Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rapid differentiation of xylazine metabolites using SLIM IM-MS†
Ralph
Aderorho
,
Shadrack Wilson
Lucas
and
Christopher D.
Chouinard
 *
*
Department of Chemistry, Clemson University, 211 S Palmetto Blvd, Clemson, SC 29634, USA. E-mail: cchouin@clemson.edu
First published on 5th June 2024
Abstract
Xylazine represents an increased threat to the recreational drug market. In this study, we present a rapid strategy for identifying xylazine and differentiating its common isomeric metabolites using Structures for Lossless Ion Manipulations (SLIM) ion mobility coupled to high-resolution/tandem mass spectrometry (IM-HRMS/MS). Chemical derivatization using dansyl chloride also assisted with separations and led to identification of resolvable reaction product atropisomers.
Xylazine, a structural analogue of clonidine, is an α2-agonist originally developed in 1962 for use as an antihypertensive agent in humans.1,2 However, its use was discontinued due to adverse effects observed during clinical trials and, since then, it has only been used therapeutically in veterinary medicine as a large animal sedative. Recently, xylazine has been introduced as an increasingly frequent additive in the illicit drug market. Commonly referred to as “tranq”, “tranq dope”, or “zombie drug”, xylazine has been detected in recreational drug mixtures including cocaine, amphetamines, heroine, morphine, and fentanyl.3 In 2021, xylazine was found in >90% of illicit drug samples in Philadelphia and has since infiltrated nearly all 50 states.4,5 As a result of its increased prevalence, its differential treatment (i.e., naloxone administration does not reverse overdose), and the continued emergence of other new psychoactive substances (NPS), there is a critical need to develop advanced analytical technologies capable of rapidly and confidently identifying such compounds and/or their biological metabolites.
Ion mobility spectrometry (IMS) has been a stalwart in drug detection for several decades.6–9 As a screening technique, IMS provides rapid feedback in a portable/handheld instrument. In order to achieve the selectivity required for differentiating similar drugs within a given class, such as isomers, IMS has been coupled with tandem and/or high-resolution mass spectrometry (i.e., IM-MS).10–16 This powerful technique has seen increased utility in clinical, forensic, and toxicological applications. Our group has reported on a recently commercialized high-resolution IM platform, Structures for Lossless Ion Manipulations (SLIM),17–19 for the rapid differentiation of synthetic cannabinoid isomers.20
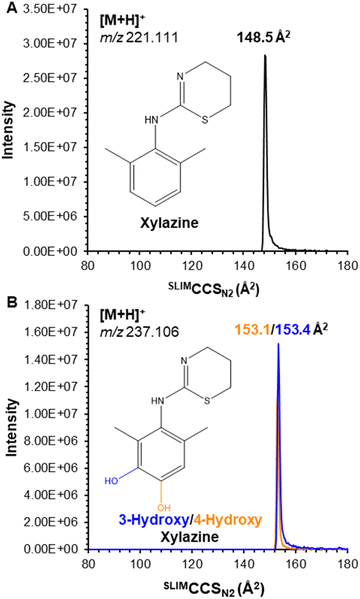
Herein, we focus on xylazine and its prominent metabolites (3-hydroxy xylazine and 4-hydroxy xylazine). These compounds, purchased from Cayman Chemical (Ann Arbor, MI) as DEA exempt preparations, were prepared as 1 μg mL−1 solutions in 50/50 water (0.1% formic acid)/methanol (v/v) and measured using a MOBILion Systems MOBIE SLIM platform (Chadd's Ford, PA) coupled to an Agilent 6546 QTOF (Santa Clara, CA). Samples were introduced via flow injection analysis (FIA) using an Agilent 1290 UHPLC autosampler and ionized with Agilent Jetstream (AJS) electrospray ionization (ESI) in positive mode. Xylazine yielded a single mobility feature (Fig. 1A) for the protonated ion at m/z 221.111 with a measured collision cross section (CCS) of SLIMCCSN2 148.5 Å2. All compounds were separately analyzed on an Agilent 6560 drift tube ion mobility spectrometry (DTIMS) instrument for direct CCS measurements; xylazine had a measured DTCCSN2 of 148.1 Å2 (ΔCCS < 0.3%), demonstrating the accuracy of SLIMCCS measurements with only external calibration using the Agilent Tune Mix and no correction factor needed. Complete details of instrumental parameters (Tables S1–S4†) and DTCCSN2 data (Table S5†) are included in the ESI.†
 | ||
| Fig. 1 Ion mobilograms for protonated (A) xylazine and (B) its isomeric metabolites 3-hydroxy/4-hydroxy xylazine. | ||
Its metabolites, 3-hydroxy and 4-hydroxy xylazine, also each yielded a single mobility feature for the protonated ion at m/z 237.106, with measured SLIMCCSN2 of 153.4 and 153.1 Å2, respectively; despite the high resolving power of SLIM (Rp ∼ 200), the isomeric metabolites were unresolved (Fig. 1B). To overcome this challenge, these compounds were derivatized using the common dansyl chloride reaction,20,21 which selectively dansylated amines and phenolic alcohols. The success of this reaction (which was performed by combining the individual metabolites with dansyl chloride in a pH 9.0 buffer solution maintained at 60 °C for 5 minutes) was monitored by the singly dansylated product at m/z 470.157 (Fig. 2A). Their products also yielded a single mobility feature for each metabolite, but these were now baseline resolved by SLIM (Fig. 2B) with measured SLIMCCSN2 of 213.5 and 218.4 Å2 for 3-hydroxy and 4-hydroxy xylazine, respectively. This ∼2% difference in CCS demonstrates the necessity for the higher resolving power of SLIM-based separations, where lower resolution instruments might not adequately separate these isomers.
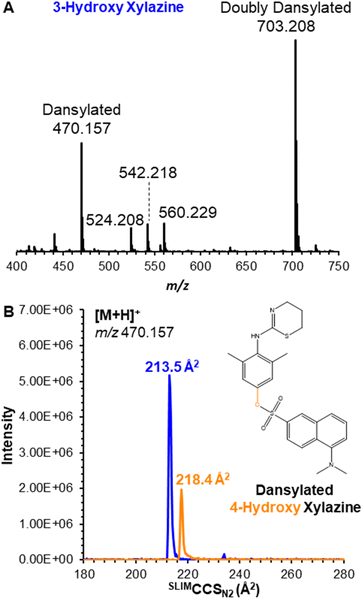 | ||
| Fig. 2 (A) Mass spectrum for the products of dansylation of 3-hydroxy xylazine. (B) Ion mobilogram for 3-hydroxy and 4-hydroxy xylazine as the protonated dansylated product at m/z 470. | ||
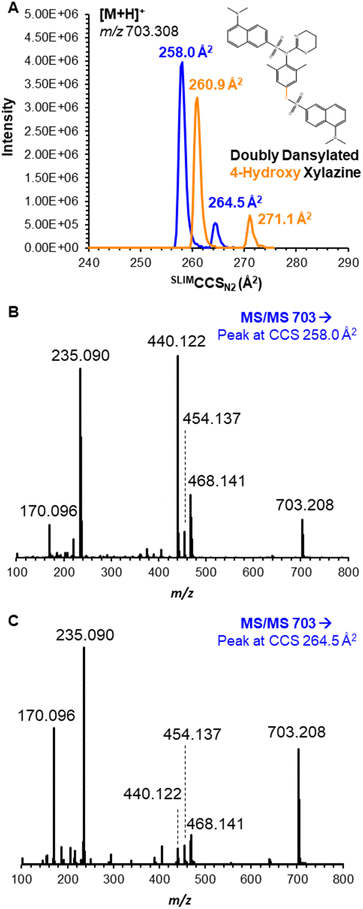
Furthermore, the doubly dansylated product (reacted at both secondary amine and phenolic oxygen) was observed prominently at m/z 703.208, but each individual compound yielded a bimodal mobility distribution (Fig. 3A). Despite the appearance of multiple peaks, the high resolving power of SLIM contributed to increased peak capacity that allowed near baseline resolution of all four species, with SLIMCCSN2 of 258.0/264.5 Å2 for 3-hydroxy xylazine and 260.9/271.1 Å2 for 4-hydroxy xylazine; an IM spectrum displaying separation of the mixture is shown in Fig. S1.† The appearance of multiple mobility peaks for a single m/z could be the result of reaction product isomers (i.e., atropisomers), gas-phase conformers, or protonation site isomers (i.e. prototropic isomers or ‘protomers’). Because the latter two scenarios would be a result of the ionization process and/or transport into the gas-phase, neither would be expected to yield multiple chromatographic peaks. However, upon analysis of these compounds with reversed-phase LC (see conditions in ESI†), unique and resolvable chromatographic features were observed at retention times of 8.8 and 9.1 minutes (Fig. S2†), respectively; this observance indicates different chemical species, presumably atropisomers, where dansylation can occur to form one of two (non-interconverting) stereoisomers with hindered rotation (most likely at the secondary amine derivatization). Proposed figures for all dansylation products, including the atropisomers of these two metabolites, are shown in Fig. S3 and S4.† This conclusion was further aided by the use of mobility-aligned fragmentation.
(MAF) performed on the individual mobility features for each reaction product, which yielded nearly identical MS/MS patterns albeit with differing relative abundances (as might be expected for differential fragmentation of atropisomers based on the stability of the two compounds).
Conclusions
In summary, we have demonstrated an LC-SLIM IM-HRMS/MS approach, combined with simple chemical derivatization, for the separation and differentiation of xylazine and the reaction products of two of its hydroxylated metabolites. This reaction improved IM resolution for the singly dansylated products. In the case of the (also prominently observed) doubly dansylated products, this strategy allowed confirmation of the formation of atropisomers, or rotationally/sterically hindered isomers. This especially demonstrates the significant structural characterization capabilities enabled by the combined LC-SLIM IM-HRMS/MS approach, which the authors expect will have significant impact not only for detection of illicit drugs but also for potential pharmaceutical applications.Author contributions
RA and SWL performed all experimental work. CDC and RA reviewed the data and prepared the manuscript. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors receive funding from MOBILion Systems, Inc., the manufacturer of the SLIM technology.Notes and references
- R. Gupta, D. R. Holtgrave and M. A. Ashburn, N. Engl. J. Med., 2023, 388, 2209–2212 CrossRef CAS PubMed
.
- S. A. Greene and J. C. Thurmon, J. Vet. Pharmacol. Ther., 1988, 11, 295–313 CrossRef CAS PubMed
.
- R. S. Alexander, B. R. Canver, K. L. Sue and K. L. Morford, Am. J. Public Health, 2022, 112, 1212–1216 CrossRef PubMed
.
- Substance Use in Philly, Xylazine/Tranq. https://www.substanceusephilly.com/tranq.
- S. L. Kacinko, A. L. A. Mohr, B. K. Logan and E. J. Barbieri, J. Anal. Toxicol., 2022, 46, 911–917 CrossRef CAS PubMed
.
- J. R. Verkouteren and J. L. Staymates, Forensic Sci. Int., 2011, 206, 190–196 CrossRef CAS
.
- E. Sisco, J. Verkouteren, J. Staymates and J. Lawrence, Forensic Chem., 2017, 4, 108–115 CrossRef CAS PubMed
.
- A. H. Lawrence, Forensic Sci. Int., 1987, 34, 73–83 CrossRef CAS PubMed
.
- L. Fytche, M. Hupé, J. Kovar and P. Pilon, J. Forensic Sci., 1992, 37, 1550–1566 CrossRef CAS
.
- C. Wu, W. F. Siems and H. H. Hill, Anal. Chem., 2000, 72, 396–403 CrossRef CAS PubMed
.
- R. Aderorho and C. D. Chouinard, Drug Test. Anal., 2024, 16, 369–379 CrossRef CAS
.
- K. E. Butler and E. S. Baker, J. Am. Soc. Mass Spectrom., 2022, 33, 1904–1913 CrossRef CAS PubMed
.
- A. B. Hall, S. L. Coy, E. Nazarov and P. Vouros, Int. J. Ion Mobility Spectrom., 2012, 15, 151–156 CrossRef CAS PubMed
.
- S. Hooshfar, S. Tchu, C. Yun and K. L. Lynch, J. Mass Spectrom. Adv. Clin. Lab, 2022, 23, 50–57 CrossRef CAS PubMed
.
- K. J. Adams, C. E. Ramirez, N. F. Smith, A. C. Muñoz-Muñoz, L. Andrade and F. Fernandez-Lima, Talanta, 2018, 183, 177–183 CrossRef CAS
.
- R. Aderorho and C. D. Chouinard, Int. J. Mass Spectrom., 2024, 496, 117185 CrossRef CAS
.
- I. K. Webb, S. V. B. Garimella, A. V. Tolmachev, T. C. Chen, X. Zhang, R. V. Norheim, S. A. Prost, B. LaMarche, G. A. Anderson, Y. M. Ibrahim and R. D. Smith, Anal. Chem., 2014, 86, 9169–9176 CrossRef CAS
.
- A. M. Hamid, Y. M. Ibrahim, S. V. B. Garimella, I. K. Webb, L. Deng, T. C. Chen, G. A. Anderson, S. A. Prost, R. V. Norheim, A. V. Tolmachev and R. D. Smith, Anal. Chem., 2015, 87, 11301–11308 CrossRef CAS PubMed
.
- A. L. Hollerbach, Y. M. Ibrahim, V. S. Lin, K. J. Schultz, A. P. Huntley, P. B. Armentrout, T. O. Metz and R. G. Ewing, J. Am. Soc. Mass Spectrom., 2024, 35, 793–803 CrossRef CAS PubMed
.
- R. Aderorho, S. W. Lucas and C. D. Chouinard, J. Am. Soc. Mass Spectrom., 2024, 35, 582–589 CrossRef CAS
.
- M. R. Anari, R. Bakhtiar, B. Zhu, S. Huskey, R. B. Franklin and D. C. Evans, Anal. Chem., 2002, 74, 4136–4144 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ay00801d |
| This journal is © The Royal Society of Chemistry 2024 |