Open Access Article
Open Access ArticleA rapid and improved method for the determination of ethyl carbamate in foodstuffs of different matrices†
Veronika
Šantrůčková
,
Jan
Fischer
 and
Jitka
Klikarová
and
Jitka
Klikarová
 *
*
Department of Analytical Chemistry, Faculty of Chemical Technology, University of Pardubice, Studentská 573, 53210 Pardubice, Czech Republic. E-mail: jitka.klikarova@upce.cz
First published on 1st July 2024
Abstract
This work deals with the rapid and simple determination of the probable carcinogen ethyl carbamate (EC), which is naturally present in fermented food products. An undemanding, robust, and rapid pre-column derivatization utilizing a 9-xanthydrol reagent has been developed. The resulting derivative was subsequently analysed by reversed-phase high-performance liquid chromatography coupled with fluorescence detection. As a result of the thorough optimisation of the chromatographic conditions, the run was completed in just 5 minutes, considerably speeding up the usual time of EC separation (30–60 min). Thanks to the fast separation, satisfactory yields (around 90%), negligible matrix effects, no interfering peaks, very low detection limit, and simple sample pre-treatment (for the very first time, the derivatization was performed in the presence of light and without any extraction step), the proposed method represents a significant improvement of the EC determination protocol used so far. After method validation, a total of fifty food samples were subjected to analysis without any additional sample pre-treatment despite their diverse matrix. Due to its robustness, simplicity, and low time, cost, and manual demands, this method is suitable for rapid screening of EC in both final food products and during their production.
1. Introduction
Ethyl carbamate (EC), also known as carbamic acid ethyl ester, urethane, or ethyl urethane, used to be commonly utilized in the textile, cosmetic, pharmaceutical, and chemical industries. However, all applications were banned after its toxicity was revealed in 1943.1,2 Besides that, EC is naturally formed in foods and beverages during their fermentation, distillation, or storage. Therefore, EC can be detected in fermented food products, such as dairy products, pastries, sauerkraut, soy sauce, vinegar, beer, or wine. However, distilled alcoholic beverages made from stone- or pome-fruits are particularly hazardous to human health, as they often contain excessive EC concentrations.3–6 In the Czech Republic, a decree establishing the maximum permitted EC level for wine and fruit spirits at 30 μg L−1 and 400 μg L−1, respectively, was in force until 2012.7 Currently, only a more lenient recommendation of the European Commission from 2010, requiring compliance with the maximum limit of 1000 μg L−1 for stone-fruit spirits, is applied.8Several specific analytical methods based on different techniques, such as gas chromatography,9–11 liquid chromatography,12–15 infrared spectrometry,16,17 nuclear magnetic resonance,18 enzymatic approaches,19–21 and electrochemical biosensors,22 have already been developed for EC analysis. However, most of them require a demanding EC isolation using various extraction techniques and/or a derivatization step.23
The aim of this work was to simplify the current time-consuming and manually demanding chromatographic determination of ethyl carbamate in foodstuffs, both in terms of sample pre-treatment and separation. Furthermore, an effort was made to screen for this hazardous substance in commonly available Czech food products and thereby determine the level of exposure of Czech consumers to this carcinogen.
2. Experimental
2.1 Chemicals and reagents
The ethyl carbamate standard (purity ≥ 98%), 9-xanthydrol (98%), 1-propanol (99%), sodium phosphate (96%), o-phosphoric acid (85%, analytical grade), ethanol (96%), and acetonitrile (analytical grade) were purchased from Merck (Darmstadt, Germany). Hydrochloric acid (35%), sodium hydroxide, and sodium acetate (all analytical grade purity) were purchased from LachNer (Neratovice, Czech Republic). High-purity water was prepared using a Milli-Q purification system (Merck Millipore, Germany).2.2 Standards and samples
A stock solution of ethyl carbamate with a concentration of 4455 mg L−1 (=50 mmol L−1) was prepared in 40% aqueous ethanol (v/v) and stored in a refrigerator at 5–8 °C. For derivatization purposes, the stock solution was further diluted with 40% aqueous ethanol to obtain a standard solution of 4455 μg L−1 (=50 μmol L−1). Subsequently, an appropriate amount of 9-xanthydrol (9-XA) derivatizing agent was dissolved in 1-propanol to prepare a solution of 3964 mg L−1 (=20 mmol L−1), which was also stored in a refrigerator.The details of each sample examined are summarized in Table 1. A total of 19 homemade fruit spirits produced between the years 2013 and 2021 in seven different small grower Czech distilleries were subjected to the analysis. These samples were mainly based on plums (nos. 1–6), cherries (nos. 7 and 8), and pears (nos. 9–11), but also included less traditional fruits, such as mirabelle plum (no. 12), currants (nos. 13), apples (no. 14), and peaches (nos. 15 and 16). Some of the spirits were bi-varietal and contained plum-gages (no. 17) or pear-carrot (no. 18). An aged spirit (no. 19) from an unknown fruit mixture was also tested. As far as the amount of alcohol was known, it was around 50%. Moreover, two producers also provided the first fraction (head fraction) of the distillation (nos. 20 and 21), which is intended for disposal and should always be removed from the main distillation fraction (heart) because of the presence of many hazardous substances. Furthermore, six commercially available plum spirits (nos. 22–27) produced by five different Czech manufacturers were analysed, all with a declared ethanol content between 40–50%. In addition to fruit spirits, EC was also monitored in other kinds of alcoholic beverages (19 samples in total). These included samples of homemade (nos. 28–31) and commercially available (nos. 32 and 33) meads, brandy (no. 34), vodka (nos. 35 and 36), whisky (nos. 37 and 38), tequila (no. 39), rum (no. 40), gin (no. 41), juniper brandy (no. 42), and white wines (nos. 43–45). One sample of concentrated grain spirit (no. 46) was also assayed. Finally, potentially hazardous foods,24,25 such as soy sauce (no. 47), vinegar (no. 48), and balsamic vinegar (nos. 49 and 50), were also examined.
| Sample no. | Homemade distilled fruit spirits; production year | Alcohol content (%) | Producer, place of origin |
|---|---|---|---|
a Abbreviations: CZ = Czech Republic, DE = Germany, HU = Hungary, and SK = Slovak republic. All sample experiments were five times (n = 5) repeated and the results were calculated and presented as confidence intervals ![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) ± s·t1−α, where ± s·t1−α, where ![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) is the arithmetic mean, s is the standard deviation, and t1−α the critical value of Student's t-distribution for five repetitions (2.776) at a significance level α of 0.05 (95% probability). is the arithmetic mean, s is the standard deviation, and t1−α the critical value of Student's t-distribution for five repetitions (2.776) at a significance level α of 0.05 (95% probability).
|
|||
| 1 | Plum | — | |
| 2 | Plum; 2013 | 51 | Grower distillery BaKaB, Hornice, CZ |
| 3 | Plum; 2020 | 50 | Grower distillery, Malé Hradisko, CZ |
| 4 | Plum; 2016 | 50 | Grower distillery, Malé Hradisko, CZ |
| 5 | Plum; 2020 | Grower distillery, Přerov, CZ | |
| 6 | Plum; 2021 | Distillery and cidery, Lipová-lázně, CZ | |
| 7 | Cherry; 2019 | 50 | Grower distillery, Veltruby, CZ |
| 8 | Cherry; 2018 | Distillery and cidery, Lipová-lázně, CZ | |
| 9 | Pear; 2013 | 50 | Grower distillery BaKaB, Hornice, CZ |
| 10 | Pear; 2018 | 50 | Grower distillery, Veltruby, CZ |
| 11 | Pear; 2021 | Distillery and cidery, Lipová-lázně, CZ | |
| 12 | Mirabelle plum; 2013 | 50 | Grower distillery, Veltruby, CZ |
| 13 | Currant | Distillery and cidery, Lipová-lázně, CZ | |
| 14 | Apple; 2018 | 50 | Grower distillery, Křinec, CZ |
| 15 | Peach; 2014 | Distillery and cidery, Lipová-lázně, CZ | |
| 16 | Peach; 2018 | Distillery and cidery, Lipová-lázně, CZ | |
| 17 | Plum-Gage; 2014 | Distillery and cidery, Lipová-lázně, CZ | |
| 18 | Pear-carrot | Distillery and cidery, Lipová-lázně, CZ | |
| 19 | Aged spirit from fruit mixture; 2021 | Grower distillery, Bohdaneč, CZ | |
| 20 | Head fraction of plum spirit | Grower distillery, Veltruby, CZ | |
| 21 | Head fraction of plum spirit No. 6 | Distillery and cidery, Lipová-lázně, CZ | |
| Sample no. | Commercially distilled fruit spirits | Alcohol content (%) | Producer/manufacturer |
|---|---|---|---|
| 22 | Plum | 50 | Žufánek distillery, CZ |
| 23 | Plum | 40 | Rudolf Jelínek distillery, CZ |
| 24 | Plum | 40 | Rudolf Jelínek distillery, CZ |
| 25 | Plum | 40 | St. Nicolaus, SK |
| 26 | Plum | 47 | Bartida, CZ |
| 27 | Spirit from maturated plums | 40 | Liqui B Blatná distillery and brewery, CZ |
| Sample no. | Other alcoholic beverages | Alcohol content (%) | Producer/manufacturer |
|---|---|---|---|
| 28 | Spring homemade mead | 11.5 | Beekeeper, Přibyslav, CZ |
| 29 | Forest homemade mead | 13.5 | Beekeeper, Přibyslav, CZ |
| 30 | Medow homemade mead | 13.5 | Beekeeper, Přibyslav, CZ |
| 31 | Forest homemade mead | 12.9 | Beekeeper, Potštejn, CZ |
| 32 | Commercial mead | 11 | Hromčík, Nivnice, CZ |
| 33 | Commercial mead | 14.5 | Medovinka, CZ |
| 34 | Brandy | 40 | Mast-Jaegermeister, CZ |
| 35 | Vodka | 40 | Brown-Forman Czechia |
| 36 | Vodka | 37.5 | Stock Plzeň-Božkov, CZ |
| 37 | Whiskey | 40 | Stock Plzeň-Božkov, CZ |
| 38 | Whiskey | 40 | Mast-Jaegermeister, DE |
| 39 | Tequila | 40 | Brown-Forman Czechia, CZ |
| 40 | Rum | 40 | Stock Plzeň-Božkov, CZ |
| 41 | Violet gin | 37.5 | Stock Plzeň-Božkov, CZ |
| 42 | Juniper brandy | 38 | St. Nicolaus, Liptovský Mikuláš, SK |
| 43 | Pálava white wine | 11.5 | Vinice Hnanice, CZ |
| 44 | Cuvéé white wine | 11.5 | Annovino Vinařství Lednice, CZ |
| 45 | Tokaji white wine | 10.5 | Grand Tokaj, HU |
| 46 | Grain spirit | 96 | Distillery Kolín, CZ |
| Sample no. | Type of foodstuff | Manufacturer/distributor | |
|---|---|---|---|
| 47 | Soy sauce | Countrylife | |
| 48 | Vinegar | Kaufland | |
| 49 | Wine vinegar | Lidl | |
| 50 | Wine vinegar I.G.P. (from Modena) | Lidl |
2.3 Sample pre-treatment
The EC derivatization reaction was based on a previously published procedure,13 which was optimised and modified. The reaction scheme is shown in Fig. 1.In 1.5 mL plastic microtubes, 600 μL 9-XA solution (c = 20 mmol L−1), 100 μL hydrochloric acid (c = 1.5 mol L−1), and 400 μL of sample or 12–345 μL of standard EC solution (c = 50 μmol L−1; together with 388–55 μL of 40% aqueous ethanol to maintain the same final volume of the derivatization mixture) were thoroughly mixed. For the least concentrated calibration solution (c = 10.1 nmol L−1), 220 μL of EC standard solution with a concentration of 50 nmol L−1 was pipetted together with 180 μL of 40% aqueous ethanol. The derivatization mixtures were always left at room temperature for 30 minutes, then filtered through a PTFE syringe filter (0.45 μm, 4 mm; Labstore, HPST, Prague, Czech Republic) and analysed.
2.4 Instrumentation and analysis
For EC determination, reversed-phase high-performance liquid chromatography coupled to fluorescence detection (RP-HPLC-FLD) was utilized. The system was equipped with two LC-30AD pumps, a DGU-20A5 degasser, an RF-20A XS fluorescence detector (all Shimadzu, Kyoto, Japan), a six-port injection valve with a 20 μL external loop (Valco-Vici, Schenkon, Switzerland), and an LCO 102 column thermostat (Ecom, Prague, Czech Republic).The optimised separation of derivatives was performed on a Luna C18 analytical column (150 × 3 mm; 3 μm particle size; Phenomenex, Torrance, USA) using a binary mobile phase consisting of sodium acetate (c = 20 mmol L−1) at pH 7.2 and 100% acetonitrile. The flow rate was 0.8 mL min−1, the column temperature 35 °C, and the injection volume 20 μL. The final mobile phase gradient was as follows: 0 min – 62% B, 4 min – 70% B, and 5 min – 100% B. The excitation and emission wavelengths of the fluorescence detector were set at 233 nm and 600 nm, respectively.
2.5 Method validation and data processing
Quantitative analysis was performed using the external calibration curve method. The calibration data were measured at nine concentration levels in the concentration range of 0.9–1400 μg L−1; each level was five times prepared and analysed (n = 5) and fitted using linear least squares regression (QC Expert 2.9, Trilobyte, Czech Republic). Influential points were identified using graphical diagnostics (Pregibon, Williams, and L-R graphs) and potential outliers were eliminated. The linearity of the calibration curves was verified using residual plots, and the significance of the intercept of the regression straight-line was tested using Student's t-test.The instrumental limits of detection (LOD) and quantification (LOQ) were calculated as the concentration yielded a signal-to-noise ratio of S/N = 3 and S/N = 10, respectively. The accuracy and precision of the method were verified by measuring the calibration solutions at three concentration levels (150 μg L−1, 700 μg L−1, and 1300 μg L−1), each level with ten repetitions (ten times prepared).
3. Results and discussion
3.1 Optimisation of the derivatization procedure
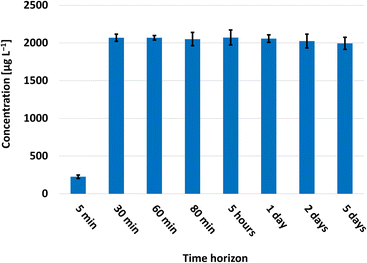
Since EC lacks a fluorophore, chromophore, and electrophore in its chemical structure and interference occurs at the selected MRM transition when using the direct HPLC-MS/MS technique, a derivatization reaction is mandatory. So far, 9-XA is the only exclusively used EC derivatization reagent, applied prior to HPLC, GC, and MS analysis. The EC reaction with 9-XA in a strongly acidic environment (hitherto always performed in the absence of light) leads to ethyl-N-xanthyl carbamate derivative (XEC),26 as depicted in Fig. 1. The XEC preparation had to be thoroughly optimised in terms of volumes and concentrations of individual derivatization components, derivatization time necessary for the quantitative reaction, stability of the resulting XEC derivatives, and effects of alcohol content, pH, and light presence on the derivatization yields. Since the absorption spectrum of 9-XA was found to be identical to that of XEC (Fig. S1 in the ESI†), the optimisation of the derivatization could not be performed by a simple and rapid spectrophotometric technique, but chromatography had to be employed.Information concerning the reaction time and stability of the derivatives varies between studies. However, already published studies agree13,26,27 that with increasing acidity of the derivatization medium, the reaction kinetic accelerates, but the derivative formed is less stable. Moreover, the reaction time also depends on the sample matrix, especially on the presence of aromatics.13 Therefore, the kinetics of the reaction and the stability of the resulting derivatives were studied in an environment of 0.15–1.5 mol L−1 hydrochloric acid, 1.5 mol L−1 acetic acid, and 0.1 mol L−1 phosphate buffer at pH 2.5. Using buffer, acetic acid, and less concentrated hydrochloric acid, no quantitative reaction occurred even within 24 hours (Fig. 2). Therefore, 1.5 mol L−1 hydrochloric acid was used for further experiments. Under these conditions, the quantitative reaction was achieved within 30 minutes at laboratory temperature and the derivative obtained was stable for at least five days (Fig. 3).
As this derivatization has so far been carried out only in the dark,13 which requires higher demands on the operator, the effect of the presence of light on the kinetics and yield of the reaction was also investigated. It was found that light elimination did not lead to a higher yield or faster reaction (Fig. 4), so subsequent experiments were conducted under light.
The literature also indicates that the derivatization yield depends on the amount of ethanol present.13,28 For this reason, a series of spiked plum spirits/standard solutions were prepared with the same EC concentration (c = 1300 μg L−1) but a different ethanol content, ranging between 10–60%. In the case of the EC standard, the relative yield decreased with increasing alcohol content (from 105% to 91%; Fig. S2a†). For the plum spirit, the relative yield fluctuated between 135% and 100% (Fig. S2b†). In general, spirits typically contain between 30% and 50% alcohol. In this concentration range of ethanol, there were no statistically significant changes in yields (98.1 ± 2.9%) in either case. A similar dependence has already been presented by a group of Chinese authors,28 but according to other authors,13 the yield of the reaction increased up to 42% ethanol and then decreased again up to 60%. For determining accurate EC concentrations in samples of different types, it would be, therefore, appropriate to always adjust the respective sample to a uniform ethanol content (corresponding to the content used to construct the calibration dependence). However, the maximum EC limits recommended by the European Commission are relatively benevolent and only applicable to distillates. Thus, it is not necessary to maintain exact ethanol concentrations for rapid EC screening of samples with different matrices in common food manufacturing companies.
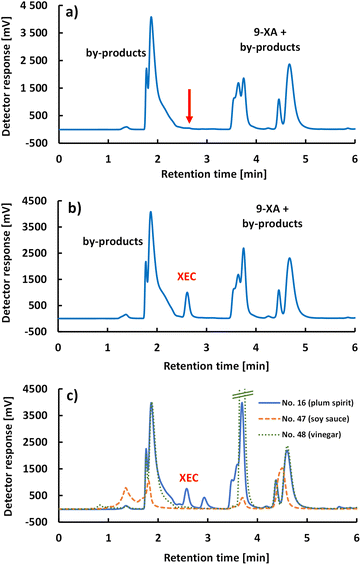
3.2 Optimisation of the chromatographic separation
HPLC-FLD separation conditions were also thoroughly optimised. Since the excitation and emission wavelengths were not uniform in the literature, it was first necessary to measure the corresponding spectra of XEC derivative (Fig. S1 and S3†), based on which the excitation and emission wavelengths of 233 nm and 600 nm, respectively, were subsequently set on the detector. The choice of column type and mobile phase was inspired by previously published data.13 In contrast, the greatest attention was paid to the optimisation of gradient elution. The aim was (i) to reduce the analysis time as much as possible so that the method is also suitable for rapid screening and routine use and (ii) to maintain sufficient peak resolution (the reaction generates a relatively large number of by-products). Thus, gradients with different initial concentrations of acetonitrile (50–62%) and different slopes were tested. The final gradient program started at 62% acetonitrile and lasted only 5 minutes (see chapter Experimental), whereas the usual analysis time has previously been between 30–50 minutes.12,13,26–28 An example of the chromatographic separation of blank solution, EC standard solution, and samples of different types after their derivatization is shown in Fig. 5. A comparison of our EC determination parameters with previously published literature data is summarized in Table 2.| Number and type of samples | Sample pre-treatment | Derivatization conditions | Stability of the derivative | Separation method; analysis time | Recovery [%] | LOD [μg L−1] | LOQ [μg L−1] | Intra-day repeatability [% RSD] | Inter-day repeatability [%RSD] | Cost; difficulty | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Abbreviations: 9-XA, 9-xanthydrol; BSTFA, bis(trimethylsilyl)trifluoroacetamide; FLD, fluorescence detection; GC, gas chromatography; HPLC, high-performance liquid chromatography; HS, head-space; LLE, liquid–liquid extraction; LOD, limit of detection; LOQ, limit of quantification; m-LLE, micro LLE; MS, mass spectrometry; MS/MS, tandem mass spectrometry; RSD, relative standard deviation; SPE, solid phase extraction; SPME, solid phase micro-extraction; and UHPLC, ultra HPLC. | |||||||||||
| 50 foodstuffs of various matrices | Derivatization with 9-XA | 30 min, room temperature, presence of light, smaller number of by-products | 5 days < | HPLC-FLD; 5 min | 84–104 | 0.25 | 0.84 | 5.7 | 6.5 | Cheap; low | Presented study |
| 19 soy sauces | LLE and SPE followed by derivatization with 9-XA | 30 min, higher temperature, in the dark, higher number of by-products | 2 hours | HPLC-FLD; 40 min | 81–95 | 3.9 | 13 | <6.6 | <8.9 | Medium expensive; high | 12 |
| 90 spirits | Derivatization with 9-XA | 50 min, room temperature, in the dark, smaller number of by-products | Not given | HPLC-FLD; 30 min | 96 | 1.8 | 5.3 | <5 | — | Cheap; low | 13 |
| 34 wines | Derivatization with 9-XA | Room temperature, in the dark, different pHs | Depends on pH | HPLC-FLD; 55 min | 93–104 | 3 | — | 1.8 | 2.1 | Cheap; low | 26 |
| 17 industrial and 15 experimental cider spirits | Derivatization with 9-XA | 30 min, room temperature, in the dark, higher number of by-products | 12 h < | HPLC-FLD; 31 min | 94–98 | 1.64 | 3.56 | <5 | — | Cheap; low | 27 |
| 26 wines and brandies | Derivatization with 9-XA | 30 min, 30 °C, in the dark, higher number of by-products | 60 min | HPLC-FLD; 50 min | 91–105 | 4.8 | 16 | <3.7 | <4.8 | Cheap; low | 28 |
| Number and type of samples | Sample pre-treatment | Derivatization conditions | Stability of the derivative | Separation method and analysis time | Recovery [%] | LOD [μg L−1] | LOQ [μg L−1] | Intra-day repeatability [%RSD] | Inter-day repeatability [%RSD] | Cost; difficulty | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 wines and 4 liquors | Without derivatization or purification | — | — | UHPLC-MS/MS, 5 min | 107–111 | 1.8 | 4 | <5 | Expensive; low | 14 | |
| 24 fortified wines | m-LLE | — | — | HPLC-MS/MS; 18 min | 93–114 | 0.17 | 0.52 | <5.6 | <8 | Expensive; medium | 15 |
| 35 kinds of alcoholic beverages | LLE followed by derivatization with BSTFA | 30 min, 80 °C, presence of light | — | GC-MS, 24 min | 71–99 | 0.3 | 5 | <8.4 | — | Expensive; high | 9 |
| 54 stone-fruit spirits | HS-SPME at 70 °C, 30 min | — | — | GC-MS/MS; 37 min | 91–109 | 30 | 110 | <4.3 | <8.2 | Expensive; high | 10 |
| — | SPE | — | — | GC-MS; 40 min | 87–93 | — | — | — | — | Expensive; medium | 11 |
| 100 foodstuffs of various matrices | Homogenization followed by SPE and other purification procedures (based on the AOAC official method11) | — | — | GC-MS; separation time not given | 66–117 | 1 | — | — | — | Expensive; high | 24 |
| 237 foodstuffs of various matrices | Homogenization followed by SPE and other purification procedures (based on the AOAC official method11) | — | — | GC-MS; separation time not given | 90–102 | — | — | — | — | Expensive; high | 25 |
3.3 Validation of the analytical method
The developed analytical method for EC determination was validated in terms of linearity of the calibration curve (given by the coefficient of determination R2), LOD, LOQ, accuracy (recovery), and precision (intra-day and inter-day repeatability). All the validation parameters are summarized and compared with the literature in Table 2.Linear dependence (in the concentration range of 0.9–1400 μg L−1) was characterized using the equation A = 2.27 (±0.02)c + 204.99 (±11.44); where A is the peak area (mV s) and c is the concentration (μg L−1) and R2 = 0.9991, representing good linearity (Fig. S4†). The LOD and LOQ values reached 0.25 μg L−1 and 0.84 μg L−1, respectively. According to the validation guideline, the recovery for our concentration range should be between 80% and 110%.29 As can be seen from Table S1,† all three matrices (plum brandy, soy sauce, and vinegar) met this range at all concentrations tested (150–1300 μg L−1), and the method can be considered sufficiently accurate. Moreover, no interfering peaks causing potential co-elution with the target analyte were observed in any of the samples (Fig. 5 and S5†), despite the completely different nature of the matrices.
Intra-day repeatability and inter-day repeatability were expressed as relative standard deviation (RSD) and reached mean values of 5.7% and 6.5%, respectively (Table S2†). According to the validation guide,29 RSD < 7.3% should be obtained for the given concentrations, which is met in both cases, and the method can thus be considered sufficiently precise. In addition, all validation data obtained are consistent with those already published or better (Table 2).
3.4 Sample analysis
EC was determined in a total of 48 food samples and 2 samples of the head fraction of the plum spirit distillation process. Out of these fifty samples analysed, EC was detected in fifteen (Table 3). The largest number of samples with detectable EC content was observed in homemade stone fruit spirits from grower distilleries. Of these 19 samples monitored, EC was present in 12 (nos. 1, 2, 5–9, 11, 12, 15, 16, and 19). However, in three samples (nos. 6, 11, and 15), the EC concentration could not be exactly determined because its level was below the limit of quantification. The other samples contained EC at concentrations ranging from 14 μg L−1 to 1500 μg L−1. Until 2012, a Czech decree allowed a maximum EC level of 400 μg L−1 in fruit spirits. This would not be met by two samples, Nos. 9 and 2, in which 516 μg L−1 and 1503 μg L−1 were found, respectively. Since 2012, a more benevolent EU recommendation has been in force in the Czech Republic, allowing EC levels up to 1000 μg L−1. From this point of view, only sample no. 2 would not comply with this recommendation and is thus considered unsafe.| Sample no. | EC quantity [μg L−1] | Sample no. | EC quantity [μg L−1] | Sample no. | EC quantity [μg L−1] |
|---|---|---|---|---|---|
a LOQ = 0.84 μg L−1; values are given as confidence intervals ![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) ± s·t1−α, where ± s·t1−α, where ![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) is the arithmetic mean, s is the standard deviation, and t1−α the critical value of Student's t-distribution for five repetitions (2.776) at a significance level α of 0.05 (95% probability). is the arithmetic mean, s is the standard deviation, and t1−α the critical value of Student's t-distribution for five repetitions (2.776) at a significance level α of 0.05 (95% probability).
|
|||||
| 1 | 81.2 ± 2.1 | 8 | 140.4 ± 1.5 | 16 | 354.0 ± 1.2 |
| 2 | 1503.4 ± 2.9 | 9 | 516.1 ± 2.3 | 19 | 150.4 ± 8.7 |
| 5 | 378.2 ± 3.3 | 11 | <LOQa | 22 | 379.5 ± 6.8 |
| 6 | <LOQa | 12 | 316.3 ± 2.2 | 31 | 467.5 ± 3.0 |
| 7 | 14.2 ± 1.7 | 15 | <LOQa | 33 | <LOQa |
Since, in an earlier study,30 a large amount of EC (up to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1) was found in the first fraction of distillation, two samples of the head distillation fraction (nos. 20 and 21) were analysed in addition to alcoholic beverages and food. However, EC was not found in any of these samples. The head fractions are rich in low-boiling substances, whereas EC has a relatively high boiling point (182–184 °C) and would thus be distilled mainly in the heart or tail fractions of distillation.8 This is also confirmed by the fact that in one distillation batch (head no. 21 and heart no. 6), EC was detected above the LOD only in the heart fraction. In the above-mentioned study,30 high findings in the head fractions were not further commented or explained. For comparison, 6 commercially available plum spirit samples were analysed in addition to homemade fruit spirits from small grower distilleries. However, these spirits are usually not 100% fruit distillates but are fortified with ethanol and often contain flavourings, dyes, and other ingredients. According to Czech legislation, a fruit distillate is a beverage produced exclusively by alcoholic fermentation of fruit with subsequent distillation of fruit leaven. It must not be aromatized or fortified with alcohol (the exception is the addition of alcohol to the fruit distillate before the final distillation, but its concentration must not be higher than 30%).31 Of the commercial plum spirit samples investigated, only two represent fruit distillates (nos. 22 and 26). The remaining four samples contained only a minimal quantity of fruit distillate (nos. 23–25, 27) and, therefore, were not expected to have significant EC concentrations, which was subsequently also confirmed. EC was found in only one sample of pure fruit distillate (no. 22), whose concentration (380 μg L−1) did not exceed the European Commission recommended maximum level.
000 μg L−1) was found in the first fraction of distillation, two samples of the head distillation fraction (nos. 20 and 21) were analysed in addition to alcoholic beverages and food. However, EC was not found in any of these samples. The head fractions are rich in low-boiling substances, whereas EC has a relatively high boiling point (182–184 °C) and would thus be distilled mainly in the heart or tail fractions of distillation.8 This is also confirmed by the fact that in one distillation batch (head no. 21 and heart no. 6), EC was detected above the LOD only in the heart fraction. In the above-mentioned study,30 high findings in the head fractions were not further commented or explained. For comparison, 6 commercially available plum spirit samples were analysed in addition to homemade fruit spirits from small grower distilleries. However, these spirits are usually not 100% fruit distillates but are fortified with ethanol and often contain flavourings, dyes, and other ingredients. According to Czech legislation, a fruit distillate is a beverage produced exclusively by alcoholic fermentation of fruit with subsequent distillation of fruit leaven. It must not be aromatized or fortified with alcohol (the exception is the addition of alcohol to the fruit distillate before the final distillation, but its concentration must not be higher than 30%).31 Of the commercial plum spirit samples investigated, only two represent fruit distillates (nos. 22 and 26). The remaining four samples contained only a minimal quantity of fruit distillate (nos. 23–25, 27) and, therefore, were not expected to have significant EC concentrations, which was subsequently also confirmed. EC was found in only one sample of pure fruit distillate (no. 22), whose concentration (380 μg L−1) did not exceed the European Commission recommended maximum level.
A diverse group of other alcoholic beverages, consisting of rum, gin, juniper brandy, vodka, tequila, whisky, brandy, wines, and meads, was also subjected to the analysis. From the total number of 19 samples, EC was quantified only in a sample of commercial mead (no. 31), with a concentration of 468 μg L−1. The other commercial mead sample (no. 33) contained EC below the quantification limit. To the best of our knowledge, the determination of EC in mead has not been performed before, and therefore, our results could not be compared. EC was not detected in other alcoholic beverages, although according to the results presented by EFSA, EC concentrations for white wine samples were up to 30 μg L−1, for gin, vodka, and rum up to 55 μg L−1, and for whisky, tequila, and brandy up to 520 μg L−1.3 Fermented foods generally contain negligible concentrations of EC. The exceptions are soy sauces and vinegars, showing EC concentrations up to 130 μg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24,25 and up to 17 μg L−1,32 respectively. For this reason, two balsamic vinegars, one fermented spirit vinegar, and one soy sauce were analysed. However, EC was not detected in either sample.
24,25 and up to 17 μg L−1,32 respectively. For this reason, two balsamic vinegars, one fermented spirit vinegar, and one soy sauce were analysed. However, EC was not detected in either sample.
4. Conclusions
A derivatization of ethyl carbamate is carried out exclusively with the 9-xanthydrol reagent. By thoroughly optimizing the existing derivatization procedure, the entire process has been considerably simplified and accelerated, with the possibility of performing it in the presence of light, without an extraction step, and regardless of the matrix of the analysed sample. In addition, the optimised reaction conditions provided fewer by-products, thus exhibiting a much smaller background. After mixing 600 μL 20 mmol L−1 9-XA, 100 μL 1.5 mol L−1 HCl, and 400 μL of the sample, this mixture was incubated in the presence of light for 30 min. The product of the derivatization reaction was a very stable fluorescent derivative (stable up to 5 days with minimal loss), which was analysed by high-performance liquid chromatography coupled with a fluorescence detector. The optimally selected separation conditions, employing a Luna C18 analytical column (150 × 3 mm; 3 μm) and a binary mobile phase consisting of 20 mmol L−1 sodium acetate at pH 7.2 (A) and 100% acetonitrile (B), with a flow rate of 0.8 mL min−1 and gradient elution of 0 min – 62% B, 4 min – 70% B, and 5 min – 100% B, significantly reduced the analysis time from the usual 30–60 min to only 5 min. The obtained validation parameters demonstrated that the developed method is sufficiently accurate, precise, robust, and sample matrix independent, with much lower limits of detection and quantification than commonly reported. This method thus represents a very promising potential for the rapid screening of EC not only in final products of the food industry but also during their production.The presence of ethyl carbamate in fifty samples, involving alcoholic beverages of different natures and origins, as well as fermented foods, was monitored using the developed derivatization and chromatographic method. No further sample preparation process was necessary. In eleven samples of spirits, ethyl carbamate was quantified in the concentration range of 14–1503 μg L−1. In the other four samples, the presence of ethyl carbamate was observed below the limit of quantification (<0.84 μg L−1).
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Veronika Šantrůčková: formal analysis, methodology, validation. Jan Fischer: conceptualization, writing – review & editing. Jitka Klikarová: methodology, supervision, conceptualization, data curation, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Faculty of Chemical Technology, University of Pardubice (project No. SGS-024-004) is gratefully acknowledged.References
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Alcohol Consumption and Ethyl Carbamate, International Agency for Research on Cancer, Lyon, France, 2010, ISBN-978-92-832-1296-6 Search PubMed.
- A. Haddow and W. A. Sexton, Influence of carbamic esters (urethanes) on experimental animal tumours, Nature, 1946, 157, 500–503 CrossRef CAS PubMed.
- J. Alexander, G. A. Auðunsson, D. Benford, A. Cockburn, J. Cravedi, E. Dogliotti, A. Di Domenico, M. L. Férnandez-Cruz, P. Fürst, J. Fink-Gremmels, C. Lodovico Galli, P. Grandjean, J. Gzyl, G. Heinemeyer, N. Johansson, A. Mutti, J. Schlatter, R. van Leeuwen, C. Van Peteghem and P. Verger, Opinion of the Scientific Panel on Contaminants in the Food chain on a request from the European Commission on ethyl carbamate and hydrocyanic acid in food and beverages., EFSA J., 2007, 551, 1–44 Search PubMed.
- J. V. Weber and V. I. Sharypov, Ethyl carbamate in foods and beverages: a review, Environ. Chem. Lett., 2009, 7, 233–247 CrossRef CAS.
- C. Wang, M. Wang and M. P. Zhang, Ethyl carbamate in Chinese liquor (Baijiu): presence, analysis, formation, and control, Appl. Microbiol. Biotechnol., 2021, 105, 4383–4395 CrossRef CAS PubMed.
- E. Abt, V. Incorvati, L. Posnick Robin and B. W. Redan, Occurrence of Ethyl Carbamate in Foods and Beverages: Review of the Formation Mechanisms, Advances in Analytical Methods, and Mitigation Strategies, J. Food Prot., 2021, 84, 2195–2212 CrossRef PubMed.
- Decree No. 305/2004 Coll., determining types of contaminating and toxic substances and their admissible levels in foodstuffs, Collection of Laws, Czech Republic, 2004, vol. 100, pp. 6398–6406 Search PubMed.
- Commission Recommendation of 2 March 2010 on the Prevention and Reduction of Ethyl Carbamate Contamination in Stone Fruit Spirits and Stone Fruit Marc Spirits and on the Monitoring of Ethyl Carbamate Levels in These Beverages: (2010/133/EU), Off. J. Eur.Union, 2010, vol. 133, pp. 53–57 Search PubMed.
- X. Xu, Y. Gao, X. Cao, X. Wang, G. Song, J. Zhao and Y. Hu, Derivatization followed by gas chromatography-mass spectrometry for quantification of ethyl carbamate in alcoholic beverages, J. Sep. Sci., 2012, 35, 804–810 CrossRef CAS PubMed.
- D. W. Lachenmeier, U. Nerlich and T. Kuballa, Automated determination of ethyl carbamate in stone-fruit spirits using headspace solid-phase microextraction and gas chromatography-tandem mass spectrometry, J. Chromatogr. A, 2006, 1108, 116–120 CrossRef CAS PubMed.
- AOAC, AOAC official method 944.07 AOAC official method 944.07: ethyl carbamate in alcoholic beverages and soy sauce, AOAC official methods of analysis, J. AOAC Int., 17th edn, 2000, pp. 14–15 Search PubMed.
- K. Zhou, Y. Liu, W. Q. Li, G. L. Liu, N. Wei, Y. M. Sun, W. D. Bai and Z. L. Xu, An improved HPLC-FLD for fast and simple detection of ethyl carbamate in soy sauce and prediction of precursors, Food Anal. Methods, 2017, 10, 3856–3865 CrossRef.
- G. Li, Q. Zhong, D. Wang, X. Zhang, H. Gao and S. Shen, Determination and formation of ethyl carbamate in chinese spirits, Food Control, 2015, 56, 169–176 CrossRef CAS.
- X. Zhao and C. Jiang, Determination of ethyl carbamate in fermented liquids by ultra high performance liquid chromatography coupled with a Q Exactive hybrid quadrupole-orbitrap mass spectrometer, Food Chem., 2015, 177, 66–71 CrossRef CAS PubMed.
- J. M. Leca, V. Pereira, A. C. Pereira and J. C. Marques, A sensitive method for the rapid determination of underivatized ethyl carbamate in fortified wine by liquid chromatography-electrospray tandem mass spectrometry, Food Anal. Methods, 2018, 11, 327–333 CrossRef.
- D. Yang, H. Zhou, Y. Ying, R. Niessner and C. Haisch, Surface-enhanced Raman scattering for quantitative detection of ethyl carbamate in alcoholic beverages, Anal. Bioanal. Chem., 2013, 405, 9419–9425 CrossRef CAS PubMed.
- D. Yang, N. E. Mircescu, H. Zhou, N. Leopold, V. Chis, M. Oltean, Y. Ying and C. Haisch, DFT study and quantitative detection by surface-enhanced Raman scattering (SERS) of ethyl carbamate, J. Raman Spectrosc., 2013, 44, 1491–1496 CrossRef CAS.
- Y. B. Monakhova, T. Kuballa and D. W. Giachmeier, Rapid quantification of ethyl carbamate in spirits using NMR spectroscopy and chemometrics, ISRN Anal. Chem., 2012, 1–5 Search PubMed.
- L. Wu, Y. Wang, S. Zhou, Y. Zhu and X. Chen, Enzyme-induced Cu2+/Cu+ conversion as the electrochemical signal for sensitive detection of ethyl carbamate, Anal. Chim. Acta, 2021, 1151, 338256 CrossRef CAS PubMed.
- X. Lu, N. Zhou and Y. Tian, Spectrophotometric determination of ethyl carbamate through bi-enzymatic cascade reactions, Anal. Methods, 2015, 7, 1261–1264 RSC.
- L. Luo, H. T. Lei, J. Y. Yang, G. L. Liu, Y. M. Sun, W. D. Bai, H. Wang, Y. D. Shen, S. Chen and Z. L. Xu, Development of an indirect ELISA for the determination of ethyl carbamate in Chinese rice wine, Anal. Chim. Acta, 2017, 950, 162–169 CrossRef CAS PubMed.
- Z. Zhang, X. Lu, Y. Tian and N. Zhou, High-sensitive electrochemical determination of ethyl carbamate using urethanase and glutamate dehydrogenase modified electrode, Electroanalysis, 2017, 29, 481–488 CrossRef CAS.
- Q. Xia, C. Yang, C. Wu, R. Zhou and Y. Li, Quantitative strategies for detecting different levels of ethyl carbamate (EC) in various fermented food matrices: An overview, Food Control, 2018, 84, 499–512 CrossRef CAS.
- S. Hasnip, C. Crews, N. Potter, J. Christy, D. Chan, T. Bondu, W. Matthews, B. Walters and K. Patelt, Survey of ethyl carbamate in fermented foods sold in the United Kingdom in 2004, J. Agric. Food Chem., 2007, 55, 2755–2759 CrossRef CAS PubMed.
- P. Wu, X. Pan, L. Wang, X. Shen and D. Yang, A survey of ethyl carbamate in fermented foods and beverages from Zhejiang, China, Food Control, 2012, 23, 286–288 CrossRef CAS.
- Z. Ajtony, N. Szoboslai, L. Bencs, E. Visket and V. G. Mihucz, Determination of ethyl carbamate in wine by high performance liquid chromatography, Food Chem., 2013, 141, 1301–1305 CrossRef CAS PubMed.
- R. R. Madrera and B. S. Valles, Determination of ethyl carbamate in cider spirits by HPLC-FLD, Food Control, 2009, 20, 139–143 CrossRef CAS.
- J. Zhang, G. Liu, Y. Zhang, Q. Gao, D. Wang and H. Liu, Simultaneous determination of ethyl carbamate and urea in alcoholic beverages by high-performance liquid chromatography coupled with fluorescence detection, J. Agric. Food Chem., 2014, 62, 2797–2802 CrossRef CAS PubMed.
- Appendix F: Guidelines for Standard Method Performance Requirements, J. AOAC Int., 2016, vol. 1, pp. 1–16.
- J. C. B. Júnior, R. C. S. Mendonca, J. M. A. T. K. Pereira, J. A. M. Pereira and N. F. F. Soares, Ethyl-carbamate determination by gas chromatography-mass spectrometry at different stages of production of a traditional Brazilian spirit, Food Chem., 2011, 129, 1383–1387 CrossRef.
- Regulation (EU) 2019/787 of the European Parliament and of the Council of 17, April, 2019 on the Definition, Description, Presentation and Labeling of Spirit Drinks, the Use of the Names of Spirit Drinks in the Presentation and Labeling of Other Foodstuffs, the Protection of Geographical Indications for Spirit Drinks, the Use of Ethyl Alcohol and Distillates of Agricultural Origin in Alcoholic Beverages, and Repealing Regulation (EC) No 110/2008, Off. J. Eur.Union, 2019, vol. 130, pp. 1–54.
- K. G. Lee, Analysis and risk assessment of ethyl carbamate in various fermented foods, Eur. Food Res. Technol., 2013, 236, 891–898 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ay00643g |
| This journal is © The Royal Society of Chemistry 2024 |