterically and electronically and new systems continue to be developed.1 Collection of concentration data over time is a crucial tool for understanding stoichiometric and catalytic transformations. Among others, NMR reaction monitoring is typically performed in situ, with an NMR tube inserted into the spectrometer, or off-line, monitoring the analyte after sampling. The former has issues of mass transfer2 while the latter suffers from delays, which compromise the kinetic information and might cause alteration of the sample. On-line reaction monitoring overcomes these limitations by connecting the reaction vessel directly with the analytical instrument: an aliquot of reaction mixture is circulated continuously between the external reaction vessel and the spectrometer.3
Benchtop NMR spectrometers (1–2.4 T; 43–100 MHz)4 are a valuable tool for on-line reaction monitoring due to their portability,5 which allows for operation close to the chemical reaction setup (e.g., in a fume hood)6 and integration into larger automated reaction setups.7 Furthermore, they are much cheaper than standard high-field NMR spectrometers (7–28 T; 300–1200 MHz), both in terms of upfront cost as well as maintenance. Applying benchtop NMR systems for on-line reaction monitoring is challenging because of their lower sensitivity and lower chemical shift resolution, when compared to high-field spectrometers. For example, the signal-to-noise ratio (SNR) drops approximately 11-fold when moving from 400 MHz to 80 MHz, while the absolute chemical shift axis in hertz is compressed by a factor of five. The resultant peak overlap can be mitigated by moving from 1H to heteronuclei such as 19F and 31P, which have wider chemical shift ranges and fewer background signals. However, shifting to these lower receptivity nuclei causes further reductions in observed SNR. These sensitivity limitations are intrinsic to the lower field strengths of benchtop NMR spectrometers, but it is possible to modify the conditions of the NMR experiment to improve SNR. In this manuscript, we take advantage of advanced pulse sequences to boost the sensitivity of 31P benchtop NMR.
Sensitive Homogeneous And Resolved PEaks in Real time (SHARPER) is a signal enhancement pulse sequence designed by the Uhrín research group,8 which has recently been adapted for the use on benchtop NMR spectrometers.9 The SHARPER sequence (Fig. S1†) simplifies the spectrum and boosts SNR by collapsing complicated multiplets into a single, narrow peak. This is achieved through multiple refocusing pulses interleaved with signal detection, where the full FID is constructed from the so-called chunks acquired between refocusing pulses. As a result of this refocusing, chemical shift information is sacrificed to increase sensitivity. Importantly, this is achieved by the application of radio frequency (RF) pulses exclusively to the target nucleus. In the related pure shift methods,10 peak multiplicities are removed while the chemical shift information is preserved. However, this is often achieved at the cost of sensitivity. For example, Zangger–Sterk11 and Pure Shift Yielded by Chirp Excitation (PSYCHE) typically recover only 0.5–10% and 3–20% of the signal, respectively.12 The SHARPER sequence not only retains the full NMR signal but also boosts the SNR by concentrating it into a single narrow peak. For benchtop NMR detection of 19F, enhancement factors of 10–30 have been reported via SHARPER, which not only refocuses scalar couplings but also residual magnetic field inhomogeneity.9
The original SHARPER sequence refocuses all signals into a single peak. Chemical specificity can be introduced through the use of the selective SHARPER (sel-SHARPER)13 variant, in which band-selective pulses are substituted for both signal excitation and refocusing within the acquisition loop. Sel-SHARPER can be further optimised by using band-selective excitation followed by non-selective refocusing pulses during acquisition.13,14 This provides good selectivity and optimised SNR enhancement while the chunk length, the duration of signal acquisition between refocussing pulses, is kept short. The band-selective approach requires each resonance of interest to be acquired in separate experiments. This limitation can be overcome by using Multi-Resonance SHARPER (MR-SHARPER),15 where the chunk length and refocusing pulse spacing are adjusted to match a multiple of the inverse of the chemical shift difference between the target resonances, in hertz.
The optimal version of the SHARPER sequences depends on the practical application. SHARPER will give the best SNR enhancement if only one resonance or set of resonances is present in the standard NMR spectrum. When the spectrum includes multiple resonances, sel-SHARPER can be used to isolate and selectively enhance the target species. For reaction monitoring applications, where it is often desirable to monitor two components simultaneously, MR-SHARPER provides an attractive solution.
Reaction monitoring using SHARPER has only been demonstrated previously using high-field NMR detection. Two examples of protodeboronation of fluorinated aryl boronic acids have been reported for the 19F nucleus.8,15 One example of phosphine oxidation has been described for the 31P nucleus.8 Of note, these cases were performed under in situ or stopped-flow conditions. Here we explore reaction monitoring using 31P SHARPER on our flow setup6 integrated with an 80 MHz benchtop NMR spectrometer (equipped with an additional channel for the 31P nucleus). Previously, we have demonstrated that the flow setup is suitable for the study of highly oxygen-sensitive organometallic complexes, stepwise additions of reactants and catalytic transformations under inert conditions.6 In this work, we apply SHARPER and its variants to single and multi-component systems, for in situ and on-line reaction monitoring with benchtop NMR detection.
Initially, we explored the performance of SHARPER on a model single-component solution of the rhodium complex [Rh((R,R)-DIPAMP)(NBD)]BF4 (DIPAMP = (1,2-bis[(o-methoxyphenyl)(phenyl) phosphino]ethane, NBD = norbornadiene, 31P{1H} NMR in MeOH: δ 49.3 ppm, JRhP = 158.4 Hz, Scheme 1), which gives rise to a doublet in the 31P{1H} NMR spectrum (Fig. 1B) due to coupling between 103Rh and 31P. Proton decoupling is essential to observe the target signal within the concentration range (9–112 mM) reported herein (Fig. 1A and B). The Nuclear Overhauser Effect (NOE) provides a modest increase of the SNR of the 31P{1H} NMR sequence (Fig. 1B and C). The previously published SHARPER 19F NMR pulse sequences9 were adapted for 31P detection and an NOE transfer step was introduced, including the necessary changes in the phase cycle list (Table S1†). When coupled to SHARPER, NOE provided a comparable boost in SNR as for standard 31P{1H} acquisition (Fig. 1H and I). As expected, higher SNR enhancement is observed when shorter selective Gaussian pulses (5 ms vs. 10 ms, Fig. 1E and G) are employed in the sel-SHARPER variant but the highest SNR enhancement is achieved with non-selective SHARPER (Fig. 1I). The maximum improvement was a factor of 9 compared to a traditional 31P{1H} NMR sequence with NOE (Fig. 1C and I). This is comparable to the previously observed enhancements for 19F benchtop NMR with SHARPER.9
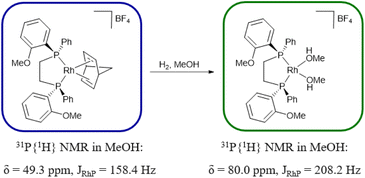 | ||
| Scheme 1 Hydrogenation of [Rh((R,R)-DIPAMP)(NBD)]BF4 to produce [Rh((R,R)-DIPAMP)(MeOH)2]BF4 in MeOH. | ||
 | ||
| Fig. 1 Comparison between 31P NMR and SHARPER experiments of [Rh((R,R)-DIPAMP)(NBD)]BF4 (112 mM in DCM-d2): 31P NMR spectrum without decoupling from 1H and without NOE (A); 31P{1H} NMR spectra, with or without NOE (B and C); sel-SHARPER 31P NMR spectra with a Gaussian-shaped selective pulse of the duration of 10 ms, with or without NOE (D and E); sel-SHARPER 31P NMR spectra with a Gaussian-shaped selective pulse of the duration of 5 ms, with or without NOE (F and G); SHARPER 31P NMR spectra, with or without NOE (H and I). Please refer to Table S2† for additional information. | ||
Moving to a model two-component solution, 31P MR-SHARPER was explored. A sample containing two diolefin complexes [Rh((R,R)-DIPAMP)(NBD)]BF4 and [Rh(DPPB)(COD)]BF4 (ratio ≈ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) gives rise to a pair of doublet resonances in the 31P{1H} NMR spectrum separated by 25 ppm (Fig. S7†). While each resonance is optimally enhanced when the offset is set on the peak itself (Fig. S7B and C,† 5-fold enhancement) the relative intensities between the peaks are non-quantitative, when compared to the standard 31P{1H} spectrum, due to RF pulse off-resonance effects. Thus, the best compromise is to set the offset in the centre of the two resonances so that they experience a consistent RF response. While setting the offset in the centre results in a slight reduction in SNR enhancement factor for the two resonances from 5 to 4, it remains a significant advantage over the traditional 31P{1H} NMR spectrum.
1) gives rise to a pair of doublet resonances in the 31P{1H} NMR spectrum separated by 25 ppm (Fig. S7†). While each resonance is optimally enhanced when the offset is set on the peak itself (Fig. S7B and C,† 5-fold enhancement) the relative intensities between the peaks are non-quantitative, when compared to the standard 31P{1H} spectrum, due to RF pulse off-resonance effects. Thus, the best compromise is to set the offset in the centre of the two resonances so that they experience a consistent RF response. While setting the offset in the centre results in a slight reduction in SNR enhancement factor for the two resonances from 5 to 4, it remains a significant advantage over the traditional 31P{1H} NMR spectrum.
The comparisons above have all considered the same number of scans. However, the repetition time is 15 s for the 31P{1H} NMR sequence and 25 s for SHARPER. A more meaningful SNR comparison is thus possible for equal total experiment time (Fig. S8†). Accordingly, the SNR enhancement factor decreases from 4, comparing equal number of scans, to 3, comparing equal experiment time.
To evaluate the potential of the optimised MR-SHARPER benchtop NMR sequence for in situ reaction monitoring, the hydrogenation of [Rh((R,R)-DIPAMP)(NBD)]BF4 in MeOH to give the Rh(I) solvent complex [Rh((R,R)-DIPAMP)(MeOH)2]BF4 (31P{1H} NMR in MeOH: δ 80.0 ppm, JRhP = 208.2 Hz, Scheme 1) was explored. Each spectrum, for both 31P{1H} NMR and SHARPER methods, was obtained in approximately 8 minutes (Fig. 2A, B and S9†). SHARPER reaction monitoring is superior in terms of SNRs (4-fold enhancement) as well as scattering of the data points observed in the kinetic profile. Repeating the same comparison but now obtaining each spectrum in approximately 1 minute, the difference between the 31P{1H} and SHARPER data is even more pronounced (Fig. 2C, D and S10†). No fitting could be performed on the data derived from the traditional 31P{1H} NMR spectra. Instead, a meaningful kinetic profile could only be obtained via the SHARPER sequence. Comparing the results from the reaction monitoring by 31P{1H} NMR where each spectrum is obtained in ca. 8 minutes (Fig. 2A) and the SHARPER sequence where each spectrum is obtained in ca. 1 minute (Fig. 2D) we observe similar data quality. Indeed, the SHARPER sequence acquired in 1 min still gives slightly better SNRs and lower dispersion errors than standard 31P{1H} NMR spectra acquired in 8 min. Therefore, SHARPER is expected to perform better in cases of either faster reactions or lower concentrations of analyte. To explore faster reactivity, in the final section monitoring was performed with an on-line setup (flow rate of 3.5 mL min−1).6
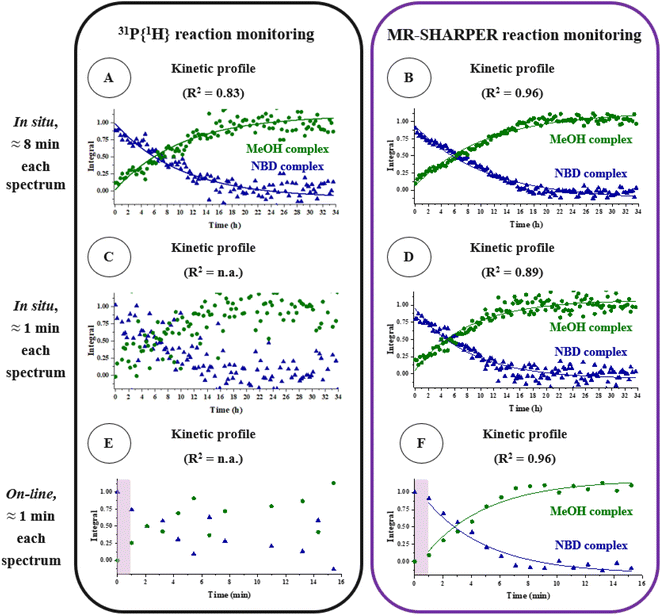 | ||
| Fig. 2 Comparison between 31P{1H} NMR (A, C, E) and MR-SHARPER 31P NMR (B, D, F) reaction monitoring of the hydrogenation of [Rh((R,R)-DIPAMP)(NBD)]BF4 (9 mM in MeOH) to produce [Rh((R,R)-DIPAMP)(MeOH)2]BF4 (Scheme 1). Experiment times: 7 min 50 s for A; 7 min 59 s for B; 51 s for C; 54 s for D; 59 s for E; 58 s for F. For the corresponding NMR spectra, please refer to Fig. S9–S11.†In situ monitoring has been performed in a J Young tube inserted into the spectrometer (A, B, C, D). On-line monitoring has been performed via a customised setup with a flow rate of 3.5 mL min−1 (E, F). The preparation time is highlighted: the two points in each kinetic profile appearing at time zero have been taken from the spectra (31P{1H} NMR and MR-SHARPER) in flow conditions of the starting material (E, F). | ||
The above-described hydrogenation of the diolefin complex [Rh((R,R)-DIPAMP)(NBD)]BF4 (Scheme 1) has been followed by traditional 31P{1H} NMR and by the MR-SHARPER technique (Fig. 2E, F and S11†). A delay at the start of each reaction monitoring experiment of ca. 1 min, herein called the preparation time, could not be avoided. It consists of the time required to stir the reaction mixture after the addition of hydrogen and to pump the reaction mixture into the spectrometer. The data obtained using 31P{1H} NMR monitoring (Fig. 2E) is not of sufficient quality for fitting of the data points. In contrast, SHARPER monitoring produced a robust kinetic profile (Fig. 2F). As in static conditions, significantly better SNRs have been obtained with the MR-SHARPER sequence for both the reactant and product resonances. Interestingly, this demonstrates that despite the reduced residence time of the sample inside the active region of the benchtop NMR spectrometer under flow conditions, the series of SHARPER refocusing pulses are able to achieve significant line-narrowing and therefore SNR enhancement.
In conclusion, we have demonstrated that 31P benchtop NMR spectroscopy with signal enhancement via SHARPER is a valuable tool for in situ and on-line reaction monitoring of organometallic systems. Focus on the heteronucleus 31P is particularly relevant for applications in main group chemistry and homogeneous catalysis. The sensitivity limitations posed by the low magnetic field of the benchtop spectrometer (1.9 T) and the relative insensitivity of 31P compared to 1H nucleus were overcome through the implementation of the SHARPER pulse sequence, demonstrating enhancements of 3 to 9-fold when compared to traditional 31P{1H} NMR spectra. Drawbacks are still present, such as the pre-knowledge of the species involved in the reaction and the limitation of the number of signals that can be monitored in a single spectrum. However, the signal enhancement provide by SHARPER generates kinetic profiles with lower dispersion, which ultimately allows for either faster analysis or analysis at lower concentrations of analyte. Indeed, a fast reaction, which could not be followed via traditional 31P{1H} NMR spectroscopy, was successfully monitored using the MR-SHARPER sequence and a customised flow setup, specifically designed for highly oxygen-sensitive organometallic species.
Data availability
The data supporting this article have been included as part of the ESI.† NMR spectroscopic details can be obtained from the authors.Author contributions
LT: conceptualisation, data curation, formal analysis, investigation, project administration, visualisation, writing (original draft). MEH: conceptualisation, software, writing (review & editing). TB: funding acquisition, supervision, writing (review & editing).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Deutsche Forschungsgemeinschaft (DFG), Grant number 452714985 and the UK Engineering and Physical Sciences Research Council (EPSRC), Grant Number: EP/X03528X/1. We thank Hans-Joachim Drexler (LIKAT) for resources and Ana Silva Terra and Daniel Taylor (University of York) for their assistance on NMR pulse programming.Notes and references
- J. F. Hartwig, Organotransition Metal Chemistry. From Bonding to Catalysis, University Science Books, Sausalito, 2010; A. L. Clevenger, R. M. Stolley, J. Aderibigbe, J. Louie, Chem. Rev. 2020, 120, 6124–6196; S. Lapointe, A. Sarbajna, V. H. Gessner, Acc. Chem. Res. 2022, 55, 770–782 Search PubMed.
- D. A. Foley, A. L. Dunn and M. T. Zell, Magn. Reson. Chem., 2016, 54, 451–456 CAS.
- R. Neudert, E. Ströfer and W. Bremser, Magn. Reson. Chem., 1986, 24, 1089–1092 CAS; A. M. R. Hall, J. C. Chouler, A. Codina, P. T. Gierth, J. P. Lowe and U. Hintermair, Catal. Sci. Technol., 2016, 6, 8406–8417 Search PubMed; C. Jacquemmoz, F. Giraud and J.-N. Dumez, Analyst, 2020, 145, 478–485 Search PubMed.
- T. A. van Beek, Phytochem. Anal., 2021, 32, 24–37 CAS; M. Grootveld, B. Percival, M. Gibson, Y. Osman, M. Edgar, M. Molinari, M. L. Mather, F. Casanova and P. B. Wilson, Anal. Chim. Acta, 2019, 1067, 11–30 Search PubMed.
- E. Danieli, J. Perlo, B. Blümich and F. Casanova, Angew. Chem., Int. Ed., 2010, 49, 4133–4135 CAS.
- L. Tadiello, H.-J. Drexler and T. Beweries, Organometallics, 2022, 41, 2833–2843 CAS.
- D. Caramelli, J. M. Granda, S. H. M. Mehr, D. Cambié, A. B. Henson and L. Cronin, ACS Cent. Sci., 2021, 7, 1821–1830 CAS.
- A. B. Jones, G. C. Lloyd-Jones and D. Uhrín, Anal. Chem., 2017, 89, 10013–10021 CAS.
- C. L. Dickson, G. Peat, M. Rossetto, M. E. Halse and D. Uhrín, Chem. Commun., 2022, 58, 5534–5537 CAS.
- For examples of applications of pure-shift methods on benchtop spectrometers: B. Gouilleux, J. Farjon and P. Giraudeau, J. Magn. Reson., 2020, 319, 106810 CAS.
- K. Zangger and H. Sterk, J. Magn. Reson., 1997, 124, 486–489 CAS.
- L. Castañar, Magn. Reson. Chem., 2017, 55, 47–53 Search PubMed.
- A. I. Silva Terra, M. Rossetto, C. L. Dickson, G. Peat, D. Uhrín and M. E. Halse, ACS Meas. Sci. Au, 2023, 3, 73–81 CAS.
- G. Peat, P. J. Boaler, C. L. Dickson, G. C. Lloyd-Jones and D. Uhrín, Nat. Commun., 2023, 14, 4410 CAS.
- M. Davy, C. L. Dickson, R. Wei, D. Uhrín and C. P. Butts, Analyst, 2022, 147, 1702–1708 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and pulse sequence details, additional figures. See DOI: https://doi.org/10.1039/d4ay00948g |
| This journal is © The Royal Society of Chemistry 2024 |
