Multivariate optimization and validation of 200 pesticide residues in the banana matrix by GC-MS/MS†
Tushar Rajaram
Ahire
 ab,
Rupal Rajesh
Thasale
ab,
Rupal Rajesh
Thasale
 a,
Ankita
Das
a,
Ankita
Das
 ac,
Nikhil Pradip
Kulkarni
ac,
Nikhil Pradip
Kulkarni
 a,
Dhyan Mineshkumar
Vyas
a,
Dhyan Mineshkumar
Vyas
 ab and
Sivaperumal
Perumal
ab and
Sivaperumal
Perumal
 *a
*a
aChemical Sciences Division, ICMR – National Institute of Occupational Health, Meghani Nagar, Ahmedabad, India. E-mail: sivaperumal.p@gov.in; sivaperum2003@yahoo.co.in
bDepartment of Biochemistry and Forensic Science, Gujarat University, Ahmedabad, India
cNational Forensic Sciences University, Tripura Campus, India
First published on 4th June 2024
Abstract
GC-MS/MS has been observed from past studies to be an appropriate choice for designing a simple, efficient and sensitive analytical technique. Accordingly, the linearity and working range, Method Limit of Detection (MLOD), Method Limit of Quantification (MLOQ), accuracy, precision (intra-day and inter-day), Matrix Effect (ME) and selectivity were analyzed for the assessment of 200 pesticide residues [organophosphorus pesticides (OPP), organochlorine pesticides (OCP), organonitrogen pesticides (ONP), synthetic pyrethroid pesticides (SPP), and herbicide methyl esters (HME)] in the banana matrix. The procedure involved QuEChERS (quick, easy, cheap, effective, rugged, and safe) extraction and clean-up with Multi-Walled Carbon Nanotubes (MWCNTs) and Primary Secondary Amine (PSA) wherein the factors were optimized using the Plackett–Burman and central composite designs. The performance of the method in order to quantitate 200 pesticides at trace levels was evaluated by matrix-matched calibration. The linearity was observed to range from 1 to 100 μg L−1 with determination coefficient (r2) > 0.99. Recovery studies were conducted at 2 levels, 10 μg kg−1 and 25 μg kg−1, and the values obtained were in the range of 71–116% and 72–119%, respectively. The Relative Standard Deviation (RSD) was observed to be less than 20% in line with the recommended guidelines (SANTE/11312/2021). The MLOD and MLOQ were found to be in the range of 0.45–6.33 μg kg−1 and 1.44–9.59 μg kg−1 respectively. The developed method was applied satisfactorily to analyse banana samples cultivated in different regions of Gujarat, India.
Introduction
Banana, an edible fruit belonging to the genus Musa is known for its nutritional as well as medicinal benefits. It is not just high in calories, providing about 90–100 kcal per 100 g of edible portion, but it also has an abundance of necessary elements.1 Banana plantations thrive in hot, wet and tropical climates, due to which they are more susceptible to pest infestations and fungal infection, especially from Thrips hawaiiensis, Hypomeces spp., Macrophoma musae, Pyricularia grisea, Mycosphaerella musicola, etc.2 It is to prevent these infestations as well as to increase the yield of bananas that agricultural professionals have over time made liberal use of pesticides.3However, it is imperative to note that repeated usage of pesticides increases the possibility of pesticide resistance and pest resurgence, in addition to retention of residues, and the resultant ecological damage. Depending on their type and interaction, potential pesticides build up in the fatty tissues of human beings and over a period of time damage the endocrine, nervous, and immune systems, sometimes even causing cancer. Serious illnesses such as kidney disease, Parkinson's disease, Alzheimer's disease etc.1,3–5 too have been linked to exposure to them. In order to mitigate these and many other adverse health effects, various organizations have proposed maximum residue levels (MRLs) so as to regulate exposure to pesticide residues in foods.1,3 Development of specific and sensitive analytical methods aids in assessing multi-pesticide residues which in turn aids in evaluating MRLs for regulatory purposes. The present study is an attempt towards achieving the same for the banana matrix.
Chromatographic techniques combined with Nitrogen–Phosphorus Detection (NPD),6 Flame Ionization Detection (FID),6 Diode-Array Detection (DAD),6 Electron Capture Detection (ECD),6 Mass Spectrometry (MS),6,7etc. have been to date commonly employed for specific pesticide residue analysis. Herein, the number of target analytes is limited, but sensitivity and confirmation too frequently fall short of trace level (sub ppb level) estimations. Gas chromatographic methods coupled with tandem mass spectrometry (MS/MS) detection have of late emerged as an excellent alternative for detecting pesticide residues at extremely low levels with exceptional sensitivity and specificity.6,8,9 In view of this, it is critical to emphasize that use of the multiple reaction monitoring (MRM) mode and electron impact ionization shortens the time of chromatographic analysis or separation and even improves the signal-to-noise ratios (S/N) thereby improving specificity and sensitivity.9,10
Determination of pesticide residues at trace levels in food matrices is challenging due to a host of factors. These include the physicochemical features of the pesticides being estimated as well as the voluminous quantity of interferents which can lead to adverse outcomes post chromatography.1,4,11,12 Over the last few years numerous procedures involving Solid Liquid Extraction (SLE),12 Solid Phase Extraction (SPE),12 Matrix Solid-Phase Dispersion (MSPD),12 Solid-Phase Micro Extraction (SPME),12 Pressurized Liquid Extraction (PLE),12 Stir Bar Sorptive Extraction (SBSE)12 and Microwave Assisted Extraction (MAE)10 have been developed and employed for extraction and clean-up, for residual pesticide analysis in fruits.10 Although these techniques have been proven to be quite effective, some of them used large amounts of solvents whereas some had high run times making these studies difficult to perform when extraction of a small range of pesticides was needed, or sometimes when multi-residue analysis7,10 was to be carried out.
The QuEChERS method developed in 2003 brought about a revolutionary change in the sample preparation method for pesticide residue analysis. This approach based on acetonitrile-based extraction and salt-assisted partitioning14 involved use of different sorbents for extraction procedures. Whilst magnesium sulphate (MgSO4) is used in this technique to lower the aqueous phase and increase partitioning of pesticides into an organic layer, the fat globules were broken down using sodium acetate (Na acetate). Cleaning up with primary secondary amine (PSA) in dispersive solid phase extraction (d-SPE) further improved the production of cleaner extracts.15,16 This in turn increased the capacity for removing sugars, organic and fatty acids and polar pigments, as well as aiding in eliminating matrix co-extractants, which could interfere with pesticide residue analysis. The QuEChERS technique incorporates multiwalled carbon nanotubes (MWCNTs) due to their advantageous physico-chemical properties, such as high porosity, surface area, and chemical stability. Furthermore, MWCNTs exhibit favourable stacking interactions between π–π molecules and a strong affinity for polycyclic molecules, making them valuable in this context.16–18 Whilst the C18 silica sorbent is employed for samples with known fat content, graphitic carbon black (GCB) is used in this technique to remove pigments such as chlorophylls and carotenoids because of their affinity for planar molecules. Usage of citrate salts is also occasionally done whenever method modification is required so as to enhance recovery of sensitive and pH-dependent pesticides or when complicated matrices1,12 are investigated. Over the last few years the QuEChERS method has seen optimization and validation for a host of matrices viz. vegetables, fruits, fruit juices, cereals, fatty foods1,12,15etc. However, during a literature review it was observed that only limited studies reported the amalgamation of d-SPE and MWCNT extraction methods for estimation of multi-class pesticide residues, especially for the banana matrix.
The Central Insecticides Board and Registration Committee (CIBRC) of India has registered under its ambit numerous pesticides, insecticides, fungicides and herbicides, the usage of which is prevalent in the country. Whilst some of these are regularly applied to bananas, others are used in broader agricultural contexts and often result in residues in banana samples via runoff, drift, or soil contamination. In order to encompass this wide spectrum of pesticides, it became pertinent to develop a method for a mixture with a vast spectrum of residues. Development and validation of such a method would help facilitate a comprehensive assessment of pesticide residues, thereby enabling detection of pesticide residues in banana to assist in implementation of risk mitigation strategies, and help promote sustainable agricultural practices.
With these objectives in mind, the current study has integrated the use of d-SPE and MWCNTs along with a central composite design (CCD) for optimisation of the QuEChERS method for determination of 200 pesticide residues in banana by GC-MS/MS in MRM mode.
Materials and methods
Chemicals and apparatus
For the purpose of this study nine groups of certified reference standard pesticide mixtures of OPP, OCP, ONP, SPP and HME (totalling 200 pesticides) at a concentration of 100 mg L−1 and 81–99% purity were obtained from RESTEK (Bellefonte, PA 16823, USA). An individual stock standard solution of each group of pesticides was prepared at a concentration of 50 mg L−1 in toluene and the mixtures were stored at a temperature of −20 °C in amber glass volumetric flasks. A total of 200 mixtures of working standard solutions were prepared at different concentrations [50 mg L−1 (main stock), 5 mg L−1 (intermediate stock) and 1 mg L−1 (working standard solution)] by appropriate dilution of the stock solutions. These were used for method optimization, validation, quantification and analysis of actual samples.HPLC grade acetonitrile (J.T. Baker, New Jersey 8865, USA), reagent plus grade acetic acid, reagent plus grade anhydrous magnesium sulphate (purity ≥99.5%), MWCNTs (with a diameter of 50–90 nm and >95% carbon basis) and activated charcoal (100–400 mesh) were used (Sigma Aldrich, Louis, MO 63103, USA). Emparta® ACS grade ethyl acetate (Merck Life Science, Rahway, NJ 07065, USA), pesticide residue grade toluene (Fisher Scientific, Loughborough LE11 5RE – UK), ExcelR grade acetone and sodium acetate from Qualigens (Maharashtra, India) and PSA from Agilent Technologies (Santa Clara, CA 95051, USA) were used for this study.
A high volume homogenizer (Robot Coupe BLIXER® 6 V.V.); analytical balance – Shimadzu AUX (220); refrigerated centrifuge (SORVALL Legend X1R, Thermo Scientific, Waltham, MA 02451, USA); Zymark Turbovap LV (Marshall Scientific); Cryocube F740hi (Eppendorf); and micropipettes (Eppendorf) were used for this study. Gas chromatography-triple quadrupole mass spectrometry (Nexis, GC 2030 and GC-MS-TQ8050NX, Shimadzu, Kanagawa 210-0821, Japan) was used for quantification and conditioning. The parameters adopted for GC-MS/MS are as described in Table 1.
| Parameters | Conditions |
|---|---|
| Column | SH-I-5SilMS, L 30 m × ID 0.25 μm × dF 0.25 μm |
| Injector port temperature | 280 °C |
| Interface temperature | 280 °C |
| Ion-source temperature | 230 °C |
| Injection mode | Splitless |
| Column flow | 1.0 mL min−1 |
| Purge flow | 3 mL min−1 |
| Solvent cut time (on delay) | 4 min |
| Collision gas | Argon (99.9999%) |
| Carrier gas | Helium (99.9999%) |
| Detector gain | +0.70 kV |
| Ionization voltage | 1.3 kV |
| Rate (°C min−1) | Temp. (°C) | Time (min) | |
|---|---|---|---|
| Column temperature program | — | 60 | 1 |
| 15 | 190 | 5 | |
| 10 | 300 | 4 | |
| Total run time | 28 min | ||
| Injection volume | 1 μL | ||
| Quantitation and confirmation mode | MRM | ||
| Split ratio | 20.0 | ||
Sample processing
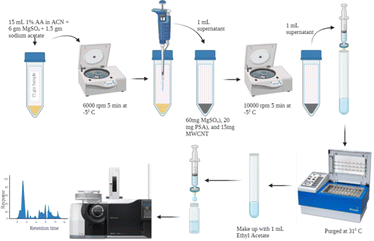
Samples of banana fruit (n = 25) were selected at random in the months of February–March 2023 from the local markets of Ahmedabad, Gujarat. A representative portion of the sample was blended using a high-volume homogenizer and mixed thoroughly. The homogenised samples were stored in 50 mL polytetrafluoroethylene (PTFE) tubes at −20 °C. Prior to initiation of the experiment, these samples were thawed at 5 °C overnight. Details of the extraction and d-SPE clean-up procedure are as shown in Fig. 1.Multivariate optimization
In line with the aim of the study a multivariate approach was chosen for optimizing the factors involved in the extraction process. The variables selected for optimization were sample weight (g), as well as the weight of MWCNTs (mg), MgSO4 (mg), PSA (mg), and activated charcoal (mg). For the purpose of optimization and representation these variables have been referred to as A, B, C, D, and E respectively in Table S1,† so as to be able to depict the interrelationships and interactions among multiple variables simultaneously. The process of multivariate optimisation involved two processes: (i) a Plackett–Burman (P–B) design (for screening), depicted in Tables S1 and S2,† followed by (ii) a central composite design (CCD) (for optimization) as shown in Table S3† for obtaining an effective and optimized extraction process for analysing multi-pesticide residues using the Minitab18.1® statistical software trial version (Minitab Inc., State College, USA).Herein, a mixture of 200 pesticides was used for the analysis. Accordingly, the average recovery of these pesticides was used as the response in this optimization. This was done so as to develop an efficient extraction method by using a mixture of pesticides.
Method validation
The developed method was validated in order to establish its reliability and suitability for quantification of multi-pesticide analytes in the banana matrix. The validation procedures were assessed as per SANTE guidelines.12,19 The linearity and working range, MLOD, MLOQ, accuracy, precision (intra-day and inter-day), selectivity and ME were evaluated. The investigation of ME involved comparing the calibration curves generated in solvent with those created in the matrix-matched solvent. This was achieved by adding the standard to the solvent and matrix-matched solvent, respectively, to assess the differences if any in the matrix effect. For the purpose of linearity, pesticide mixtures were evaluated at 8 levels (viz. 1, 2.5, 5, 10, 25, 50, 75 and 100 μg L−1). Accuracy and precision were evaluated by examining seven replicates of a blank banana matrix spiked at concentrations of 10, 25, and 50 μg L−1 before sample extraction. The assessment was based on predefined acceptance criteria: recovery rates between 70% and 120% and a Relative Standard Deviation (RSD) of less than 20%. Inter-day precision was assessed and observed to be 25 μg L−1. Post this seven spiked samples were examined for intra-day precision. The MLOD and MLOQ were evaluated by extracting seven replicates of the sample spiked with a mixture of pesticides at a concentration of 10 μg L−1. The selectivity was evaluated based on extraction and analysis of a ‘blank’ and ‘reagent blank’.Results and discussion
Optimization of GC-MS/MS
GC-MS/MS parameters were optimized through Solution ver. 4.45 software and Microsoft Excel™ based files (MRM Optimization Tool and pesticide database ver. 1.03). For optimization of MS parameters, all compounds were first monitored in full scan mode in the range of 45–550 m/z using the Shimadzu SMART MRM Optimization Tool. The three most intense transitions for each pesticide and their optimal Collision Energies (CE) were selected for MRM mode. The working parameters of the GC-MS/MS have been tabulated in Tables 1 and 3 respectively.The MRM mode was used, since it has been observed to be very specific, selective, and sensitive.20 It was also observed that the peaks obtained were precisely separated and that retention times (RTs) too were within a deviation of ± 0.2 min from the estimated retention durations. Based on the separation obtained, the total ion chromatogram was used for screening whereas MRM was used for quantitation of pesticide residues in banana samples.
Plackett–Burman (P–B) design
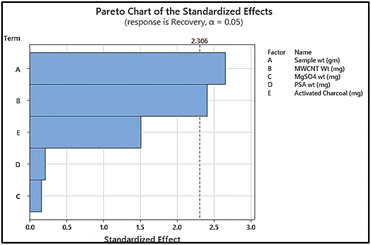
Sample preparation was optimized using the Plackett–Burman design and was used to screen for the most significant parameters. A permutation of the five effective parameters viz. (a) sample wt: 5–15 g; (b) MWCNTs wt: 5–15 mg; (c) MgSO4 wt: 30–60 mg; (d) PSA wt: 20–40 mg and (e) activated charcoal wt: 0–5 mg was carried out to investigate their potential impact on sample preparation. The effectiveness was investigated in 15 runs at 3 levels (−1, 0, +1) using Analysis of Variance (ANOVA) at the 95% confidence level (p value < 0.05). Systematic random sampling was carried out for the samples for the said procedure so as to avoid bias. Results obtained from the P–B design have been depicted in the form of a Pareto chart in Fig. 2. The results indicated that, for sample preparation of the target analytes, sample weight and quantity of MWCNT were the only significant variables amongst all the parameters. It was also observed that MWCNTs were more significant as compared to PSA during the clean-up step, due to their ability to remove pigments and matrix interfering substances whilst at the same time maintaining pH for improving method sensitivity.21 The weight of (c) MgSO4, (d) PSA and (e) activated charcoal had no significant impact on the response and hence their values were fixed based on the response they produced during the screening design. Accordingly, (c) weight of MgSO4 and (d) weight of PSA were fixed at 60 mg and 20 mg respectively. The smaller quantity of MgSO4 was chosen since it tended to agglomerate at higher quantities and also produced an exothermic reaction, which could in turn cause loss of thermally sensitive pesticides.22 It was also noticed that, as the amount of activated charcoal increased, recovery of analytes decreased indicating that use of activated charcoal as an adsorbent led to the deterioration of recovery of pesticide analytes in the matrix. Rather, higher recovery and efficiency were observed without including this adsorbent in this method.Further, so as to deduce the optimum values of the two most significant factors the central composite design was utilised.
| Recovery = 87.93 + 0.407a − 0.369b − 0.0083c − 0.0164d − 0.462e + 1.31CtPt |
Central composite design (CCD)
The two significant factors obtained from the screening design were further optimized using a CCD and were studied at different levels of low (−1), medium (0), and high (+1) as well as at the axial points (−α, +α). The ANOVA test was employed in order to elicit a response and to ascertain the optimum value for each factor at a 95% (p value < 0.05) confidence level (Table 2). It was observed that the interaction between the sample weight and weight of MWCNTs had a significant impact on the extraction efficiency with p-values of 0.01 and 0.05 respectively. The ‘lack of fit’ observed in this model was statistically not significant with a p-value of 0.5, indicating that the model satisfactorily captured the relationship between design and data.| Source | DF | Adj. SS | Adj. MS | f-Value | P-Value |
|---|---|---|---|---|---|
| a DF – degree of freedom; adj. SS – adjusted sum of square; adj. MS – adjusted mean square. | |||||
| Model | 5 | 1025.42 | 205.083 | 3.9 | 0.052 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Linear Linear |
2 | 916.82 | 458.408 | 8.72 | 0.013 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sample wt (g) Sample wt (g) |
1 | 628.33 | 628.333 | 11.95 | 0.011 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MWCNTs wt (mg) MWCNTs wt (mg) |
1 | 288.48 | 288.483 | 5.49 | 0.052 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Square Square |
2 | 17.4 | 8.699 | 0.17 | 0.851 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sample wt (g) × sample wt (g) Sample wt (g) × sample wt (g) |
1 | 13.16 | 13.157 | 0.25 | 0.632 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MWCNT wt (mg) × MWCNT wt (mg) MWCNT wt (mg) × MWCNT wt (mg) |
1 | 2.46 | 2.461 | 0.05 | 0.835 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-Way interaction 2-Way interaction |
1 | 91.2 | 91.202 | 1.74 | 0.229 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sample wt (g) × MWCNT wt (mg) Sample wt (g) × MWCNT wt (mg) |
1 | 91.2 | 91.202 | 1.74 | 0.229 |
| Error | 7 | 367.96 | 52.656 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lack-of-fit Lack-of-fit |
3 | 147.28 | 49.092 | 0.89 | 0.519 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pure error Pure error |
4 | 220.68 | 55.17 | ||
| Total | 12 | 1393.37 | |||
The two-level full factorial CCD for 2 factors consisted of 13 randomized experimental runs including the center points. The randomized runs were performed so as to aid in mitigating the influence of a host of unpredictable and uncontrollable variables in the multi-residual extraction procedure experimental runs as shown in Table S3.† [The design of these runs consisted of 1 base block with cube point 4, a center point in cube 5 and the axial point 4.] The randomized runs were produced in line with the following equation viz.
| N = 2K + 2k + Cp |
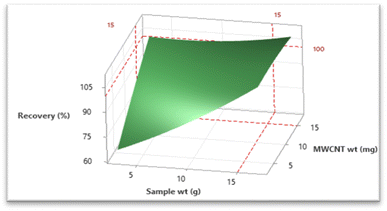
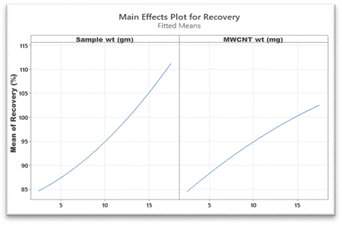
The three-dimensional surface plot depicted in Fig. 3 portrays the influence of the two significant factors viz. wt of MWCNTs and wt of the sample on recovery. The surface plot also revealed that maximal recovery of pesticide residues could be achieved with an increase in sample weight and also by maintaining the weight of MWCNTs at a higher value. The main effects plot (Fig. 4) for recovery showcases a graphical representation depicting the optimal values for each independent variable which could maximize recovery. The analysis revealed that there existed a positive correlation between the increase in the weight of MWCNTs and sample weight which in turn led to an improvement in recovery of target analytes. Overall, gradual enhancements in the recovery of analytes were observed as the weights of the MWCNTs and the sample were increased. However, post reaching a threshold level (viz. wt of MWCNTs: 15 mg and wt of sample: 15 g) a further increase in their weights did not significantly enhance analyte recovery.
A regression equation for recovery of target analytes was also derived using a CCD since this equation could aid in prediction of response variables based on values of the factors and their resultant interaction within the experimental range.
The resultant equation obtained was:
| Recovery = 49.2 + 2.58x + 3.59y + 0.055x × x − 0.024y × y − 0.191x × y |
The regression analysis coefficients were observed to offer insights into both the direction and magnitude of the impacts of the factors, including their interactions on the response variables. The results obtained led to the identification of optimal conditions for the efficient and effective extraction of pesticides viz.: a sample weight of 15 g, weight of MWCNTs: 15 mg, weight of MgSO4: 60 mg, and weight of PSA: 20 mg.
QuEChERS in conjunction with MWCNTs and PSA was selected for sample extraction chiefly due to its simplicity, ease of use, and associated benefits.16,17,24 It is important to note here that the associated minimalism played a key role in influencing the results positively. These have been described below in method validation as well as in Table 4.
Method validation
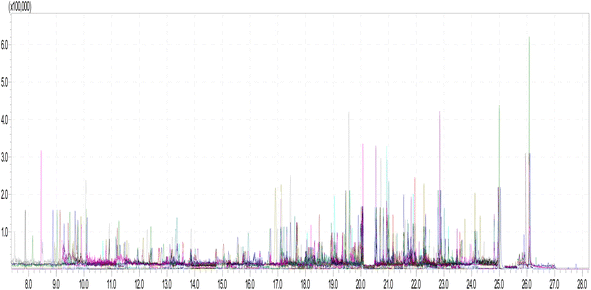
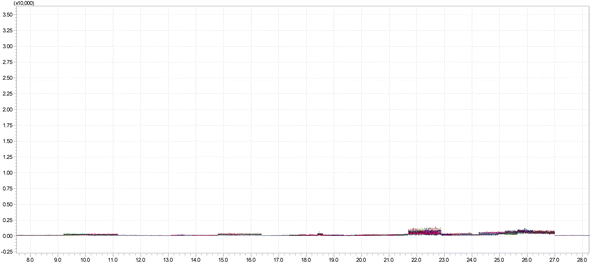
Performance of the developed method was evaluated by validating several parameters, viz. linearity, recovery, precision, accuracy, matrix effect, MLOD and MLOQ. These parameters were assessed in order to determine the efficiency of the developed and validated method for determination of multi-pesticide residue in banana samples. Fig. 5 shows the chromatogram generated for quantitative analysis of 200 pesticide residues in MRM mode whereas the blank chromatogram is presented in Fig. 6. Parameters and validation parameters for all the pesticide residues analysed are summarized in Tables 3 and 4.| Compound name | Chemical class | RTb | Quantitation | Confirmation | ||||
|---|---|---|---|---|---|---|---|---|
| MRM transitionc | CEd | MRM transitionc | CEd | MRM transitionc | CEd | |||
| a OPP: organophosphorus pesticides; OCP: organochlorine pesticides; ONP: organonitrogen pesticides; SPP: synthetic pyrethroid pesticides; HME: herbicide methyl esters. b R.T.: retention time. c MRM: multiple reaction monitoring (m/z). d CE: collision energy. | ||||||||
| (E)-Chlorfenvinphos | OPP | 18.315 | 323.00 > 267.00 | 16 | 267.00 > 159.00 | 18 | 267.00 > 203.00 | 12 |
| (Z)-Chlorfenvinphos | OPP | 19.425 | 323.00 > 267.00 | 16 | 267.00 > 159.00 | 18 | 267.00 > 203.00 | 12 |
| 2,3,5,6-Tetrachloroaniline | ONP | 11.195 | 228.90 > 158.00 | 18 | 230.90 > 158.00 | 22 | 230.90 > 160.00 | 18 |
| 2,4′-Methoxychlor | OCP | 21.940 | 227.10 > 121.10 | 16 | 121.10 > 78.00 | 22 | 121.10 > 91.00 | 12 |
| 2-Phenylphenol | HME | 10.060 | 170.10 > 141.10 | 24 | 141.10 > 115.10 | 18 | 170.10 > 115.10 | 28 |
| 3,4-Dichloroaniline | ONP | 9.270 | 161.00 > 99.00 | 22 | 161.00 > 90.00 | 18 | 161.00 > 126.00 | 14 |
| 4,4′-Dichlorobenzophenone | OCP | 17.445 | 139.00 > 111.00 | 14 | 139.00 > 75.00 | 26 | 249.90 > 139.00 | 16 |
| 4,4′-Methoxychlor olefin | OCP | 21.540 | 238.10 > 223.10 | 12 | 308.00 > 238.10 | 16 | 238.10 > 195.10 | 20 |
| Acequinocyl | HME | 26.000 | 342.20 > 188.10 | 14 | 188.10 > 131.10 | 22 | 188.10 > 160.10 | 8 |
| Acetochlor | ONP | 15.210 | 174.10 > 146.10 | 12 | 223.10 > 132.10 | 22 | 223.10 > 147.10 | 10 |
| Acrinathrin-1 | SPP | 24.085 | 181.10 > 152.10 | 26 | 289.10 > 93.00 | 14 | 181.10 > 127.10 | 28 |
| Alachlor | ONP | 15.580 | 188.10 > 160.10 | 10 | 188.10 > 132.10 | 18 | 160.10 > 132.10 | 10 |
| Aldrin | OCP | 17.975 | 262.90 > 191.00 | 34 | 262.90 > 193.00 | 28 | 292.90 > 219.90 | 26 |
| Allethrin-3,4 | SPP | 18.450 | 123.10 > 81.10 | 10 | 136.10 > 93.10 | 14 | 123.10 > 95.10 | 8 |
| Allidochlor | ONP | 7.875 | 132.10 > 56.00 | 8 | 138.10 > 96.00 | 6 | 132.10 > 49.00 | 24 |
| Alpha-BHC | OCP | 12.130 | 180.90 > 144.90 | 16 | 218.90 > 182.90 | 8 | 218.90 > 144.90 | 20 |
| Alpha-endosulfan | OCP | 14.810 | 194.90 > 160.00 | 8 | 194.90 > 125.00 | 24 | 194.90 > 123.00 | 22 |
| Anthraquinone | SPP | 17.110 | 180.10 > 152.10 | 14 | 208.10 > 180.10 | 10 | 208.10 > 152.10 | 22 |
| Atrazine | ONP | 12.700 | 215.10 > 58.00 | 14 | 215.10 > 173.10 | 6 | 200.10 > 104.10 | 18 |
| Azinphos-ethyl | OPP | 24.295 | 132.10 > 77.00 | 14 | 160.10 > 132.10 | 4 | 160.10 > 77.00 | 18 |
| Azinphos-methyl | OPP | 23.640 | 160.10 > 132.10 | 6 | 160.10 > 77.00 | 20 | 132.10 > 77.00 | 14 |
| Benfluralin | ONP | 11.535 | 292.10 > 264.00 | 8 | 292.10 > 160.00 | 22 | 292.10 > 206.00 | 12 |
| Beta-BHC | OCP | 12.785 | 180.90 > 144.90 | 16 | 218.90 > 182.90 | 8 | 218.90 > 144.90 | 20 |
| Beta-endosulfan | OCP | 20.800 | 194.90 > 160.00 | 8 | 194.90 > 125.00 | 24 | 194.90 > 123.00 | 22 |
| Bifenthrin | SPP | 22.845 | 181.10 > 166.10 | 12 | 181.10 > 179.10 | 12 | 181.10 > 153.10 | 8 |
| Biphenyl | ONP | 8.870 | 154.10 > 128.10 | 22 | 154.10 > 115.10 | 24 | 152.10 > 126.10 | 24 |
| Bromfenvinfos-methyl | OPP | 18.340 | 294.90 > 109.00 | 16 | 296.90 > 109.00 | 16 | 294.90 > 266.90 | 6 |
| Bromfenvinphos | OPP | 19.425 | 266.90 > 159.00 | 14 | 268.90 > 161.00 | 16 | 322.90 > 266.90 | 12 |
| Bromophos | OPP | 17.655 | 330.90 > 315.90 | 14 | 328.90 > 313.90 | 18 | 330.90 > 285.90 | 28 |
| Bromophos-ethyl | OPP | 18.910 | 358.90 > 302.90 | 16 | 302.90 > 284.90 | 18 | 358.90 > 330.90 | 10 |
| Bromopropylate | HME | 22.895 | 340.90 > 182.90 | 18 | 340.90 > 184.90 | 20 | 340.90 > 157.00 | 30 |
| Bupirimate | ONP | 20.090 | 273.10 > 108.10 | 16 | 273.10 > 193.10 | 8 | 316.10 > 208.10 | 10 |
| Captafol | ONP | 22.300 | 79.00 > 77.00 | 14 | 79.00 > 51.00 | 20 | 183.10 > 79.00 | 18 |
| Captan | ONP | 18.520 | 149.10 > 79.10 | 14 | 149.10 > 105.10 | 4 | 149.10 > 70.00 | 18 |
| Carbophenothion | OPP | 21.525 | 157.00 > 45.00 | 18 | 341.90 > 157.00 | 14 | 341.90 > 199.00 | 8 |
| Carfentrazone-ethyl | HME | 21.455 | 340.10 > 312.10 | 14 | 312.10 > 151.10 | 24 | 340.10 > 151.10 | 28 |
| Chlorbenside | OCP | 18.930 | 125.00 > 89.00 | 16 | 125.00 > 99.00 | 18 | 127.00 > 89.00 | 18 |
| Chlorfenapyr | ONP | 20.325 | 247.10 > 227.00 | 16 | 139.00 > 102.00 | 12 | 247.10 > 200.00 | 24 |
| Chlorfenson | OCP | 19.580 | 175.00 > 111.00 | 12 | 175.00 > 75.00 | 28 | 301.90 > 175.00 | 8 |
| Chlorobenzilate | HME | 20.712 | 139.00 > 111.00 | 16 | 251.00 > 139.00 | 14 | 139.00 > 75.00 | 26 |
| Chloroneb | OCP | 9.885 | 206.00 > 191.00 | 12 | 206.00 > 141.00 | 20 | 193.00 > 113.00 | 18 |
| Chlorothalonil | ONP | 13.625 | 263.90 > 168.00 | 24 | 263.90 > 228.80 | 18 | 265.90 > 168.00 | 22 |
| Chlorpropham | HME | 11.430 | 127.10 > 65.00 | 22 | 213.10 > 171.10 | 6 | 127.10 > 92.00 | 18 |
| Chlorpyrifos | OPP | 16.975 | 196.90 > 168.90 | 14 | 313.90 > 257.90 | 14 | 313.90 > 285.90 | 8 |
| Chlorpyrifos-methyl | OPP | 15.265 | 285.90 > 93.00 | 22 | 287.90 > 93.00 | 22 | 285.90 > 270.90 | 14 |
| Chlorthal-dimethyl | HME | 17.145 | 298.90 > 220.90 | 24 | 300.90 > 222.90 | 26 | 300.90 > 272.90 | 14 |
| Chlorthiophos | OPP | 20.300 | 256.90 > 239.00 | 14 | 256.90 > 193.00 | 22 | 256.90 > 165.00 | 26 |
| Chlozolinate | HME | 18.245 | 330.90 > 258.90 | 6 | 258.90 > 188.00 | 14 | 330.90 > 186.00 | 20 |
| cis-Chlordane | OCP | 19.325 | 374.80 > 265.90 | 26 | 372.80 > 263.90 | 28 | 372.80 > 265.90 | 22 |
| cis-Nonachlor | OCP | 20.940 | 406.80 > 299.90 | 24 | 406.80 > 109.00 | 22 | 406.80 > 334.90 | 16 |
| Clomazone | ONP | 12.860 | 204.10 > 107.00 | 20 | 204.10 > 78.00 | 26 | 204.10 > 68.00 | 24 |
| Coumaphos | OPP | 24.950 | 362.00 > 109.00 | 16 | 362.0 > 109.0 | 14 | 362.00 > 226.00 | 14 |
| Cycloate | ONP | 11.255 | 154.20 > 83.10 | 8 | 154.20 > 55.00 | 18 | 154.20 > 72.00 | 6 |
| Cyfluthrin | SPP | 25.355 | 163.10 > 127.10 | 6 | 163.10 > 91.00 | 14 | 226.10 > 206.10 | 14 |
| Cypermethrin | SPP | 25.715 | 163.10 > 127.10 | 6 | 163.10 > 91.00 | 14 | 181.10 > 152.10 | 22 |
| Cyprodinil | ONP | 18.035 | 224.10 > 208.10 | 16 | 224.10 > 197.10 | 22 | 224.10 > 131.10 | 14 |
| Delta-BHC | OCP | 14.003 | 180.90 > 144.90 | 16 | 218.90 > 182.90 | 8 | 218.90 > 144.90 | 20 |
| Deltamethrin | SPP | 27.760 | 180.90 > 151.90 | 22 | 252.90 > 93.00 | 20 | 252.90 > 171.90 | 8 |
| Di-allate | ONP | 11.905 | 234.10 > 150.00 | 20 | 234.10 > 192.10 | 14 | 128.00 > 86.00 | 4 |
| Diazinon | OPP | 13.355 | 304.10 > 179.10 | 10 | 179.10 > 137.10 | 18 | 179.10 > 122.10 | 24 |
| Dichlofluanid | ONP | 16.660 | 223.90 > 123.10 | 8 | 167.10 > 124.10 | 10 | 167.10 > 97.00 | 22 |
| Dichlobenil | ONP | 8.445 | 171.00 > 136.00 | 13 | 171.00 > 100.00 | 25 | 136.00 > 100.00 | 10 |
| Dicloran | ONP | 12.460 | 206.00 > 176.00 | 10 | 176.00 > 148.00 | 12 | 206.00 > 124.00 | 24 |
| Dieldrin | OCP | 20.015 | 276.90 > 241.00 | 8 | 262.90 > 193.00 | 34 | 262.90 > 228.00 | 24 |
| Dimethachlor | ONP | 15.015 | 197.10 > 148.10 | 10 | 197.10 > 120.10 | 22 | 199.10 > 148.10 | 10 |
| Diphenamid | ONP | 17.675 | 167.10 > 152.10 | 20 | 239.10 > 167.10 | 8 | 239.10 > 72.00 | 16 |
| Diphenylamine | ONP | 11.141 | 169.10 > 66.00 | 24 | 167.10 > 139.10 | 28 | 169.10 > 77.00 | 28 |
| Disulfoton | OPP | 13.790 | 153.00 > 97.00 | 10 | 153.00 > 125.00 | 6 | 186.00 > 153.00 | 6 |
| Edifenphos | OPP | 21.595 | 173.00 > 109.00 | 10 | 310.00 > 173.00 | 14 | 310.00 > 109.00 | 26 |
| Endosulfan ether | OCP | 14.820 | 240.90 > 205.90 | 16 | 238.90 > 203.90 | 16 | 240.90 > 203.90 | 18 |
| Endosulfan sulfate | OCP | 21.705 | 271.80 > 236.90 | 18 | 386.80 > 252.90 | 16 | 386.80 > 288.80 | 10 |
| Endrin | OCP | 20.565 | 262.90 > 191.00 | 30 | 262.90 > 193.00 | 28 | 244.90 > 173.00 | 32 |
| Endrin aldehyde | OCP | 21.150 | 249.80 > 214.90 | 26 | 344.90 > 244.90 | 16 | 344.90 > 242.90 | 14 |
| Endrin ketone | OCP | 22.760 | 316.90 > 244.90 | 20 | 314.90 > 242.90 | 18 | 316.90 > 101.00 | 16 |
| EPN | OPP | 22.860 | 169.10 > 140.90 | 8 | 156.90 > 77.00 | 24 | 169.10 > 77.00 | 22 |
| Ethalfluralin | ONP | 11.293 | 276.00 > 202.00 | 18 | 316.10 > 276.00 | 10 | 276.00 > 248.00 | 10 |
| Ethion | OPP | 20.910 | 153.00 > 97.00 | 14 | 230.90 > 129.00 | 24 | 153.00 > 125.00 | 6 |
| Ethylan | OCP | 20.545 | 223.00 > 167.00 | 12 | 223.00 > 179.00 | 22 | 223.00 > 193.00 | 28 |
| Etofenprox | ONP | 26.050 | 163.10 > 135.10 | 10 | 163.10 > 107.10 | 18 | 135.10 > 107.10 | 10 |
| Etridiazole | ONP | 9.420 | 210.90 > 182.90 | 10 | 182.90 > 139.90 | 18 | 210.90 > 139.90 | 22 |
| Fenamiphos | OPP | 19.455 | 303.10 > 195.10 | 8 | 288.10 > 260.10 | 6 | 303.10 > 154.10 | 18 |
| Fenarimol | ONP | 24.180 | 251.00 > 139.00 | 14 | 330.00 > 139.00 | 8 | 251.00 > 111.00 | 26 |
| Fenchlorphos | OPP | 15.930 | 284.90 > 269.90 | 16 | 286.90 > 271.90 | 18 | 284.90 > 239.90 | 26 |
| Fenitrothion | OPP | 16.425 | 277.00 > 260.00 | 6 | 277.00 > 109.10 | 14 | 260.00 > 125.10 | 12 |
| Fenpropathrin | ONP | 23.045 | 181.10 > 152.10 | 22 | 265.10 > 210.10 | 12 | 181.10 > 127.10 | 28 |
| Fenson | OCP | 17.600 | 141.00 > 77.00 | 16 | 267.90 > 141.00 | 6 | 267.90 > 77.00 | 20 |
| Fenthion | OPP | 17.100 | 278.00 > 109.00 | 20 | 278.00 > 169.00 | 14 | 278.00 > 125.00 | 20 |
| Fenvalerate | SPP | 26.755 | 225.10 > 119.10 | 20 | 225.10 > 147.10 | 10 | 419.10 > 225.10 | 6 |
| Fipronil | ONP | 18.150 | 366.90 > 212.90 | 30 | 368.90 > 214.90 | 30 | 366.90 > 254.90 | 22 |
| Fluazifop-P-butyl | HME | 20.515 | 282.10 > 91.00 | 18 | 282.10 > 238.10 | 18 | 383.10 > 282.10 | 14 |
| Fluchloralin | ONP | 13.425 | 306.00 > 264.00 | 8 | 326.00 > 63.00 | 16 | 328.00 > 65.00 | 16 |
| Flucythrinate | SPP | 25.885 | 199.10 > 157.10 | 10 | 157.10 > 107.10 | 12 | 199.10 > 107.10 | 22 |
| Fludioxonil | ONP | 19.625 | 248.00 > 127.00 | 26 | 248.00 > 154.00 | 20 | 182.00 > 127.00 | 16 |
| Fluquinconazole | ONP | 24.980 | 340.00 > 298.00 | 20 | 340.00 > 313.00 | 14 | 342.00 > 300.00 | 22 |
| Fluridone | ONP | 26.310 | 328.10 > 259.00 | 24 | 328.10 > 313.00 | 22 | 328.10 > 127.00 | 24 |
| Flusilazole | ONP | 20.040 | 233.10 > 165.10 | 14 | 206.10 > 151.10 | 16 | 233.10 > 152.10 | 14 |
| Flutolanil | ONP | 19.545 | 173.00 > 145.00 | 14 | 173.00 > 95.00 | 26 | 281.10 > 173.00 | 12 |
| Flutriafol | ONP | 19.385 | 219.10 > 123.10 | 14 | 219.10 > 95.00 | 28 | 164.10 > 95.00 | 28 |
| Folpet | ONP | 18.690 | 259.90 > 130.00 | 14 | 261.90 > 130.00 | 18 | 261.90 > 233.90 | 10 |
| Fonofos | OPP | 13.335 | 137.10 > 109.10 | 8 | 246.00 > 137.10 | 6 | 246.00 > 109.10 | 18 |
| Gamma-BHC | OCP | 13.115 | 180.90 > 144.90 | 16 | 218.90 > 182.90 | 8 | 218.90 > 144.90 | 20 |
| Heptachlor | OCP | 15.870 | 271.80 > 236.90 | 20 | 273.80 > 238.90 | 16 | 271.80 > 117.00 | 32 |
| Heptachlor-exo-epoxide | OCP | 18.295 | 352.80 > 262.90 | 14 | 354.80 > 264.90 | 20 | 352.80 > 316.90 | 10 |
| Hexachlorobenzene | OCP | 12.275 | 283.80 > 248.80 | 24 | 283.80 > 213.80 | 28 | 285.80 > 250.80 | 22 |
| Hexazinone | ONP | 21.880 | 171.10 > 71.00 | 16 | 171.10 > 85.00 | 16 | 128.10 > 83.00 | 10 |
| Iodofenphos | OPP | 19.585 | 376.90 > 361.80 | 22 | 376.90 > 331.80 | 32 | 378.90 > 363.80 | 24 |
| Iprodione | ONP | 22.660 | 314.00 > 245.00 | 12 | 314.00 > 56.00 | 22 | 316.00 > 247.00 | 12 |
| Isazofos | OPP | 13.815 | 257.00 > 162.00 | 8 | 257.00 > 119.00 | 18 | 285.00 > 161.00 | 12 |
| Isodrin | OCP | 17.990 | 192.90 > 157.00 | 20 | 192.90 > 123.00 | 26 | 262.90 > 192.90 | 28 |
| Isopropalin | ONP | 17.840 | 280.10 > 238.10 | 8 | 280.10 > 133.10 | 18 | 280.10 > 165.10 | 16 |
| Lambda-cyhalothrin | SPP | 23.925 | 181.10 > 152.10 | 24 | 163.10 > 91.00 | 22 | 163.10 > 127.00 | 14 |
| Lenacil | ONP | 21.670 | 153.10 > 136.10 | 14 | 153.10 > 82.10 | 16 | 153.10 > 110.10 | 16 |
| Leptophos | OPP | 23.605 | 376.90 > 361.90 | 24 | 374.90 > 359.90 | 24 | 376.90 > 268.90 | 36 |
| Linuron | ONP | 16.620 | 248.00 > 61.00 | 16 | 248.0 > 61.0 | 16 | 250.00 > 61.00 | 16 |
| Malathion | OPP | 16.730 | 173.10 > 99.00 | 14 | 173.10 > 127.00 | 6 | 158.10 > 125.00 | 10 |
| Metalaxyl | HME | 15.810 | 249.20 > 190.10 | 8 | 206.10 > 132.10 | 20 | 249.20 > 146.10 | 22 |
| Metazachlor | ONP | 18.065 | 209.10 > 132.10 | 18 | 133.10 > 117.10 | 24 | 211.10 > 132.10 | 20 |
| Methacrifos | OPP | 9.770 | 208.00 > 180.00 | 8 | 240.00 > 208.00 | 4 | 208.00 > 110.00 | 18 |
| Methoxychlor | ONP | 23.000 | 227.10 > 169.10 | 24 | 227.10 > 212.10 | 14 | 227.10 > 141.10 | 28 |
| Metolachlor | ONP | 16.905 | 162.10 > 133.10 | 16 | 238.10 > 162.10 | 12 | 238.10 > 133.10 | 26 |
| Mevinphos | OPP | 9.125 | 127.00 > 109.00 | 12 | 192.00 > 127.00 | 12 | 127.00 > 95.00 | 18 |
| MGK 264 | ONP | 17.735 | 164.10 > 93.00 | 10 | 111.10 > 82.00 | 8 | 164.10 > 80.00 | 24 |
| Mirex | OCP | 24.095 | 271.80 > 236.80 | 18 | 273.80 > 238.80 | 18 | 271.80 > 234.80 | 18 |
| Myclobutanil | ONP | 19.965 | 179.10 > 125.00 | 14 | 179.10 > 152.00 | 8 | 150.00 > 123.00 | 18 |
| N-(2,4-Dimethylphenyl)formamide | ONP | 9.667 | 149.10 > 121.10 | 6 | 149.10 > 106.10 | 16 | 120.10 > 77.00 | 18 |
| Nitralin | ONP | 22.295 | 316.10 > 274.00 | 8 | 274.00 > 169.00 | 12 | 274.00 > 216.00 | 8 |
| Nitrofen | ONP | 20.470 | 202.00 > 139.00 | 24 | 282.90 > 253.00 | 12 | 282.90 > 162.00 | 24 |
| Norflurazon | ONP | 21.555 | 145.00 > 95.00 | 18 | 303.00 > 145.00 | 22 | 145.00 > 75.00 | 28 |
| o,p′-DDD | OCP | 20.055 | 235.00 > 165.00 | 24 | 237.00 > 165.00 | 28 | 235.00 > 199.00 | 16 |
| o,p′-DDE | OCP | 19.035 | 246.00 > 176.00 | 30 | 248.00 > 176.00 | 28 | 246.00 > 211.00 | 22 |
| o,p′-DDT | OCP | 21.010 | 235.00 > 165.00 | 24 | 237.00 > 165.00 | 28 | 235.00 > 199.00 | 16 |
| Oxadiazon | ONP | 19.902 | 258.00 > 175.00 | 8 | 302.00 > 175.00 | 14 | 258.00 > 112.00 | 28 |
| Oxyfluorfen | ONP | 20.100 | 252.00 > 196.00 | 22 | 361.00 > 300.00 | 14 | 361.00 > 317.00 | 6 |
| p,p′-DDD | OCP | 20.935 | 235.00 > 165.00 | 24 | 237.00 > 165.00 | 28 | 235.00 > 199.00 | 16 |
| p,p′-DDE | OCP | 19.900 | 246.00 > 176.00 | 30 | 317.90 > 248.00 | 24 | 246.00 > 211.00 | 22 |
| p,p′-DDT | OCP | 21.805 | 235.00 > 165.00 | 24 | 237.00 > 165.00 | 28 | 235.00 > 199.00 | 16 |
| Parathion | OPP | 17.210 | 139.00 > 109.00 | 8 | 291.10 > 109.00 | 14 | 291.10 > 137.00 | 6 |
| Parathion-methyl | OPP | 15.480 | 263.00 > 109.00 | 14 | 125.00 > 47.00 | 12 | 125.00 > 79.00 | 8 |
| Pebulate | ONP | 9.485 | 128.10 > 57.00 | 6 | 128.10 > 72.00 | 4 | 161.10 > 128.10 | 6 |
| Penconazole | ONP | 18.185 | 248.10 > 157.10 | 26 | 159.10 > 123.10 | 22 | 248.10 > 192.10 | 14 |
| Pendimethalin | ONP | 18.015 | 252.10 > 162.10 | 10 | 252.10 > 191.10 | 8 | 252.10 > 208.10 | 6 |
| Pentachloroaniline | ONP | 14.750 | 262.90 > 191.90 | 22 | 264.90 > 193.90 | 18 | 264.90 > 191.90 | 18 |
| Pentachloroanisole | OCP | 12.410 | 264.80 > 236.80 | 16 | 279.90 > 236.80 | 26 | 279.90 > 264.80 | 12 |
| Pentachlorobenzene | OCP | 10.107 | 249.90 > 214.90 | 18 | 249.90 > 178.90 | 28 | 249.90 > 176.90 | 26 |
| Pentachlorobenzonitrile | ONP | 13.090 | 274.80 > 239.80 | 18 | 272.80 > 202.90 | 30 | 274.80 > 204.90 | 32 |
| Permethrine | SPP | 24.805 | 183.10 > 153.10 | 14 | 163.10 > 127.10 | 6 | 183.10 > 168.10 | 14 |
| Phenothrin | SPP | 23.340 | 123.10 > 81.00 | 8 | 183.10 > 153.10 | 14 | 183.10 > 168.10 | 14 |
| Phorate | OPP | 11.935 | 260.00 > 75.00 | 8 | 231.00 > 129.00 | 24 | 231.00 > 175.00 | 12 |
| Phosalone | OPP | 23.565 | 182.00 > 111.00 | 14 | 182.00 > 138.00 | 8 | 182.00 > 102.00 | 14 |
| Phosmet | OPP | 22.785 | 160.00 > 77.00 | 24 | 160.00 > 133.00 | 14 | 160.00 > 105.00 | 18 |
| Piperonyl butoxide | OPP | 22.275 | 176.10 > 131.10 | 12 | 176.10 > 117.10 | 20 | 176.10 > 103.10 | 24 |
| Pirimiphos ethyl | OPP | 17.995 | 304.10 > 168.10 | 12 | 318.10 > 166.10 | 12 | 318.10 > 182.10 | 12 |
| Pirimiphos-methyl | OPP | 16.340 | 290.10 > 125.00 | 22 | 290.10 > 233.10 | 12 | 305.10 > 180.10 | 8 |
| Pretilachlor | ONP | 19.720 | 262.10 > 202.10 | 10 | 238.10 > 162.10 | 10 | 238.10 > 146.10 | 10 |
| Prochloraz | ONP | 25.030 | 180.10 > 138.10 | 12 | 180.10 > 69.00 | 20 | 180.10 > 95.00 | 20 |
| Procymidone | ONP | 18.615 | 283.00 > 96.00 | 10 | 285.00 > 96.00 | 10 | 283.00 > 68.00 | 24 |
| Prodiamine | ONP | 16.450 | 321.10 > 279.10 | 6 | 321.10 > 203.10 | 10 | 321.10 > 205.10 | 14 |
| Profenofos | OPP | 19.775 | 338.90 > 268.90 | 18 | 336.90 > 266.90 | 14 | 338.90 > 310.90 | 6 |
| Profluralin | ONP | 13.015 | 318.10 > 199.10 | 16 | 318.10 > 55.00 | 22 | 330.10 > 69.00 | 20 |
| Propachlor | ONP | 10.905 | 120.00 > 77.00 | 20 | 176.10 > 57.00 | 8 | 120.00 > 92.00 | 8 |
| Propanil | ONP | 15.065 | 217.00 > 161.00 | 10 | 160.90 > 99.00 | 24 | 160.90 > 90.00 | 22 |
| Propargite | ONP | 22.130 | 135.10 > 107.10 | 16 | 135.10 > 77.00 | 24 | 135.10 > 95.00 | 14 |
| Propisochlor | ONP | 15.735 | 162.10 > 120.10 | 14 | 162.10 > 147.10 | 14 | 162.10 > 144.10 | 12 |
| Propyzamide | ONP | 13.300 | 172.90 > 144.90 | 16 | 172.90 > 109.00 | 26 | 172.90 > 74.00 | 28 |
| Prothiofos | OPP | 19.675 | 266.90 > 238.90 | 10 | 309.00 > 238.90 | 14 | 266.90 > 220.90 | 20 |
| Pyraclofos | OPP | 24.440 | 194.00 > 138.00 | 22 | 360.10 > 194.00 | 14 | 360.10 > 139.00 | 14 |
| Pyrazophos | OPP | 24.125 | 221.10 > 193.10 | 12 | 221.10 > 149.10 | 14 | 221.10 > 177.10 | 16 |
| Pyridaben | ONP | 25.005 | 147.10 > 117.10 | 22 | 147.10 > 132.10 | 14 | 147.10 > 119.10 | 10 |
| Pyridaphenthion | OPP | 22.625 | 340.00 > 199.10 | 8 | 199.10 > 92.00 | 16 | 199.10 > 77.00 | 24 |
| Pyrimethanil | ONP | 13.555 | 198.10 > 183.10 | 14 | 198.10 > 118.10 | 28 | 198.10 > 158.10 | 18 |
| Pyriproxyfen | ONP | 23.745 | 136.10 > 78.00 | 20 | 136.10 > 96.00 | 14 | 226.10 > 186.10 | 14 |
| Quinalphos | OPP | 18.490 | 146.10 > 118.00 | 10 | 146.10 > 91.00 | 24 | 157.10 > 129.00 | 14 |
| Quintozene | ONP | 12.980 | 264.80 > 236.80 | 10 | 294.80 > 236.80 | 16 | 294.80 > 264.80 | 12 |
| Resmethrin | SPP | 22.195 | 143.10 > 128.10 | 10 | 171.10 > 143.10 | 6 | 171.10 > 128.10 | 12 |
| Sulfotep | OPP | 11.605 | 322.00 > 202.00 | 10 | 322.00 > 174.00 | 18 | 322.00 > 294.00 | 4 |
| Sulprofos | OPP | 21.300 | 156.00 > 141.00 | 18 | 322.00 > 156.00 | 8 | 156.00 > 108.00 | 28 |
| Tau-fluvalinate | SPP | 26.890 | 250.10 > 55.00 | 18 | 250.1 > 55.0 | 16 | 250.10 > 200.10 | 16 |
| Tebuconazole | ONP | 22.095 | 250.10 > 125.10 | 22 | 125.10 > 89.00 | 18 | 250.10 > 153.10 | 12 |
| Tebufenpyrad | ONP | 23.155 | 333.10 > 171.10 | 20 | 333.10 > 276.10 | 8 | 318.10 > 131.10 | 18 |
| Tecnazene | ONP | 10.790 | 260.90 > 202.90 | 14 | 202.90 > 142.90 | 22 | 202.90 > 85.00 | 24 |
| Tefluthrin | SPP | 13.855 | 177.00 > 127.10 | 16 | 177.00 > 137.10 | 16 | 197.00 > 141.10 | 14 |
| Terbacil | ONP | 13.740 | 161.00 > 144.00 | 14 | 161.00 > 88.00 | 20 | 117.00 > 76.00 | 8 |
| Terbufos | OPP | 13.175 | 231.00 > 128.90 | 26 | 231.00 > 174.90 | 14 | 231.00 > 202.90 | 8 |
| Terbuthylazine | ONP | 13.165 | 229.10 > 173.10 | 6 | 214.10 > 71.00 | 16 | 214.10 > 132.10 | 8 |
| Tetradifon | OCP | 23.455 | 226.90 > 199.00 | 16 | 355.90 > 159.00 | 18 | 355.90 > 228.90 | 12 |
| Tetramethrin | SPP | 22.715 | 164.10 > 107.10 | 14 | 164.10 > 77.00 | 22 | 164.10 > 135.10 | 8 |
| THPI (tetrahydrophthalimide) | ONP | 9.755 | 151.10 > 79.00 | 18 | 151.10 > 123.10 | 4 | 151.10 > 77.00 | 28 |
| Tolclofos-methyl | OPP | 15.550 | 264.90 > 249.90 | 14 | 264.90 > 93.00 | 24 | 264.90 > 219.90 | 22 |
| Tolylfluanid | ONP | 18.280 | 238.00 > 137.10 | 14 | 181.10 > 138.10 | 10 | 181.10 > 94.10 | 18 |
| trans-Chlordane | OCP | 18.960 | 374.80 > 265.90 | 26 | 372.80 > 263.90 | 28 | 372.80 > 265.90 | 22 |
| Transfluthrin | SPP | 15.670 | 163.10 > 127.10 | 6 | 163.10 > 143.10 | 16 | 163.10 > 91.00 | 12 |
| trans-Nonachlor | OCP | 19.410 | 406.80 > 299.90 | 24 | 406.80 > 334.90 | 16 | 406.80 > 109.00 | 22 |
| Triadimefon | ONP | 17.315 | 208.10 > 181.00 | 10 | 208.10 > 111.00 | 22 | 208.10 > 127.00 | 14 |
| Triadimenol | ONP | 18.580 | 168.10 > 70.00 | 10 | 128.10 > 65.00 | 22 | 128.10 > 100.10 | 14 |
| Tri-allate | ONP | 14.100 | 268.10 > 184.00 | 20 | 270.10 > 186.00 | 20 | 268.10 > 226.00 | 14 |
| Triazophos | OPP | 21.255 | 161.00 > 134.00 | 8 | 161.00 > 106.00 | 14 | 257.00 > 162.00 | 8 |
| Tricyclazole | ONP | 19.705 | 189.00 > 161.90 | 12 | 189.00 > 135.00 | 18 | 161.90 > 135.00 | 8 |
| Triflumizole | ONP | 18.672 | 206.10 > 179.10 | 14 | 278.10 > 73.00 | 6 | 206.10 > 186.10 | 8 |
| Trifluralin | ONP | 11.470 | 306.10 > 264.10 | 8 | 264.10 > 160.10 | 18 | 264.10 > 206.10 | 8 |
| Vinclozolin | ONP | 15.410 | 212.00 > 172.00 | 16 | 285.00 > 212.00 | 12 | 212.00 > 145.00 | 24 |
| Compound name | r 2 | Precisionb | Spiking levelc (μg kg−1) | MLODd | MLOQe | % MEf | ||
|---|---|---|---|---|---|---|---|---|
| CV(r) | CV(R) | 10 | 25 | |||||
| a r 2: linearity. b Precision: CV(R): between-day precision (n = 7), CV(r): within-day precision (n = 7). c % recovery (n = 7) calculated from spiking concentration. d MLOD: method limit of detection (μg kg−1). e MLOQ: method limit of quantification (μg kg−1). f % ME: matrix effect (%). | ||||||||
| (E)-Chlorfenvinphos | 0.999 | 3 | 1 | 100 | 109 | 1 | 2 | 13.9 |
| (Z)-Chlorfenvinphos | 0.999 | 4 | 5 | 96 | 112 | 2 | 7 | 16.5 |
| 2,3,5,6-Tetrachloroaniline | 0.999 | 2 | 2 | 99 | 112 | 1 | 3 | 11.1 |
| 2,4′-Methoxychlor | 0.997 | 2 | 2 | 90 | 101 | 1 | 2 | 12.0 |
| 2-Phenylphenol | 0.999 | 2 | 6 | 211 | 221 | 13 | 42 | 128.2 |
| 3,4-Dichloroaniline | 0.999 | 4 | 8 | 101 | 116 | 1 | 5 | 19.7 |
| 4,4′-Dichlorobenzophenone | 0.998 | 3 | 2 | 97 | 113 | 1 | 3 | 18.0 |
| 4,4′-Methoxychlor olefin | 0.999 | 3 | 3 | 100 | 113 | 1 | 3 | 20.1 |
| Acequinocyl | 0.995 | 12 | 22 | 59 | 53 | 2 | 6 | −43.7 |
| Acetochlor | 0.999 | 2 | 11 | 107 | 114 | 3 | 8 | 21.5 |
| Acrinathrin-1 | 0.994 | 4 | 6 | 88 | 102 | 3 | 9 | 20.2 |
| Alachlor | 0.999 | 3 | 16 | 91 | 109 | 1 | 4 | 15.1 |
| Aldrin | 0.991 | 4 | 6 | 96 | 110 | 2 | 8 | 27.0 |
| Allethrin-3,4 | 0.999 | 10 | 31 | 107 | 88 | 3 | 9 | −8.6 |
| Allidochlor | 0.999 | 2 | 1 | 102 | 116 | 1 | 4 | 15.5 |
| Alpha-BHC | 0.999 | 1 | 4 | 92 | 109 | 1 | 3 | 8.1 |
| Alpha-endosulfan | 0.997 | 15 | 19 | 94 | 99 | 3 | 9 | −9.8 |
| Anthraquinone | 0.998 | 2 | 3 | 105 | 111 | 1 | 3 | 20.6 |
| Atrazine | 0.997 | 4 | 5 | 99 | 116 | 3 | 9 | 13.9 |
| Azinphos-ethyl | 0.996 | 12 | 2 | 104 | 114 | 2 | 6 | 34.7 |
| Azinphos-methyl | 0.994 | 7 | 4 | 97 | 97 | 2 | 6 | 22.2 |
| Benfluralin | 0.998 | 2 | 1 | 97 | 111 | 1 | 4 | 20.4 |
| Beta-BHC | 0.999 | 4 | 3 | 103 | 112 | 1 | 4 | 12.5 |
| Beta-endosulfan | 0.998 | 8 | 7 | 102 | 107 | 3 | 9 | 6.8 |
| Bifenthrin | 0.999 | 3 | 1 | 100 | 113 | 1 | 2 | 19.5 |
| Biphenyl | 0.999 | 3 | 1 | 698 | 320 | 6 | 20 | 212.0 |
| Bromfenvinfos-methyl | 0.997 | 3 | 5 | 87 | 106 | 2 | 6 | 16.5 |
| Bromfenvinphos | 0.999 | 3 | 1 | 89 | 106 | 1 | 4 | 8.1 |
| Bromophos | 0.998 | 2 | 3 | 108 | 113 | 1 | 3 | 18.0 |
| Bromophos-ethyl | 0.998 | 4 | 5 | 96 | 113 | 2 | 5 | 19.8 |
| Bromopropylate | 0.997 | 3 | 3 | 110 | 116 | 1 | 4 | 24.3 |
| Bupirimate | 0.999 | 5 | 6 | 99 | 111 | 2 | 6 | 14.1 |
| Captafol | 0.993 | 70 | 47 | 328 | 273 | 18 | 58 | 125.3 |
| Captan | 0.992 | 21 | 36 | ND | 99 | 15 | 46 | 16.0 |
| Carbophenothion | 0.998 | 3 | 3 | 101 | 111 | 1 | 5 | 23.3 |
| Carfentrazone-ethyl | 0.999 | 2 | 4 | 103 | 112 | 1 | 5 | 16.2 |
| Chlorbenside | 0.998 | 4 | 2 | 106 | 106 | 1 | 3 | 9.2 |
| Chlorfenapyr | 0.996 | 13 | 14 | 91 | 103 | 3 | 9 | 12.7 |
| Chlorfenson | 0.999 | 2 | 3 | 101 | 113 | 1 | 2 | 18.2 |
| Chlorobenzilate | 0.997 | 3 | 3 | 103 | 115 | 1 | 2 | 20.0 |
| Chloroneb | 0.997 | 3 | 1 | 1295 | 490 | 19 | 62 | 371.4 |
| Chlorothalonil | 0.995 | 4 | 8 | 53 | 72 | 1 | 3 | −24.3 |
| Chlorpropham | 0.999 | 2 | 1 | 108 | 114 | 1 | 4 | 16.7 |
| Chlorpyrifos | 0.999 | 3 | 5 | 108 | 109 | 2 | 5 | 9.6 |
| Chlorpyrifos-methyl | 0.999 | 4 | 2 | 98 | 113 | 2 | 5 | 19.4 |
| Chlorthal-dimethyl | 0.998 | 1 | 3 | 93 | 112 | 1 | 3 | 15.3 |
| Chlorthiophos | 0.996 | 4 | 16 | 105 | 110 | 2 | 7 | 16.0 |
| Chlozolinate | 0.995 | 7 | 3 | 112 | 178 | 2 | 7 | 80.8 |
| cis-Chlordane | 0.994 | 10 | 3 | 90 | 106 | 2 | 5 | 6.3 |
| cis-Nonachlor | 0.999 | 4 | 5 | 98 | 111 | 2 | 5 | 16.8 |
| Clomazone | 0.999 | 2 | 4 | 92 | 110 | 1 | 2 | 12.2 |
| Coumaphos | 0.996 | 4 | 3 | 112 | 107 | 1 | 4 | 20.3 |
| Cycloate | 0.999 | 1 | 2 | 100 | 113 | 1 | 3 | 14.3 |
| Cyfluthrin | 0.998 | 16 | 11 | 100 | 101 | 2 | 8 | 19.7 |
| Cypermethrin | 0.997 | 16 | 21 | 100 | 98 | 2 | 7 | 15.8 |
| Cyprodinil | 0.999 | 3 | 2 | 105 | 114 | 1 | 4 | 20.3 |
| Delta-BHC | 0.998 | 2 | 6 | 98 | 111 | 1 | 4 | 11.0 |
| Deltamethrin | 0.994 | 2 | 4 | 88 | 109 | 2 | 7 | 26.8 |
| Di-allate | 0.999 | 3 | 3 | 101 | 111 | 1 | 3 | 9.4 |
| Diazinon | 0.995 | 5 | 5 | 111 | 120 | 2 | 8 | 38.6 |
| Dichlofluanid | 0.999 | 4 | 9 | 72 | 95 | 2 | 6 | −3.0 |
| Dichlobenil | 0.999 | 3 | 3 | 381 | 179 | 5 | 15 | 77.2 |
| Dicloran | 0.995 | 3 | 2 | 99 | 111 | 1 | 4 | 3.0 |
| Dieldrin | 0.999 | 6 | 7 | 114 | 110 | 2 | 7 | 10.1 |
| Dimethachlor | 0.997 | 2 | 3 | 97 | 111 | 1 | 2 | 13.0 |
| Diphenamid | 0.998 | 3 | 5 | 102 | 112 | 2 | 7 | 15.6 |
| Diphenylamine | 0.999 | 2 | 2 | 108 | 110 | 1 | 3 | 15.6 |
| Disulfoton | 0.998 | 11 | 25 | 80 | 93 | 3 | 8 | −5.7 |
| Edifenphos | 0.996 | 2 | 1 | 86 | 96 | 1 | 4 | 9.1 |
| Endosulfan ether | 0.997 | 2 | 2 | 100 | 119 | 2 | 6 | 22.7 |
| Endosulfan sulfate | 0.995 | 3 | 3 | 93 | 106 | 1 | 4 | 20.4 |
| Endrin | 0.993 | 2 | 9 | 85 | 108 | 2 | 7 | 10.2 |
| Endrin aldehyde | 0.995 | 15 | 53 | 191 | 98 | 38 | 121 | 7.1 |
| Endrin ketone | 0.992 | 10 | 9 | 98 | 106 | 2 | 6 | 22.4 |
| EPN | 0.996 | 5 | 5 | 108 | 108 | 3 | 9 | 21.3 |
| Ethalfluralin | 0.998 | 2 | 4 | 99 | 109 | 1 | 4 | 15.8 |
| Ethion | 0.999 | 1 | 1 | 102 | 111 | 1 | 3 | 18.0 |
| Ethylan | 0.998 | 1 | 1 | 97 | 112 | 1 | 2 | 19.0 |
| Etofenprox | 0.999 | 2 | 2 | 102 | 115 | 1 | 2 | 15.3 |
| Etridiazole | 0.999 | 1 | 3 | 94 | 106 | 1 | 2 | 14.2 |
| Fenamiphos | 0.999 | 4 | 6 | 110 | 103 | 2 | 7 | 11.4 |
| Fenarimol | 0.999 | 2 | 2 | 106 | 116 | 1 | 4 | 21.3 |
| Fenchlorphos | 0.997 | 3 | 2 | 101 | 117 | 1 | 4 | 15.2 |
| Fenitrothion | 0.998 | 3 | 2 | 116 | 119 | 2 | 6 | 23.3 |
| Fenpropathrin | 0.994 | 7 | 9 | 102 | 109 | 2 | 8 | 16.1 |
| Fenson | 0.999 | 2 | 4 | 99 | 114 | 1 | 2 | 16.0 |
| Fenthion | 0.999 | 2 | 4 | 115 | 123 | 1 | 2 | 25.9 |
| Fenvalerate | 0.997 | 3 | 4 | 96 | 113 | 1 | 4 | 20.8 |
| Fipronil | 0.997 | 3 | 4 | 103 | 116 | 3 | 8 | 22.3 |
| Fluazifop-P-butyl | 0.999 | 2 | 1 | 100 | 113 | 1 | 3 | 18.7 |
| Fluchloralin | 0.997 | 5 | 6 | 89 | 100 | 2 | 6 | 2.5 |
| Flucythrinate | 0.997 | 5 | 4 | 107 | 119 | 2 | 6 | 33.1 |
| Fludioxonil | 0.998 | 5 | 5 | 104 | 109 | 1 | 4 | 14.5 |
| Fluquinconazole | 0.999 | 2 | 3 | 104 | 110 | 1 | 2 | 16.2 |
| Fluridone | 0.993 | 7 | 7 | 108 | 120 | 3 | 9 | 49.8 |
| Flusilazole | 0.997 | 3 | 4 | 92 | 110 | 2 | 5 | 17.3 |
| Flutolanil | 0.998 | 3 | 2 | 105 | 112 | 1 | 4 | 20.6 |
| Flutriafol | 0.992 | 2 | 3 | 108 | 117 | 2 | 5 | 10.9 |
| Folpet | 0.990 | 11 | 9 | 71 | 75 | 1 | 3 | −7.7 |
| Fonofos | 0.999 | 2 | 2 | 103 | 113 | 1 | 3 | 16.2 |
| Gamma-BHC | 0.997 | 1 | 1 | 99 | 111 | 1 | 4 | 14.6 |
| Heptachlor | 0.998 | 3 | 2 | 95 | 108 | 1 | 3 | 7.5 |
| Heptachlor-exo-epoxide | 0.998 | 4 | 6 | 107 | 130 | 3 | 9 | 27.3 |
| Hexachlorobenzene | 0.997 | 2 | 4 | 109 | 115 | 1 | 2 | 16.8 |
| Hexazinone | 0.999 | 2 | 2 | 99 | 113 | 1 | 3 | 19.5 |
| Iodofenphos | 0.997 | 3 | 3 | 90 | 108 | 1 | 4 | 25.4 |
| Iprodione | 0.994 | 5 | 10 | 88 | 97 | 3 | 9 | 12.6 |
| Isazofos | 0.999 | 4 | 18 | 105 | 115 | 2 | 5 | 18.8 |
| Isodrin | 0.993 | 4 | 4 | 100 | 112 | 1 | 2 | 16.6 |
| Isopropalin | 0.994 | 2 | 1 | 110 | 115 | 1 | 3 | 34.1 |
| Lambda-cyhalothrin | 0.996 | 7 | 2 | 102 | 87 | 1 | 3 | −6.7 |
| Lenacil | 0.997 | 6 | 5 | 105 | 113 | 1 | 3 | 24.9 |
| Leptophos | 0.997 | 2 | 3 | 101 | 110 | 1 | 4 | 18.4 |
| Linuron | 0.998 | 4 | 4 | 88 | 113 | 3 | 8 | 10.7 |
| Malathion | 0.998 | 2 | 3 | 94 | 110 | 3 | 9 | 18.4 |
| Metalaxyl | 0.996 | 5 | 9 | 99 | 114 | 3 | 8 | 14.5 |
| Metazachlor | 0.997 | 3 | 2 | 101 | 113 | 1 | 4 | 23.5 |
| Methacrifos | 0.999 | 1 | 2 | 101 | 113 | 1 | 3 | 12.5 |
| Methoxychlor | 0.997 | 4 | 3 | 85 | 93 | 1 | 4 | 4.0 |
| Metolachlor | 0.999 | 2 | 2 | 97 | 111 | 1 | 2 | 14.5 |
| Mevinphos | 0.999 | 1 | 1 | 99 | 108 | 1 | 3 | 11.5 |
| MGK 264 | 0.997 | 4 | 16 | 100 | 113 | 2 | 6 | 19.4 |
| Mirex | 0.998 | 2 | 2 | 98 | 110 | 1 | 2 | 14.9 |
| Myclobutanil | 0.995 | 2 | 2 | 99 | 110 | 1 | 3 | 12.2 |
| N-(2,4-Dimethylphenyl)formamide | 0.998 | 4 | 4 | 108 | 111 | 3 | 8 | 10.4 |
| Nitralin | 0.995 | 12 | 9 | 81 | 83 | 2 | 6 | −14.8 |
| Nitrofen | 0.997 | 2 | 3 | 101 | 112 | 1 | 3 | 17.3 |
| Norflurazon | 0.997 | 5 | 2 | 100 | 109 | 1 | 4 | 17.2 |
| o,p′-DDD | 0.998 | 2 | 2 | 97 | 115 | 1 | 3 | 19.1 |
| o,p′-DDE | 0.998 | 2 | 2 | 100 | 111 | 1 | 2 | 12.8 |
| o,p′-DDT | 0.997 | 2 | 5 | 88 | 98 | 1 | 4 | 9.3 |
| Oxadiazon | 0.997 | 4 | 2 | 100 | 112 | 2 | 5 | 19.9 |
| Oxyfluorfen | 0.993 | 7 | 6 | 93 | 108 | 3 | 9 | 43.0 |
| p,p′-DDD | 0.999 | 1 | 2 | 101 | 114 | 1 | 2 | 20.9 |
| p,p′-DDE | 0.998 | 1 | 4 | 96 | 110 | 1 | 4 | 13.1 |
| p,p′-DDT | 0.997 | 4 | 3 | 86 | 96 | 0 | 1 | 8.9 |
| Parathion | 0.998 | 5 | 4 | 111 | 115 | 2 | 8 | 18.9 |
| Parathion-methyl | 0.998 | 5 | 4 | 109 | 109 | 2 | 6 | 21.2 |
| Pebulate | 0.999 | 1 | 2 | 102 | 112 | 1 | 4 | 11.4 |
| Penconazole | 0.998 | 3 | 4 | 103 | 114 | 1 | 4 | 16.6 |
| Pendimethalin | 0.994 | 5 | 2 | 113 | 113 | 1 | 4 | 32.8 |
| Pentachloroaniline | 0.997 | 2 | 2 | 94 | 113 | 2 | 7 | 13.1 |
| Pentachloroanisole | 0.998 | 2 | 3 | 100 | 114 | 1 | 3 | 15.3 |
| Pentachlorobenzene | 0.998 | 2 | 3 | 96 | 111 | 1 | 3 | 14.5 |
| Pentachlorobenzonitrile | 0.999 | 2 | 4 | 111 | 119 | 1 | 4 | 20.4 |
| Permethrine | 0.998 | 4 | 6 | 94 | 111 | 2 | 7 | 15.6 |
| Phenothrin | 0.997 | 3 | 7 | 94 | 108 | 3 | 9 | 12.9 |
| Phorate | 0.997 | 1 | 5 | 113 | 114 | 3 | 8 | 15.7 |
| Phosalone | 0.997 | 4 | 4 | 101 | 109 | 2 | 6 | 19.1 |
| Phosmet | 0.995 | 4 | 4 | 91 | 97 | 1 | 3 | 13.1 |
| Piperonyl butoxide | 0.999 | 2 | 2 | 107 | 115 | 1 | 3 | 22.0 |
| Pirimiphos ethyl | 0.998 | 3 | 4 | 107 | 116 | 2 | 6 | 24.2 |
| Pirimiphos-methyl | 0.996 | 3 | 2 | 107 | 112 | 1 | 3 | 16.2 |
| Pretilachlor | 0.996 | 2 | 2 | 93 | 112 | 2 | 6 | 20.8 |
| Prochloraz | 0.998 | 7 | 9 | 106 | 109 | 2 | 7 | 20.4 |
| Procymidone | 0.998 | 4 | 3 | 95 | 113 | 1 | 3 | 14.2 |
| Prodiamine | 0.999 | 3 | 5 | 100 | 117 | 2 | 5 | 25.5 |
| Profenofos | 0.991 | 4 | 4 | 91 | 104 | 1 | 5 | 7.8 |
| Profluralin | 0.995 | 2 | 9 | 101 | 117 | 3 | 9 | 42.4 |
| Propachlor | 0.999 | 3 | 2 | 94 | 109 | 1 | 4 | 11.4 |
| Propanil | 0.998 | 11 | 3 | 107 | 106 | 3 | 10 | 12.6 |
| Propargite | 0.991 | 21 | 7 | 94 | 95 | 2 | 8 | 3.0 |
| Propisochlor | 0.997 | 2 | 3 | 104 | 127 | 3 | 9 | 30.9 |
| Propyzamide | 0.998 | 2 | 1 | 106 | 114 | 1 | 5 | 18.7 |
| Prothiofos | 0.998 | 2 | 3 | 99 | 113 | 1 | 3 | 21.3 |
| Pyraclofos | 0.994 | 7 | 4 | 105 | 102 | 2 | 7 | 11.0 |
| Pyrazophos | 0.998 | 5 | 3 | 94 | 108 | 1 | 3 | 22.5 |
| Pyridaben | 0.998 | 4 | 9 | 108 | 110 | 3 | 9 | 23.4 |
| Pyridaphenthion | 0.998 | 3 | 6 | 107 | 114 | 2 | 6 | 29.3 |
| Pyrimethanil | 0.998 | 4 | 7 | 87 | 108 | 3 | 9 | 9.0 |
| Pyriproxyfen | 0.998 | 6 | 9 | 96 | 105 | 3 | 9 | 11.1 |
| Quinalphos | 0.999 | 3 | 4 | 100 | 116 | 2 | 7 | 22.2 |
| Quintozene | 0.997 | 17 | 13 | 98 | 112 | 2 | 5 | 26.8 |
| Resmethrin | 0.994 | 6 | 18 | 99 | 106 | 2 | 7 | 8.1 |
| Sulfotep | 0.999 | 3 | 4 | 98 | 113 | 1 | 4 | 17.0 |
| Sulprofos | 0.997 | 2 | 3 | 117 | 115 | 1 | 3 | 20.3 |
| Tau-fluvalinate | 0.993 | 3 | 3 | 100 | 107 | 1 | 4 | 25.8 |
| Tebuconazole | 0.999 | 3 | 1 | 100 | 112 | 1 | 2 | 14.8 |
| Tebufenpyrad | 0.999 | 3 | 3 | 94 | 112 | 1 | 3 | 14.7 |
| Tecnazene | 0.999 | 4 | 4 | 106 | 115 | 1 | 3 | 15.5 |
| Tefluthrin | 0.997 | 2 | 3 | 78 | 111 | 1 | 4 | 13.2 |
| Terbacil | 0.997 | 2 | 33 | 104 | 115 | 3 | 9 | 19.6 |
| Terbufos | 0.999 | 2 | 2 | 112 | 127 | 2 | 7 | 27.6 |
| Terbuthylazine | 0.998 | 9 | 8 | 104 | 110 | 1 | 4 | 9.1 |
| Tetradifon | 0.998 | 3 | 1 | 87 | 108 | 3 | 8 | 9.3 |
| Tetramethrin | 0.999 | 6 | 7 | 100 | 111 | 2 | 8 | 17.9 |
| THPI (tetrahydrophthalimide) | 0.995 | 3 | 7 | 107 | 116 | 2 | 6 | 20.8 |
| Tolclofos-methyl | 0.998 | 4 | 2 | 92 | 110 | 2 | 5 | 11.6 |
| Tolylfluanid | 0.998 | 5 | 8 | 73 | 93 | 1 | 4 | −3.9 |
| trans-Chlordane | 0.995 | 5 | 3 | 92 | 106 | 1 | 2 | 12.4 |
| Transfluthrin | 0.999 | 6 | 3 | 99 | 111 | 1 | 3 | 8.1 |
| trans-Nonachlor | 0.992 | 2 | 12 | 88 | 103 | 2 | 7 | 29.1 |
| Triadimefon | 0.997 | 5 | 10 | 84 | 113 | 2 | 7 | 12.5 |
| Triadimenol | 0.997 | 13 | 10 | 73 | 97 | 22 | 71 | 1.8 |
| Tri-allate | 0.999 | 1 | 3 | 97 | 112 | 3 | 9 | 9.8 |
| Triazophos | 0.996 | 3 | 6 | 105 | 113 | 2 | 5 | 32.6 |
| Tricyclazole | 0.997 | 15 | 14 | 100 | 101 | 3 | 8 | 10.7 |
| Triflumizole | 0.992 | 3 | 4 | 92 | 115 | 1 | 4 | 10.2 |
| Trifluralin | 0.999 | 3 | 1 | 102 | 111 | 1 | 2 | 18.6 |
| Vinclozolin | 0.996 | 7 | 4 | 105 | 111 | 2 | 5 | 24.5 |
Linearity and working range
Linearity of the method was assessed by analysing the relationship between the concentration of the analytes and their corresponding response signals. A linear curved graph was obtained during the matrix match calibration over the concentration range of 1–100 μg L−1, indicating a strong linearity of 0.995 in the range of 0.990–0.999.Studies of a similar kind have been reported for estimating 733 pesticide residues in fruits and vegetables. The correlation co-efficient of r2 ≥ 0.995 was recorded for 96.1% of the pesticides by LC-Q-TOFMS and 92.4% by GC-Q-TOFMS.4 Another study wherein 31 compounds were analysed by GC-MS/MS1 reported a linearity of 0.995 in the range of 0.9570–0.9992.
Selectivity
In line with the SANTE guidelines, selectivity of a method is verified by identification and confirmation at the Screening Detection Limit (SDL).19 The procedure is referred to as selective, when the analyte is detected in tests and is not obscured by the presence of other chemicals. Hence, accordingly, analysis was done on blank banana samples in order to check for interference peaks, if any. Further, in order to verify the presence, if any, of other compounds which could interfere in peak detection, blank and spiked samples were also compared.With the exception of one instance, every pesticide was accurately identified in the examined samples. None reported any interference. In the sole instance wherein interference was observed, six pesticides (2-phenyl phenol, captafol, captan, choroneb, endrin aldehyde and triadimenol) were reported in the banana matrix. Hence, these six compounds could be considered as not complying with the validation parameters of the SANTE guidelines. This could be attributed to ion enhancement. Since this was observed only for 06 out of the 200 pesticides, the study can be deemed reliable since the results are within the permissible range.
Method limit of detection (MLOD)
The MLOD of this method was determined by using seven replicates (n = 7) of pesticide fortified solutions at a concentration of 10 μg L−1. The MLOD of the method was observed to range between 1 and 6 μg kg−1 for 194 compounds while for the remaining 6 compounds (chloroneb, 2-phenylphenol, captan, triadimenol, endrin aldehyde, and captafol) it was found to be at higher than 10 μg kg−1 levels. A study conducted in 2013 in Brazil for the banana matrix reported the LOD ranging between 5.0 and 7.5 μg kg−1 for 128 pesticide residues.3,13Method limit of quantification (MLOQ)
The MLOQ for the current method was reported to be in the range of 1–10 μg kg−1 for 191 compounds. The remaining 9 compounds (dichlobenil, biphenyl, chloroneb, 2-phenylphenol, captan, triadimenol, endrin aldehyde, acequinocyl and captafol) could not be quantified accurately due to matrix interference being observed at lower concentrations (<25 μg kg−1). On correlating the current study with other studies,1,3 it was observed that the LOQs obtained in this study were less than 10 μg kg−1 for a majority of pesticides, with the exception of fenamiphos and mevinphos for which the obtained LOQ was 25 μg kg−1. The values of MLOD and MLOQ obtained in this study have been detailed in Table 4. A comparison of the obtained MLOQ values with the established MRLs for specific pesticides indicated that the MLOQs obtained by this method were comparatively lower.Recovery, accuracy and precision
Mean recovery rates were obtained by spiking the standards at 2 levels, viz. 10 and 25 μg kg−1 before sample extraction. The mean recovery rates for 191 pesticide residues varied between 71 and 117% when fortified at 10 μg kg−1 before sample extraction. The recovery range for some of the pesticides viz. dichlobenil, biphenyl, chloroneb, 2-phenylphenol etc. was observed to be outside the prescribed range despite the RSD being within the range. When the standards were spiked at 25 μg kg−1, the method recovery was observed to be in the range of 72–120% for 190 pesticide residues. Precision of the method was assessed by calculating the RSD of seven replicate measurements at specific concentrations. Additionally, to evaluate the precision across different levels, intraday (CVr) and interday (CVR) studies were conducted. The average RSD yield (2–10%) of the developed method was found to be below 20% for all the compounds in the CVr study. In the CVR study, the RSD for all compounds ranged between 1 and 20%, and was observed to be below 20% except for three pesticide residues where it exceeded 20%. The obtained responses were satisfactory, as the precision and recovery data from the developed method fulfilled the requirements of 70–120% recovery along with RSD values being ≤20% for the pesticides in accordance with the SANTE guidelines.19Matrix effect
The results of the matrix effect analysis indicated that of all the analyzed compounds 145 of the total 200 pesticide residues fell within the range of −20% to +20%, as prescribed by the SANTE guidelines.19 This was suggestive of the fact that the sample matrix had minimal impact on the measurement of the majority of compounds as most of these values were close to the repeatability limit (Table 4). Only 6 compounds showed strong matrix effects i.e. beyond the −50 to +50% range of matrix effects. However, the RSD for these compounds was well within the acceptable limit except for captafol. These findings demonstrate a strong level of accuracy and reliability in the analysis, indicating that interference from the matrix on most compounds was minimal. When ME was below −20%, it indicated ion suppression as the sample matrix was causing a decrease in the signal intensity of the analyte and when it was above 20%, it indicated ion enhancement, suggestive of the fact that the sample matrix was causing an increase in the signal intensity of the analyte.Several recent studies (Table S4†) carried out over the last 3 years have investigated various methodologies for pesticide analysis. A study conducted by Mohammed Almutairi et al. analysed 294 pesticides with the QuEChERS-dSPE method.25 A similar study using the QuEChERS-dSPE-GC-QMS method was also carried out by Hiago de O. Gomes et al. but only for three pesticides. The LOD, LOQ, recovery and RSD in this study were reported to be 0.01–0.05 mg kg−1, 0.03–0.10 mg kg−1, 74.78% and 99.98% and <20 respectively.26 Saihao Ren et al. investigated the presence of single compound fluopyram using GC-MS/MS in a range of crops that included tomato, cucumber, cowpea, pepper, eggplant, potato, banana, grape, and citrus. The extraction method employed was QuEChERS, with the clean-up using PSA, C18, and MWCNTs-NH2. The recovery for this study ranged from 87.02% to 101.42%, RSD was 9.25%, and ME ranged from −1.41% to 17.67%.27 Ahmed S. Afify et al. conducted an analysis for 49 pesticides encompassing commodities such as tomato, cucumber, zucchini, etc. This analysis utilized the QuEChERS dSPE + GCB method in conjunction with GC-MS/MS. Key parameters such as LOD (0.0005–0.0024 mg kg−1), LOQ (0.0011–0.0047 mg kg−1), recovery (78–107%), RSD (<20%), and r2 (0.99) were determined. However, it is pertinent to note that only 49 pesticides were extracted.28 Xiao Shu et al. conducted a study on hawk tea utilizing the EMR-lipid material-QuEChERS for the extraction of 186 pesticides. MgSO4, PSA, and MWCNTs were employed for clean-up and quantification was performed using a GCMS-TQ8050 triple-quadrupole mass spectrometer, with parameters including LOD (0.001–0.02 mg kg−1), LOQ (0.005–0.05 mg kg−1), recovery (70–120%), RSD (0.3–14.4%), and r2 (0.99). However, it is significant to note that the authors validated and estimated their results using 1200 mg MgSO4, 400 mg PSA, and 200 mg MWCNTs which is much higher than the usage reported in the current study.29 Hiago de O. Gomes et al. analyzed three compounds, namely azoxystrobin, difenoconazole, and propiconazole, using a modified QuEChERS-dSPE-GC/MS method. Results obtained indicated LOD values of 0.018, 0.066, and 0.007, LOQ values of 0.054, 0.199, and 0.022, and r2 values of 0.9985, 0.9966, and 0.9997, respectively.30 Sun-Il Choi et al. conducted an analysis of thiabendazole in banana and citrus fruits using HPLC-PDA and LC-MS/MS. The results reported LOD, LOQ, recovery, RSD, and r2 of 0.009 and 0.017 μg mL−1, 0.028 and 0.052 μg mL−1, 93.61 to 98.08%, 1.33% and 0.999 respectively.31
Salient features of similar studies carried out over the last few years have been tabulated in Table S4.† A sneak peek into these studies indicated that those which examined up to 294 analytes did not specify the validation parameters. This study which used gas chromatography tandem mass spectrometry, in contrast, has details about recovery, RSD, LOD, LOQ, and ME employed for validation, all of which fell within acceptable ranges for the compounds analyzed. Further, the usage of MWCNTs too conferred a significant advantage due to their cost-effectiveness as well as superior recovery rates across all analytes. By employing a simplistic combination of MWCNTs and PSA, this study was able to achieve an MLOD range of 0.45–6.33 μg kg−1 and an MLOQ range of 1.44–9.59 μg kg−1.
The matrix effect characterization provided valuable information about the impact of the sample matrix on the accuracy and reliability of the analysis for each specific pesticide residue thereby implying that the analytical method employed was well-suited for analysing pesticide residue levels in vegetable and fruit samples.
Application to real samples
In order to ascertain the applicability of the method and ease of sample treatment procedure, a total of 25 bananas were analysed by the proposed method. The banana samples were collected from the local markets of Ahmedabad, Gujarat. The outcomes of the examined samples are tabulated in Table 5.| Sample no. | Fluchloralin | Isazofos | Tri-allate | Allethrin-3,4 | Ethion | Tebuconazole | Propargite | Bifenthrin | Phenothrin | Pyriproxyfen | Cypermethrin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Concentration of analytes and MRL: μg kg−1, <LOQ: less than limit of quantification, BA: banana, MRL: maximum residual level. | |||||||||||
| Compound | |||||||||||
| BA 1 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 26 | <LOQ | <LOQ | <LOQ |
| BA 2 | 11 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| BA 3 | <LOQ | <LOQ | 16 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| BA 4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 37 | 12 | <LOQ |
| BA 5 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 18 | <LOQ | <LOQ |
| BA 6 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 17 | <LOQ | <LOQ | <LOQ |
| BA 8 | <LOQ | 11 | <LOQ | 17 | <LOQ | <LOQ | <LOQ | <LOQ | 14 | <LOQ | <LOQ |
| BA 10 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 21 | <LOQ | <LOQ | <LOQ | <LOQ |
| BA 12 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 21 | <LOQ | <LOQ | <LOQ | <LOQ |
| BA 13 | <LOQ | <LOQ | <LOQ | <LOQ | 27 | <LOQ | <LOQ | <LOQ | 24 | 25 | <LOQ |
| BA 14 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 54 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| BA 15 | <LOQ | <LOQ | 16 | <LOQ | <LOQ | <LOQ | <LOQ | 40 | <LOQ | <LOQ | <LOQ |
| BA 16 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 31 | 11 | <LOQ | <LOQ |
| BA 17 | <LOQ | <LOQ | <LOQ | 15 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| BA 18 | <LOQ | <LOQ | <LOQ | 22 | <LOQ | <LOQ | <LOQ | 25 | <LOQ | <LOQ | <LOQ |
| BA 19 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 18 |
| BA 20 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 20 |
| BA 22 | <LOQ | <LOQ | <LOQ | 11 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| BA 23 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 15 |
| BA 24 | <LOQ | <LOQ | <LOQ | 15 | <LOQ | 252 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| BA 25 | 11 | <LOQ | 16 | <LOQ | <LOQ | 121 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| MRL | |||||||||||
| EU | — | — | 100 | — | 10 | 1500 | 10 | 100 | 20 | 700 | 50 |
| CODEX | — | — | — | — | — | 1500 | — | 100 | — | — | — |
| FSSAI | — | — | — | — | 2000 | 1500 | — | — | — | — | — |
Of all the samples analyzed, 84% (n = 21) of bananas reported the presence of pesticide residues at or below the MRLs established by different agencies (EU, Codex, FSSAI).32–34 The remaining 16% (n = 4) of the samples reported no known pesticide residues used in the study. A total of 16% (n = 4) of samples reported pesticide residues above EU prescribed MRLs. Those commonly found were propargite (8%) and phenothrin (8%) followed by ethion (4%).
Similar findings were echoed in a study conducted by Carneiro et al. in Brazil, wherein 3 out of 10 samples were found to be contaminated with boscalid, carbendazin and imidacloprid residues.3,13 A similar study conducted in Spain3 too showed the presence of chlorpyriphos, malathion, fenitrothion and buprofezine in banana samples.
Conclusions
The enhanced and validated modified QuEChERS-d-SPE method employed a multivariate approach and demonstrated successful extraction of diverse pesticide residues from bananas. The samples were subjected to quantitative analysis using gas chromatography-tandem mass spectrometry. The incorporation of a P–B design and Central Composite Design (CCD) in the multivariate approach significantly enhanced the efficiency of the extraction process as this approach aided in screening and optimizing factors. This resulted in effective removal of pigments as co-extractives and minimized the matrix effect during the clean-up step. The developed method demonstrated simplicity and required minimal amounts of sorbents as compared to other methods. The approach also enhanced the suitability of monitoring multi-pesticide residues in banana via application of developed method to real samples. The optimized method was validated in terms of recovery, linearity, precision and sensitivity with favourable outcomes achieved for all validation parameters. To conclude, results showed that the proposed method is simple, efficient, selective, reproducible, cost-effective, and environmentally friendly.Author contributions
Tushar Rajaram Ahire – investigation, validation, writing – original draft; Rupal Rajesh Thasale – data curation, methodology, validation, visualization; Ankita Das – formal analysis; Nikhil P. Kulkarni – writing – review & editing; Dhyan M. Vyas – formal analysis; Sivaperumal Perumal – conceptualisation, funding acquisition, project administration, supervision.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The research was supported by the ICMR – National Institute of Occupational Health (ICMR-NIOH). Further, the authors are greatly thankful to the Director, ICMR – National Institute of Occupational Health (ICMR-NIOH) for guidance and support.Notes and references
- M. Tankiewicz and A. Berg, Improvement of the QuEChERS method coupled with GC–MS/MS for the determination of pesticide residues in fresh fruit and vegetables, Microchem. J., 2022, 181, 107794 CrossRef CAS
.
- Z. Tang, Y. Wei, D. Wang, J. Huang, N. Wan, J. Wei and B. Li, Risk assessment of 369 pesticide residues in banana from Hainan province of China through UPLC-Q-TOF/MS, J. Food Compos. Anal., 2023, 123, 105638 CrossRef CAS
.
- H. de O. Gomes, J. M. C. Menezes, J. G. M. da Costa, H. D. M. Coutinho, R. N. P. Teixeira and R. F. do Nascimento, Evaluating the presence of pesticides in bananas: An integrative review, Ecotoxicol. Environ. Saf., 2020, 189, 110016 CrossRef CAS PubMed
.
- G. Pang, Q. Chang, R. Bai, C. Fan, Z. Zhang, H. Yan and X. Wu, Simultaneous Screening of 733 Pesticide Residues in Fruits and Vegetables by a GC/LC-Q-TOFMS Combination Technique, Engineering, 2020, 6, 432–441 CrossRef CAS
.
- V. P. Kalyabina, E. N. Esimbekova, K. V. Kopylova and V. A. Kratasyuk, Pesticides: formulants, distribution pathways and effects on human health – a review, Toxicol. Rep., 2021, 8, 1179–1192 CrossRef CAS PubMed
.
- E. Hakme, A. Lozano, C. Ferrer, F. J. Díaz-Galiano and A. R. Fernández-Alba, Analysis of pesticide residues in olive oil and other vegetable oils, TrAC, Trends Anal. Chem., 2018, 100, 167–179 CrossRef CAS
.
- S. Mandal, R. Poi, I. Ansary, D. K. Hazra, S. Bhattacharyya and R. Karmakar, Validation of a modified QuEChERS method to determine multiclass multipesticide residues in apple, banana and guava using GC–MS and LC–MS/MS and its application in real sample analysis, SN Appl. Sci., 2020, 2, 1–14 Search PubMed
.
- F. kardani, A. zarei jelyani and M. dahanzadeh, Determination of 250 pesticide residues in the Iranian vegetables assessed during 2019–2020 using the modified QuEChERS method along with gas chromatography - mass spectrometry, Appl. Food Res., 2022, 2, 100106 CrossRef CAS
.
- K. Pszczolińska, N. Shakeel and H. Barchanska, A simple approach for pesticide residues determination in green vegetables based on QuEChERS and gas chromatography tandem mass spectrometry, J. Food Compos. Anal., 2022, 114, 104783 CrossRef
.
- M. M. Alshehri, M. A. Ouladsmane, T. A. Aouak, Z. A. ALOthman and A. Y. Badjah Hadj Ahmed, Determination of phthalates in bottled waters using solid-phase microextraction and gas chromatography tandem mass spectrometry, Chemosphere, 2022, 304, 135214 CrossRef CAS PubMed
.
- E. Hakme, A. Lozano, S. Uclés, M. M. Gómez-Ramos and A. R. Fernández-Alba, High-throughput gas chromatography-mass spectrometry analysis of pesticide residues in spices by using the enhanced matrix removal-lipid and the sample dilution approach, J. Chromatogr. A, 2018, 1573, 28–41 CrossRef CAS PubMed
.
- T. M. Rizzetti, M. Kemmerich, M. L. Martins, O. D. Prestes, M. B. Adaime and R. Zanella, Optimization of a QuEChERS based method by means of central composite design for pesticide multiresidue determination in orange juice by UHPLC-MS/MS, Food Chem., 2016, 196, 25–33 CrossRef CAS PubMed
.
- R. P. Carneiro, F. A. S. Oliveira, F. D. Madureira, G. Silva, W. R. de Souza and R. P. Lopes, Development and method validation for determination of 128 pesticides in bananas by modified QuEChERS and UHPLC-MS/MS analysis, Food Control, 2013, 33, 413–423 CrossRef CAS
.
- A. Santana-Mayor, R. Rodríguez-Ramos, A. V. Herrera-Herrera, B. Socas-Rodríguez and M. A. Rodríguez-Delgado, Updated overview of QuEChERS applications in food, environmental and biological analysis (2020–2023), TrAC, Trends Anal. Chem., 2023, 169, 117375 CrossRef CAS
.
- I. S. Jeong, B. M. Kwak, J. H. Ahn and S. H. Jeong, Determination of pesticide residues in milk using a QuEChERS-based method developed by response surface methodology, Food Chem., 2012, 133, 473–481 CrossRef CAS PubMed
.
- X. Xu, X. Xu, M. Han, S. Qiu and X. Hou, Development of a modified QuEChERS method
based on magnetic multiwalled carbon nanotubes for the simultaneous determination of veterinary drugs, pesticides and mycotoxins in eggs by UPLC-MS/MS, Food Chem., 2019, 276, 419–426 CrossRef CAS PubMed
.
- J. Wang, H. L. Duan, L. Fan, Y. M. Lin, J. N. Sun and Z. Q. Zhang, Magnetic tetraethylenepentamine modified multi-walled carbon nanotubes as matrix clean-up materials for organophosphorus pesticide residues analysis in cucumber, Food Control, 2021, 124, 107904 CrossRef CAS
.
- F. Makkliang, P. Kanatharana, P. Thavarungkul and C. Thammakhet, A novel miniaturized zinc oxide/hydroxylated multiwalled carbon nanotubes as a stir-brush microextractor device for carbamate pesticides analysis, Anal. Chim. Acta, 2016, 917, 27–36 CrossRef CAS PubMed
.
- European Comission, Document no. SANTE 11312/2021. Analytical quality control and method validation procedures for pesticide residues analysis in food and feed, 2021, pp. 1–57, accessible at: https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=727 Search PubMed.
- D. Kottadiyil, T. Mehta, R. Thasale and S. P, Determination and dietary risk assessment of 52 pesticide residues in vegetable and fruit samples by GC-MS/MS and UHPLC-QTOF/MS from Gujarat, India, J. Food Compos. Anal., 2023, 115, 104957 CrossRef CAS
.
- S. T. Narenderan, S. N. Meyyanathan and V. V. S. R. Karri, Experimental design in pesticide extraction methods: A review, Food Chem., 2019, 289, 384–395 CrossRef CAS PubMed
.
- Y. Farina, M. P. Abdullah, N. Bibi and W. M. A. W. M. Khalik, Determination of pesticide residues in leafy vegetables at parts per billion levels by a chemometric study using GC-ECD in Cameron Highlands, Malaysia, Food Chem., 2017, 224, 186–192 CrossRef PubMed
.
- A. Asati, G. N. V. Satyanarayana, V. T. Srivastava and D. K. Patel, Determination of organochlorine compounds in fish liver by ultrasound-assisted dispersive liquid–liquid microextraction based on solidification of organic droplet coupled with gas chromatography-electron capture detection, J. Chromatogr. A, 2018, 1561, 20–27 CrossRef CAS PubMed
.
- M. Anastassiades, S. J. Lehotay, D. Štajnbaher and F. J. Schenck, Fast and easy multiresidue method employing acetonitrile extraction/partitioning and ‘dispersive solid-phase extraction’ for the determination of pesticide residues in produce, J. AOAC Int., 2003, 86, 412–431 CrossRef CAS PubMed
.
- M. Almutairi, T. Alsaleem, H. Al Herbish, A. A. Al Sayari and A. M. Alowaifeer, LC-MS/MS and GC-MS/MS analysis of pesticide residues in Ecuadorian and Filipino Cavendish bananas imported into Saudi Arabia, Food Addit. Contam., Part A: Chem., Anal., Control, Exposure Risk Assess., 2021, 38, 1376–1385 CrossRef CAS PubMed
.
- H. de O. Gomes, R. da S. Cardoso, C. de F. A. Nonato, V. P. A. da Silva, C. de A. Nobre, J. G. M. da Costa, R. N. P. Teixeira and R. F. do Nascimento, Optimization and Validation of a Method Using GC–MS and QuEChERS for Pesticide Determination in Banana Pulp, Food Anal. Methods, 2023, 16, 125–131 CrossRef
.
- S. Ren, Y. Zhang, S. Zhang, H. Lu, X. Liang, L. Wang, M. Wang and C. Zhang, Residue behavior and dietary risk assessment of fluopyram in cowpea and determination in nine foodstuffs, Front. Environ. Sci., 2023, 11, 1–11 Search PubMed
.
- A. S. Afify, M. Abdallah, S. A. Ismail, M. Ataalla, M. A. S. Abourehab, S. T. Al-Rashood and M. A. Ali, Development of GC–MS/MS method for environmental monitoring of 49 pesticide residues in food commodities in Al-Rass, Al-Qassim region, Saudi Arabia, Arabian J. Chem., 2022, 15, 104199 CrossRef CAS
.
- X. Shu, N. Chu, X. Zhang, X. Yang, X. Meng, J. Yang and N. Wang, Rapid Analysis of Residues of 186 Pesticides in Hawk Tea Using Modified QuEChERS Coupled with Gas Chromatography Tandem Mass Spectrometry, Int. J. Environ. Res. Public Health, 2022, 19(19), 12639 CrossRef CAS PubMed
.
- H. de O. Gomes, R. da S. Cardoso, J. G. M. da Costa, V. P. Andrade da Silva, C. de A. Nobre, R. N. Pereira Teixeira and R. F. do Nascimento, Statistical evaluation of analytical curves for quantification of pesticides in bananas, Food Chem., 2021, 345, 128768 CrossRef CAS PubMed
.
- S. Il Choi, X. Han, S. J. Lee, X. Men, G. Oh, D. S. Lee and O. H. Lee, Validation of an Analytical Method for the Determination of Thiabendazole in Various Food Matrices, Separations, 2022, 9(6), 135 CrossRef
.
- I. MoH, Food Safety and Standards (Contaminants, Toxin and Residues), Food Saf. Stand. Auth. India, 2011, 1–19 Search PubMed
.
-
EU MRL Database, https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/products/details/4 Search PubMed
.
-
Codex MRL Database, https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticides/en/ Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ay00703d |
| This journal is © The Royal Society of Chemistry 2024 |