Open Access Article
Open Access ArticleA validated HPLC-MS/MS method for the quantification of systemic mifepristone after subcutaneous application in mice†
Julia
Tevini
 a,
Sepideh
Aminzadeh-Gohari
abc,
Daniela D.
Weber
a,
Sepideh
Aminzadeh-Gohari
abc,
Daniela D.
Weber
 a,
Luca
Catalano
a,
Victoria E.
Stefan
a,
Luca
Catalano
a,
Victoria E.
Stefan
 ad,
Elisa
Redl
bc,
Chiara
Herzog
ad,
Elisa
Redl
bc,
Chiara
Herzog
 bc,
Roland
Lang
bc,
Roland
Lang
 e,
Martin
Widschwendter
bcfg,
Thomas K.
Felder
e,
Martin
Widschwendter
bcfg,
Thomas K.
Felder
 *hi and
Barbara
Kofler
*a
*hi and
Barbara
Kofler
*a
aResearch Program for Receptor Biochemistry and Tumor Metabolism, Department of Pediatrics, University Hospital of the Paracelsus Medical University, Salzburg, Austria. E-mail: b.kofler@salk.at
bEuropean Translational Oncology Prevention and Screening (EUTOPS) Institute, University Innsbruck, Hall in Tirol, Innsbruck, Austria
cInstitute for Biomedical Aging Research, University Innsbruck, Innsbruck, Austria
dDepartment of Biosciences and Medical Biology, University of Salzburg, Salzburg, Austria
eDepartment of Dermatology and Allergology, University Hospital of the Paracelsus Medical University, Salzburg, Austria
fDepartment of Women's and Children's Health, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden
gDepartment of Women's Cancer, University College London, London, UK
hDepartment of Laboratory Medicine, Paracelsus Medical University, Salzburg, Austria. E-mail: t.felder@salk.at
iInstitute of Pharmacy, Paracelsus Medical University, Salzburg, Austria
First published on 16th July 2024
Abstract
Mifepristone (RU486, MIF) is a synthetic steroidal hormone with progesterone and glucocorticoid receptor antagonistic characteristics. MIF is commonly used for pharmalogical abortions, but also for the treatment of endometrial and endocrine disorders. The goal of the study was to establish and validate a targeted HPLC-MS/MS method for the quantification of MIF and one of its active metabolites metapristone (MET) in plasma after subcutaneous implantation of slow-release MIF pellets in female BALB/c mice. Additionally, we aimed to apply the analytical method to tissue of several organs to understand the tissue-specific distribution of both analytes after release into systemic circulation. Sample preparation comprised a simple liquid–liquid extraction with diethylether and required 100 μl of plasma or homogenates of approximately 50 mg of tissue. The presented HPLC-MS/MS method showed high sensitivity with baseline separation of MIF, MET, and the internal standard levonorgestrel within a run time of only 8.0 minutes and comparable limits of quantification for plasma and tissue homogenates ranging from 40 pg ml−1 to 105 pg ml−1 for MIF and MET. The presented study is suitable for murine plasma and tissues and can be easily applied to human samples.
Introduction
The synthetic steroidal hormone mifepristone (RU486, MIF) is a progesterone receptor as well as a glucocorticoid receptor antagonist.1 MIF is now widely approved as a first-line drug for emergency contraception and medical management of early pregnancy termination.2,3 Additional applications in clinical routine are the symptomatic treatment of endometriosis and endocrine disorders such as Cushing's disease.4,5 In addition to the aspects mentioned above, there are reports that demonstrate an antiproliferative and antimetastatic effect of MIF on different cancer cell lines in vitro.6,7 Moreover, several clinical trials have investigated the efficacy of MIF in humans, underpinning its potential role beyond traditional treatments for various cancer types.8–11After oral administration, MIF is metabolized into three biologically active metabolites, namely N-monodemethyl mifepristone (RU42633, metapristone, MET), N-didemethyl mifepristone (RU42848), and hydroxylated mifepristone (RU42698), with MET being the most predominant metabolite.12,13 The high affinity of MIF for progesterone and glucocorticoid receptors and the stable levels of the metabolites in the blood suggest a combined biological effect of MIF and its metabolites.13
MIF is usually administered orally in single or multiple doses up to 800 mg per day.14,15 Drug-loaded implants, representing an alternative for long-term treatment of gynecological diseases such as endometriosis, have been tested in preclinical studies.16,17 Such drug delivery systems not only allow a steady release of the drug, but also avoid the hepatic first-pass elimination effects of the analyte itself.16
Targeted high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) has been commonly used for the qualitative and quantitative analyses of biological specimens.18,19 Advantages such as low time consumption, superior analytical sensitivity and specificity, and the possibility to measure over a wide concentration range often override traditional assays such as immunoassays.20 We aimed to establish and test a robust targeted HPLC-MS/MS method to quantify MIF and its active metabolite MET in plasma after slow-release, custom-made pellets were implanted under the skin of female BALB/c mice. Furthermore, the analytical method was applied to tissue samples of different organs to ultimately trace the analytes after release into systemic circulation and also to elucidate the tissue-specific distribution of the analytes.
Results
Method validation
Product ion scan experiments of MIF, MET, and the internal standard (ISTD) levonorgestrel (LNG) in 50/50 (vol/vol) methanol/water containing 0.1% formic acid (FA) were used to optimize mass transitions for Selected Reaction Monitoring (SRM). Product ion scan spectra for MIF and MET are shown in Fig. 1. We used the fragments produced upon collision-induced dissociation (CID) with m/z 372.3 and m/z 358.3 as quantifier transitions, as well as fragments with m/z 134.2 and m/z 120.2 as qualifier transitions for MIF and MET, respectively. Product ion scans of the ISTD also provided meaningful spectra (data not shown, see Table 3 for parameters). The retention times of MIF, MET, and LNG were 3.45, 3.20, and 5.06 minutes, respectively. A representative overlay of Extracted Ion Chromatograms (EICs) for MIF, MET, and LNG is shown in Fig. 2.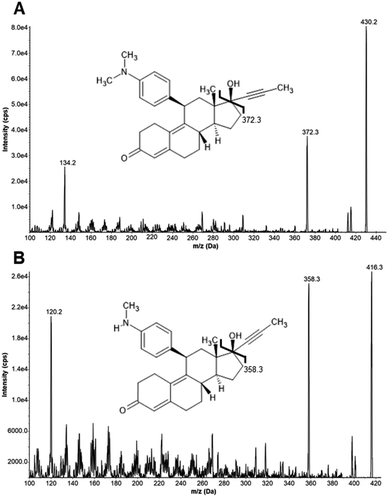 | ||
| Fig. 1 MS/MS product ion scan of MIF (A) and MET (B) at collision energies of 30 and 25 in positive ion mode. MIF: mifepristone, MET: metapristone. | ||
Method selectivity was demonstrated by the analysis of six extracted double blank human plasma samples without added ISTD. No interference was observed in any of the tested samples at the respective retention times (data not shown). Additionally, six extracted blank human plasma samples with added ISTD were analyzed, and again, no interfering peaks were observed (data not shown). Measurements of blank mouse and human plasma showed no difference with regard to potential interferences throughout the duration of the analysis (data not shown).
Subsequent solvent injections following the highest calibrator had minimal carry-over, with less than 0.5% in comparison to the directly preceding highest calibrator. During the validation process, the average ion ratio of quantifier and qualifier for calibrators and QC samples in plasma was 0.692 (95% C.I.: 0.672–0.711) for MIF, 0.770 (95% C.I.: 0.735–0.805) for MET, and 0.692 (95% C.I.: 0.689–0.696) for LNG. The mean ion ratio of quantifier and qualifier for calibrators and QC samples in neat solvent solution were 0.710 (95% C.I.: 0.697–0.724) for MIF, 0.770 (95% C.I.: 0.752–0.787) for MET, and 0.691 (95% C.I.: 0.688–0.693) for LNG.
Average ion ratio of quantifier and qualifier in plasma samples from mice was 0.718 (C.I.: 0.665–0.771) for MIF, 0.900 (C.I.: 0.845–0.954) for MET, and 0.735 (C.I.: 0.727–0.743) for LNG.
Average ion ratio of quantifier and qualifier in tissue samples from mice was 0.718 (C.I.: 0.710–0.731) for MIF, 0.750 (C.I.: 0.698–0.802) for MET, and 0.742 (C.I.: 0.728–0.756) for LNG. Evaluation of the greenness and practicality of the validated method achieved final scores of 0.6 (S-Fig. 1A) and 65.0 (S-Fig. 1B), respectively.
We obtained comparable levels for the lower limit of quantification (LLOQ) and limit of detection (LOD) for MIF and MET in both, plasma and neat solvent solution, respectively. The LLOQ and LOD in plasma for MIF was 0.040 ng ml−1, and 0.013 ng ml−1, 0.045 ng ml−1 and 0.015 ng ml−1 in neat solvent solution. On the other hand, MET had greater LLOQs and LODs in the corresponding matrices. LLOQ and LOD for MET were 0.096 ng ml−1 and 0.032 ng ml−1 in plasma, and 0.105 ng ml−1 and 0.035 ng ml−1 in neat solvent solution.
| Analyte | QC level | Plasma | Neat solvent solution | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Target value, mean area (cps) | Intra-day | Inter-day | Inter-day | |||||||
| Mean area (cps) | Trueness (%) | CV (%) | Mean area (cps) | Trueness (%) | CV (%) | Mean area (cps) | CV (%) | |||
| a Mean, trueness and CV were calculated using independent measurements of n = 6. MIF: mifepristone, MET: metapristone. | ||||||||||
| Mifepristone (MIF) | Low | 5.0 × 103 | 5.8 × 103 | 116 | 11 | 7.4 × 103 | 148 | 25 | 6.4 × 103 | 8 |
| High | 1.2 × 106 | 1.6 × 106 | 133 | 1 | 1.7 × 106 | 142 | 34 | 1.6 × 106 | 9 | |
| Metapristone (MET) | Low | 4.6 × 103 | 3.6 × 103 | 78 | 10 | 4.5 × 103 | 98 | 34 | 5.0 × 103 | 9 |
| High | 9.2 × 105 | 1.1 × 106 | 120 | 2 | 1.0 × 106 | 109 | 39 | 1.3 × 106 | 7 | |
| Condition | Storage time | QC level | Mifepristone (area/area ISTD) | Metapristone (area/area ISTD) | ||
|---|---|---|---|---|---|---|
| Recovery (%) | CV (%) | Recovery (%) | CV (%) | |||
| a Mean percentage recovery compared to reference values on day 0 using independent measurements of n = 4–6. ISTD: internal standard. b Indicates samples below LOQ for the respective QC level. | ||||||
| 4 °C | ||||||
| Autosampler stability | 24 hours | Low | 102 | 25 | 101 | 27 |
| High | 96 | 49 | 98 | 46 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| −20 °C | ||||||
| Freeze/thaw stability | 28 days | Low | 67b | 17 | 41b | 9 |
| High | 134 | 27 | 83 | 19 | ||
| Processed sample stability | 28 days | Low | 123b | 28 | 66b | 26 |
| High | 93 | 35 | 55 | 31 | ||
| 42 days | Low | 127b | 39 | 35b | 15 | |
| High | 98 | 12 | 65 | 30 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| −80 °C | ||||||
| Processed sample stability | 28 days | Low | 160 | 25 | 103 | 22 |
| High | 157 | 22 | 138 | 23 | ||
| 42 days | Low | 147 | 57 | 110 | 32 | |
| High | 81 | 47 | 64 | 37 | ||
Both analytes were stable for short-term storage up to 24 hours at 4 °C (i.e., autosampler stability) in processed samples. Stability was found to be 67% and 134% for MIF as well as 41% and 83% for MET for low and high QC samples after three repeated freeze/thaw cycles within 28 days compared to the first analytical run, respectively. MIF showed acceptable stability in processed samples regardless of sample storage conditions for up to 42 days at both low and high QC levels, respectively. Interestingly, MIF accumulated when stored as processed sample. In general, MET showed a higher degree of instability than MIF in processed samples and was more prone to degradation. Recoveries for MET were lower than for MIF in processed samples stored at −20 °C or −80 °C. Especially, some samples spiked with low QC levels stored at −20 °C were below the LOD.
Application of the validated method
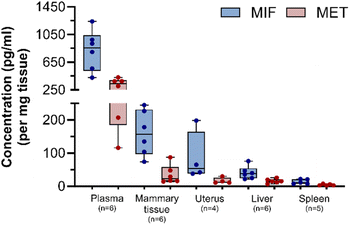
After method validation, six plasma samples and 21 samples derived from different murine tissues were processed to ultimately trace and quantify MIF and MET levels. Mice were treated with 3 mg of subcutaneously implanted MIF pellets. Concentrations of MIF and MET in plasma, mammary glands, uterus, liver, and spleen from MIF-treated animals are shown in Fig. 3. The highest levels of MIF were found in plasma, with mean plasma concentrations of 820 ± 295 pg ml−1 for MIF and 301 ± 119 pg ml−1 for MET. The levels of MIF and MET in tissues upon continuous release were highest in fat-rich organs like mammary glands, with lower amounts detected in the uterus and liver, and the lowest levels in the spleen.Discussion
Beside the use of MIF as an abortifacient and contraceptive drug in women, several studies have demonstrated the antiproliferative effects in breast cancer cells in vitro, but also in experimental models and in human breast tissue after (low-dose) MIF treatment.21,22 A study by Poole et al. reported that treatment of Brca1/p53-deficient mice with MIF delayed or even prevented breast cancer formation.23We hereby established a robust HPLC-MS/MS method for the quantification of MIF and one of its active metabolites, MET, in plasma after subcutaneous MIF pellet implantation in female BALB/c mice. Furthermore, the analytical method was applied to different tissue types, such as mammary glands, uterus, liver, and spleen, to ultimately trace the analytes after release in the systemic circulation.
We chose positive ESI ionization mode to yield extensive fragmentation patterns at an acceptable level of chemical noise. We used the most abundant fragment ions at m/z 372.3, m/z 358.3 and m/z 245.2 as quantifier ions for MIF, MET, and LNG as ISTD, respectively. Our method proved high selectivity, as no endogenous or exogenous substances compromised the ability of the method to specifically capture MIF, MET, and the ISTD.
Several complex sample preparation procedures have been described for the extraction of MIF and derived metabolites from plasma, which require larger sample volumes, which were not feasible in our preclinical setting.24,25 To keep sample preparation as straightforward as possible, we used a liquid–liquid extraction (LLE) protocol with diethylether as the extraction solvent for plasma and tissue homogenates, as described earlier by Homer et al.26 The data obtained with LLE and described herein resulted in reliable detection of the analytes, and, therefore, solid phase extraction was omitted.
Reported LODs and LLOQs for MIF with LLE were 30 and 50 pg on column,26 respectively. The LLOQ of our method for MIF (40 pg ml−1) and MET (96 pg ml−1) in plasma was approximately five-to ten-fold lower than reported for MIF and its metabolites, with a LOQ of 0.5 ng ml−1 in maternal whole blood after pharmacological abortion.27 The LOQ in a neat solvent solution for MIF and MET in tissue was similar as determined for plasma. A targeted method for the analysis of 11 contraceptive progestins and four steroids (i.e., progesterone, testosterone, androstenedione and cortisol) in plasma by high-resolution LC-MS reported LOQs ranging from 2.4 to 78.1 pg ml−1, which are in line with our data.19 Therefore, we conclude that the sensitivity of our approach is within the range of earlier reports for comparable analytes.
Stable isotopically labelled (SIL) ISTDs are chemically identical to the compound and are normally the preferred ISTD option for any quantitative assessment by mass spectrometry. However, there are also several reports using structural analogs as additional variants for ISTDs.28,29 We implemented the structural analog LNG as an ISTD. LNG shows a great structural similarity and exhibits comparable physico–chemical properties as the two analytes of interest. There are some reasons for the implementation of a SIL ISTD instead of a structural analog, such as higher degrees of accuracy, precision, and post-preparation stability.30 The overestimation of MIF in processed samples could be attributed to the implemented structural analog instead of a SIL compound as ISTD. Reasons for an overestimation of an analyte could be a higher degradation rate of the analogous ISTD compared to the SIL variant or the possible elimination of glucuronide-conjugates during storage, which might result in higher levels of MIF. Implementation of deuterated MIF as an ISTD could potentially correct for the overestimation of the compound in processed samples after long-term storage.
The method validation involved QC levels spiked with low and high concentrations of MIF and MET. Stability experiments indicated lower recoveries for QC material, particularly spiked with low levels of MET. These lower recoveries in MET may not only be the consequence of the low spiked concentrations of MET, which were to be expected borderline to the LOQ. In general, MET showed a higher degradation rate at prolonged storage in processed samples compared to MIF, which showed moderate stability when stored at −20 °C and −80 °C, suggesting immediate measurements after sample preparation and no long storage from sample collection to analysis.
Comparing the intermediate precision of plasma and neat solvent solutions showed a larger matrix effect in plasma, leading to lower CVs (in %).
After full validation, the analytical method was effectively applied to samples derived from a preclinical study. MIF is normally administered orally at different doses, and MIF could be detected for at least 4 days in humans after one single administration.31 After oral administration, plasma concentrations of the active metabolite MET are normally higher compared to the parent drug MIF.31 We determined the bioavailability of MIF upon subcutaneous pellet implantation in female BALB/c mice. This approach allows a steady, low-dose-release of MIF for long-term treatments of any progesterone-dependent diseases. MIF pellet implantation led to its metabolization into MET and to a systemic distribution via the blood stream to the organs, with the highest MIF levels in the mammary glands followed by the uterus and liver, whereas the spleen showed the lowest levels of MIF and MET. These observed differences in abundancies of MIF and MET support the assumption that different organs have different capabilities to accumulate MIF, and that MIF accumulates at the desired side of action, namely mammary tissue and uterus, when used as a therapeutic option in breast cancer and endometriosis or even as a potential breast cancer prevention strategy.
To the best of our knowledge, no study has assessed MIF levels in tissues after subcutaneous implantation. Taken together, the validated analytical method enables a fast and simultaneous detection of MIF and MET in plasma and tissue homogenates. Further experiments attributed to MIF's anticancer properties in a preclinical, disease-related setting are currently under investigation.
Experimental
Materials
Mifepristone (MIF, RU486), levonorgestrel (LNG, internal standard (ISTD)) and metapristone (MET, monodemethyl-mifepristone, RU42633) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Axon Medchem (Groningen, Netherlands), respectively. Water, acetonitrile, methanol, and ethanol absolute (>99.7%) as well as formic acid (FA, ≥99%) were obtained from VWR International (Rosny-sous-Bois, France). Diethylether (>99.9%) was purchased from Sigma-Aldrich. All chemicals and solvents were HPLC-MS grade and were used without any further purification.The study was conducted in accordance with the local legislation and institutional requirements. Left-over plasma samples from healthy, female volunteers were used for the validation procedure. Written informed consent was obtained from all volunteers.
Methods
Regarding MIF and MET quantification in tissue samples and the lack of appropriate matrix calibrants, neat standard solutions were used as calibrants (0–40.0 ng ml−1) and QC samples (0.098, 1.5 and 25 ng ml−1).
Sample preparation consisted of a liquid–liquid extraction protocol as described by Homer et al.26 For this purpose, 2 μl of LNG (1 μg ml−1) as an internal standard was added to 100 μl plasma, vortexed briefly before the addition of 900 μl diethylether. After vortexing for 1 minute, all samples were centrifuged at 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 10 minutes at 4 °C. Then, 850 μl of clear supernatant was transferred to a new tube and dried at room temperature under nitrogen. Dried residues were dissolved in 50 μl of 20/80 (vol/vol) acetonitrile/water containing 0.1% FA, vortexed for 1 minute, and centrifuged at 21
000×g for 10 minutes at 4 °C. Then, 850 μl of clear supernatant was transferred to a new tube and dried at room temperature under nitrogen. Dried residues were dissolved in 50 μl of 20/80 (vol/vol) acetonitrile/water containing 0.1% FA, vortexed for 1 minute, and centrifuged at 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 10 minutes at 4 °C. Upper phases were transferred to glass vials and stored at 4 °C until injection.
000×g for 10 minutes at 4 °C. Upper phases were transferred to glass vials and stored at 4 °C until injection.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 10 minutes at 4 °C. The tissue homogenates were then dried at room temperature under nitrogen and reconstituted in 100 μl diethylether. MIF and MET extractions from tissue homogenates were performed as stated above.
000×g for 10 minutes at 4 °C. The tissue homogenates were then dried at room temperature under nitrogen and reconstituted in 100 μl diethylether. MIF and MET extractions from tissue homogenates were performed as stated above.
Selected Reaction Monitoring (SRM) for MIF, MET, and the internal standard LNG in the samples were performed on a TripleQuad5500+ (Sciex, Darmstadt, Germany) in positive ion mode. Quantifier and qualifier ions for MIF, MET, and LNG were recorded with the settings shown in Table 3. Additional mass spectrometric parameters were set as follows: source temperature of 500 °C, collision gas of 9 (AU), curtain gas of 40 (AU), ion source gas 1 of 40 (AU), ion source gas 2 of 60 (AU), ion spray voltage of 5500 V, and entrance potential of 10 V. Analyst software 1.7.1 was used for the acquisition of data. SciexOS (version 1.7.0.36606) was used for data analysis and quantification.
| Analyte | Precursor ion (m/z) | Product ion (m/z) | Dwell time (ms) | Declustering potential (V) | Collision energy (V) | Collision cell exit potential | |
|---|---|---|---|---|---|---|---|
| Mifepristone (MIF) | Quantifier | 430.2 | 372.3 | 80 | 161 | 31 | 20 |
| Qualifier | 430.2 | 134.2 | 80 | 161 | 37 | 16 | |
| Metapristone (MET) | Quantifier | 416.3 | 358.3 | 80 | 131 | 27 | 18 |
| Qualifier | 416.3 | 120.2 | 80 | 131 | 31 | 14 | |
| Levonorgestrel (LNG) | Quantifier | 313.1 | 245.2 | 80 | 136 | 27 | 10 |
| Qualifier | 313.1 | 109.0 | 80 | 136 | 29 | 16 | |
Six double blank human plasma samples without internal standard and six blank human plasma samples with internal standard were injected to address selectivity. The SRM traces of MIF, MET, and the ISTD for all samples were inspected for any interfering substances.
Carry-over effects were evaluated by solvent injections after the injection of the highest calibrant. The SRM traces of MIF, MET, and ISTD in solvent injections were checked for any carry-over peaks.
To assess the linearity of calibration in the range of 0.039–40 ng ml−1, six independent calibration curves were analyzed on six different days. Calibration curves were derived from ratios of the peak areas of MIF or MET to the ISTD using 1/χ-weighted linear least-squares regression of the area ratio versus the concentration ratio. Analytical limits such as limit of detection (LOD) and limit of quantification (LOQ) were theoretically calculated based on and defined as three-fold and nine-fold signal-to-noise ratios (S/N), respectively.
Intra-day, time-dependent intermediate trueness and precision were evaluated by analyzing at least five replicates of low and high QC samples during short-term intervals or on at least six independent days of measurements. Absolute peak areas were compared to peak areas of the analytical run on day 0, where the first and initial analytical run took place.
The robustness of the analytical method was tested by small changes in the parameters of the method like increased column temperature (45 °C instead of 35 °C) and replacement of mobile phase additive from water or 5/95 (vol/vol) acetonitrile containing 0.1% FA to 1 mM ammonium formate in water or 5/95 (vol/vol) acetonitrile.
Moreover, evaluation of the short- and long-term stability of MIF and MET was performed for processed (i.e., post-preparation) plasma QC samples spiked with low and high concentrations of the analytes. Processed sample stability was analyzed after 28 and 42 days of storage at −20 °C and −80 °C. Additionally, the evaluation of processed samples after three freeze/thaw cycles within 28 days as well as the concentrations of samples retained in the autosampler rack for 24 hours were determined. Ratios of absolute peak areas of the analytes to absolute peak areas of the ISTD (area/area ISTD) were compared to ratios of the analytical run on day 0, where stability samples were prepared.
Furthermore, we assessed the greenness and the applicability of the analytical method with the freely available online software tools Analytical GREEnness calculator (AGREE) and blue applicability grade index (BAGI), respectively33,34.
Conclusions
We developed, validated and applied a rapid and robust method to quantify the amount of MIF and its metabolite MET in plasma and tissue samples from BALB/c mice that had a MIF pellet implanted subcutaneously. Sample preparation consisted of a LLE with diethylether and required 100 μl of plasma or homogenates of approximately 50 mg of tissue, resulting in sufficient intensities for subsequent quantification of the analytes. The presented HPLC-MS/MS method showed high sensitivity with baseline separation within a run time of 8.0 min resulting in low LOQs for plasma and tissue extracts. The presented method has been validated for murine plasma and tissue samples but can be easily applied to human samples.Data availability
The data supporting this article have been included as part of the main article and the ESI.†Author contributions
Conceptualization, BK, TKF; methodology, JT, SAG; formal analysis, JT; resources, TKF; investigation, JT, SAG, DDW, LC, VES; data curation, JT; software, JT; validation, JT; visualization, JT; supervision, RL, TKF, BK; writing – original draft, JT; writing – review & editing, SAG, DDW, LC, VES, ER, CH, RL, MW, TKF, BK; funding acquisition, MW, BK; project administration, BK.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the Austrian Cancer Foundation Salzburg and the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Program (grant agreement No. 742432; BRCA-ERC).References
- F. Díaz-Castro, M. Monsalves-Álvarez, L. E. Rojo, A. del Campo and R. Troncoso, Mifepristone for Treatment of Metabolic Syndrome: Beyond Cushing’s Syndrome, Front. Pharmacol., 2020, 11, 429 CrossRef PubMed.
- L. Silvestre, C. Dubois, M. Renault, Y. Rezvani, E. E. Baulieu and A. Ulmann, Voluntary interruption of pregnancy with mifepristone (RU 486) and a prostaglandin analogue. A large-scale French experience, N. Engl. J. Med., 1990, 322(10), 645–648 CrossRef CAS PubMed.
- M. W. Rodger and D. T. Baird, Induction of therapeutic abortion in early pregnancy with mifepristone in combination with prostaglandin pessary, Lancet, 1987, 2(8573), 1415–1418 CrossRef CAS PubMed.
- H. L. Xue, N. Yu, J. Wang, W. J. Hao, Y. Li and M. Y. Liu, Therapeutic effects of mifepristone combined with Gestrinone on patients with endometriosis, Pak. J. Med. Sci., 2016, 32(5), 1268–1272 Search PubMed.
- D. Ciato, A. G. Mumbach, M. Paez-Pereda and G. K. Stalla, Currently used and investigational drugs for Cushing's disease, Expert Opin. Invest. Drugs, 2017, 26(1), 75–84 CrossRef CAS.
- J. Chen, J. Wang, J. Shao, Y. Gao, J. Xu and S. Yu, et al., The Unique Pharmacological Characteristics of Mifepristone (RU486): From Terminating Pregnancy to Preventing, Cancer Metastasis, 2014, 34(5), 979–1000 CAS.
- S. Ponandai-Srinivasan, P. G. Lalitkumar, L. Garcia, S. J. Varghese, J. W. Carlson, K. Gemzell-Danielsson and A. Floter Radestad, Mifepristone mediates anti-proliferative effect on ovarian mesenchymal stem/stromal cells from female BRCA(1-/2-) carriers, Acta Obstet. Gynecol. Scand., 2019, 98(2), 250–261 CrossRef CAS PubMed.
- T. E. Bartlett, I. Evans, A. Jones, J. E. Barrett, S. Haran and D. Reisel, et al., Antiprogestins reduce epigenetic field cancerization in breast tissue of young healthy women, Genome Med., 2022, 14(1), 64 CrossRef CAS PubMed.
- A. Elia, L. Saldain, S. Lovisi, P. Martinez Vazquez, J. Burruchaga and C. A. Lamb, et al., Steroid profile in patients with breast cancer and in mice treated with mifepristone, Endocr.-Relat. Cancer, 2024, 31(2), e230238 CAS.
- A. Elia, L. Saldain, S. I. Vanzulli, L. A. Helguero, C. A. Lamb and V. Fabris, et al., Beneficial Effects of Mifepristone Treatment in Patients with Breast Cancer Selected by the Progesterone Receptor Isoform Ratio: Results from the MIPRA Trial, Clin. Cancer Res., 2023, 29(5), 866–877 CrossRef CAS PubMed.
- M. Llaguno-Munive, M. I. Vazquez-Lopez, R. Jurado and P. Garcia-Lopez, Mifepristone Repurposing in Treatment of High-Grade Gliomas, Front. Oncol., 2021, 11, 606907 CrossRef CAS PubMed.
- O. Heikinheimo, P. L. A. Laähteenmaäki, E. Koivunen, D. Shoupe, H. Croxatto, T. Luukkainen and P. Laähteenmaäki, Metabolism and serum binding of Ru 486 in women after various single doses, Hum. Reprod., 1987, 2(5), 379–385 CrossRef CAS PubMed.
- O. Heikinheimo, Clinical pharmacokinetics of mifepristone, Clin. Pharmacokinet., 1997, 33(1), 7–17 CrossRef CAS.
- O. Heikinheimo, M. Tevilin, D. Shoupe, H. Croxatto and P. Lähteenmäki, Quantitation of RU 486 in human plasma by HPLC and RIA after column chromatography, Contraception, 1986, 34(6), 613–624 CrossRef CAS PubMed.
- Y. E. Shi, Z. H. Ye, C. H. He, G. Q. Zhang, J. Q. Xu, P. F. Van Look and K. Fotherby, Pharmacokinetic study of RU 486 and its metabolites after oral administration of single doses to pregnant and non-pregnant women, Contraception, 1993, 48(2), 133–149 CrossRef CAS PubMed.
- L. Mei, J. Bao, L. Tang, C. Zhang, H. Wang and L. Sun, et al., A novel mifepristone-loaded implant for long-term treatment of endometriosis: in vitro and in vivo studies, Eur. J. Pharm. Sci., 2010, 39(5), 421–427 CrossRef CAS PubMed.
- M. Guarnieri, B. M. Tyler, L. Detolla, M. Zhao and B. Kobrin, Subcutaneous implants for long-acting drug therapy in laboratory animals may generate unintended drug reservoirs, J. Pharm. BioAllied Sci., 2014, 6(1), 38–42 CrossRef PubMed.
- P. J. Jannetto and R. L. Fitzgerald, Effective Use of Mass Spectrometry in the Clinical Laboratory, Clin. Chem., 2016, 62(1), 92–98 CrossRef CAS PubMed.
- C. F. Laszlo, J. Paz Montoya, M. Shamseddin, F. De Martino, A. Beguin and R. Nellen, et al., A high resolution LC-MS targeted method for the concomitant analysis of 11 contraceptive progestins and 4 steroids, J. Pharm. Biomed. Anal., 2019, 175, 112756 CrossRef CAS PubMed.
- M. L. Cawood, H. P. Field, C. G. Ford, S. Gillingwater, A. Kicman, D. Cowan and J. H. Barth, Testosterone measurement by isotope-dilution liquid chromatography-tandem mass spectrometry: validation of a method for routine clinical practice, Clin. Chem., 2005, 51(8), 1472–1479 CrossRef CAS PubMed.
- J. G. M. Klijn, B. Setyono-Han and J. A. Foekens, Progesterone antagonists and progesterone receptor modulators in the treatment of breast cancer, Steroids, 2000, 65(10), 825–830 CrossRef CAS PubMed.
- M. Engman, L. Skoog, G. Soderqvist and K. Gemzell-Danielsson, The effect of mifepristone on breast cell proliferation in premenopausal women evaluated through fine needle aspiration cytology, Hum. Reprod., 2008, 23(9), 2072–2079 CrossRef CAS PubMed.
- A. J. Poole, Y. Li, Y. Kim, S. C. Lin, W. H. Lee and E. Y. Lee, Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist, Science, 2006, 314(5804), 1467–1470 CrossRef CAS PubMed.
- C. Tang, H. C. Bi, G. P. Zhong, X. Chen, Z. Y. Huang and M. Huang, Simultaneous determination of mifepristone and monodemethyl-mifepristone in human plasma by liquid chromatography-tandem mass spectrometry method using levonorgestrel as an internal standard: application to a pharmacokinetic study, Biomed. Chromatogr., 2009, 23(1), 71–80 CrossRef CAS PubMed.
- J. Z. Chen, J. C. Wang, Y. Gao, R. J. Zeng, Z. Jiang and Y. W. Zhu, et al., A novel UPLC/MS/MS method for rapid determination of metapristone in rat plasma, a new cancer metastasis chemopreventive agent derived from mifepristone (RU486), J. Pharm. Biomed. Anal., 2014, 95, 158–163 CrossRef CAS PubMed.
- N. Z. Homer, R. M. Reynolds, C. Mattsson, M. A. Bailey, B. R. Walker and R. Andrew, Quantitative analysis of RU38486 (mifepristone) by HPLC triple quadrupole mass spectrometry, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877(5–6), 497–501 CrossRef CAS PubMed.
- P. Szpot, O. Wachelko, T. Jurek and M. Zawadzki, Determination of Mifepristone (RU-486) and Its Metabolites in Maternal Blood Sample after Pharmacological Abortion, Molecules, 2022, 27(21), 7605 CrossRef CAS PubMed.
- Z. Guo, S. Wang, D. Wei and J. Zhai, Development of a high-performance liquid chromatographic method for the determination of mifepristone in human plasma using norethisterone as an internal standard: application to pharmacokinetic study, Contraception, 2007, 76(3), 228–232 CrossRef CAS PubMed.
- K. A. Smith, S. D. Merrigan and K. L. Johnson-Davis, Selecting a Structural Analog as an Internal Standard for the Quantification of 6-Methylmercaptopurine by LC-MS/MS, J. Appl. Lab. Med., 2018, 3(3), 384–396 CrossRef CAS PubMed.
- E. Stokvis, H. Rosing and J. H. Beijnen, Stable isotopically labeled internal standards in quantitative bioanalysis using liquid chromatography/mass spectrometry: necessity or not?, Rapid Commun. Mass Spectrom., 2005, 19(3), 401–407 CrossRef CAS PubMed.
- Y. N. Teng, R. Q. Dong, B. J. Wang, H. J. Liu, Z. M. Jiang and C. M. Wei, et al., Determinations of mifepristone and its metabolites and their pharmacokinetics in healthy female Chinese subjects, Yaoxue Xuebao, 2011, 46(10), 1241–1245 CAS.
- B. Magnusson and U. Örnemark, Eurachem Guide: the Fitness for Purpose of Analytical Methods - A Laboratory Guide to Method Validation and Related Topics, 2nd edn, 2014, https://www.eurachem.org/ Search PubMed.
- N. Manousi, W. Wojnowski, J. Płotka-Wasylka and V. Samanidou, Blue applicability grade index (BAGI) and software: a new tool for the evaluation of method practicality, Green Chem., 2023, 25(19), 7598–7604 RSC.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, AGREE-Analytical GREEnness Metric Approach and Software, Anal. Chem., 2020, 92(14), 10076–10082 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ay00546e |
| This journal is © The Royal Society of Chemistry 2024 |