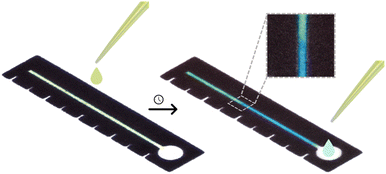
Distance-based detection of paracetamol in microfluidic paper-based analytical devices for forensic application†
Nikaele S.
Moreira
a,
Kemilly M. P.
Pinheiro
 a,
Lucas R.
Sousa
ab,
Gabriel D. S.
Garcia
a,
Federico
Figueredo
a,
Lucas R.
Sousa
ab,
Gabriel D. S.
Garcia
a,
Federico
Figueredo
 b and
Wendell K. T.
Coltro
b and
Wendell K. T.
Coltro
 *abc
*abc
aInstituto de Química, Universidade Federal de Goiás, 74690-900, Goiânia, GO, Brazil. E-mail: wendell@ufg.br
bLaboratorio de Biosensores y Bioanálisis (LABB), Departamento de Química Biológica e IQUIBICEN – CONICET, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires (UBA), Pabellón 2, Ciudad Universitaria, Ciudad Autónoma de Buenos Aires, Argentina
cInstituto Nacional de Ciência e Tecnologia de Bioanalítica, 13084-971, Campinas, SP, Brazil
First published on 20th November 2023
Abstract
Whisky adulteration is a prevalent practice driven by the high cost of these beverages. Counterfeiters commonly dilute whisky with less expensive alcoholic beverages, water, food additives, drugs or pharmaceuticals. Paracetamol (PAR), an analgesic drug that mitigates hangovers and headaches, is commonly used to adulterate whisky. Currently, the primary method for quantifying PAR levels is high-performance liquid chromatography, but this technique is both time consuming and usually generates more residues. In this context, the utilization of miniaturized and portable analytical devices becomes imperative for conducting point-of-care/need analyses. These devices offer several advantages, including portability, user-friendliness, low cost, and minimal material wastage. This study proposes the selective distance-based PAR quantification on whisky samples using a paper-based microfluidic analytical device (μPAD). Colorimetric detection on paper-based platforms offers great benefits such as affordability, portability, and the ability to detect PAR without complicated instrumentation. The optimal detection conditions were achieved by introducing 5 μL of a mixture containing 7.5 mmol L−1 of Fe(III) and K3[Fe(CN)6] into the detection zone, along with 12 μL of whisky samples into the sample zone. The method exhibited linear behavior within the concentration range from 15 to 120 mg L−1, with a determination coefficient of 0.998. PAR was quantified in adulterated samples. The results obtained with the paper-based devices were compared with a referenced method, and no significant differences were observed at a confidence level of 95%. The μPAD allowed to determine ca. 1 drop of pharmaceutical medicine PAR of 200 mg mL−1 in 1 L of solution, demonstrating excellent sensitivity. This method offers cost-effective and rapid analysis, reducing the consumption of samples, reagents, and wastes. Consequently, it could be considered a viable and portable alternative for analyzing beverages at criminal scenes, customs, and police operations, thereby enhancing the field of forensics.
Introduction
Acetyl-p-aminophenol, commonly known as paracetamol (PAR), has emerged as a cornerstone of over-the-counter pain and fever management medications since its clinical introduction in the 1950s.1–3 Paracetamol can be found in diverse formulations, including capsules, effervescent tablets, and dissolved in liquids.4 Initially, its popularity stemmed from its ability to supplant phenacetin while exhibiting lower nephrotoxicity.1 The oral administration results in rapid absorption, with peak plasma distribution achieved in approximately 90 minutes after ingesting. The half-life can be estimated from 1.5 to 2.5 hours, which is related to its first-order kinetics and bioavailability.5 The chemical structure ensures a broad tissue distribution, crosses both the placental and blood–brain barriers, and can be excreted in breast milk.6Paracetamol acts primarily by inhibiting cyclooxygenase (COX) enzymes in the central nervous system, which is critical for intervening in inflammatory mechanisms by converting arachidonic acid to prostaglandins. Although PAR is a freely marketed drug, its sale and dosage depend on local legislation and clinical prescription. Overdoses caused for this drug can lead to various clinical problems, with doses above 150 mg kg−1 causing serious problems, particularly liver failure and nephrological disorders.7
The aetiology of paracetamol use is highly varied, spanning a spectrum of contexts. While it holds significant pharmaceutical importance from both clinical and therapeutic perspectives,8,9 its relevance within forensic investigations should not be underestimated.10,11 This can be associated with a notable increase in notification of overdose-related fatalities and poisonings associated with its consumption.10,11 Moreover, its potential interaction with other toxic substances can further complicate poisoning cases.12,13 Consequently, this medication can serve as a target analyte in criminal investigations, potentially shedding light on intentional or inadvertent overdose scenarios in specific instances. A notable example is the pivotal role in authenticating alcoholic beverages that PAR has assumed nowadays. Many studies report the use of PAR to mask the effects of hangovers arising from cases of high-value alcoholic beverages being adulterated with lower-cost substitutes, particularly for whisky.14–16 Then, swift and precise monitoring of this compound, particularly in biological specimens or commercial substrates is imperative.
As defined by the Pharmacopoeias, high-performance liquid chromatography (HPLC) is the recommended method to quantify PAR.17–19 However, it requires a bulky and relatively expensive instrumentation. On the other hand, there is a growing interest in simpler and less expensive alternatives for the quantitative analysis of PAR outside the laboratory. Recently, electrochemical tools have received the most attention in this regard.20–23 Shiroma et al.20 proposed a microfluidic device for the electrochemical detection of PAR and 4-aminophenol using readily available materials. Kavai et al.21 also developed a sensor based on graphene and polyetherimide (PEI) substrate. While these devices offer advantages such as low resource consumption, high sensitivity, affordability, compact size, and portability, they may still require more robust equipment and trained personnel for data interpretation.
Colorimetric techniques provide a cost-effective and simplified approach for analysing PAR.24 Paper-based microfluidic analytical devices (μPADs) are ideal for such analyses, especially for point-of-care applications.25–27 Among the colorimetric methods, distance-based detection has gained attention due to its practicality in instrument-free semi-quantitative analysis.27 Distance-based assays require only two steps: sample addition and analysis. The operational process begins with the fabrication of the device using traditional μPAD techniques,28,29 with a designated loading zone at the end for the sample.30 Colorimetric detection reagents are then applied along the flow microchannel by either spray application or pipetting. In general, the color formation progresses to a certain distance in the channel, allowing quantitative assessment of concentration.24,27,30
Cate et al.30 pioneered this technique; they developed a μPAD to measure glucose, nickel, and glutathione using three different chemical detectors: enzymatic reactions, metal complexation, and nanoparticle aggregation, respectively. This technique is versatile and finds applications in clinical analysis and areas such as food quality control,31 separation,28 and environmental analysis.29,32 This technique has been particularly reported for the analysis of some target compounds such as iron,29 copper,29 aluminum,33 nickel,29 potassium,34 calcium,35 chloride,36,37 ascorbic acid38 and mercury,39 where complexation reactions occur with their specific chromogen. Examples of classical acid-base,40 argentometric,37,41 and redox42 titrations have been also reported using distance-based measurements on paper microfluidic devices.
Associated with the advantage of paper platforms, the present study presents the development of μPADs through wax printing43 for the colorimetric analysis of PAR by distance-based measurements. The colorimetric protocol is an adaptation of the spectrophotometric method reported by Pourakarim et al.44 based on the redox reaction using the Fe(III) and K3[Fe(CN)6] systems as colorimetric reagents. This adaptation brings, in an unprecedented way, the use of an environmentally friendly platform for the analysis of PAR at the point-of-care/need, reducing the need for equipment as well as the generation of waste.
Materials and methods
Chemicals reagents
Iron chloride, potassium hexacyanoferrate(III), ethanol, PAR, (−)-scopolamine N-butyl bromide, and ibuprofen were purchased from Sigma Aldrich Co. (Saint Louis, MO, USA). Midazolam maleate was acquired by a donation from the National Institute of Criminalistics from Federal Police (Brasília, DF, Brazil). All reagents were analytical grade and used without further purification. Analytical solutions were prepared with ultrapure water processed through a water purification system (Direct-Q3, Millipore, Darmstadt, Germany) with a resistivity of ≥18.2 MΩ cm. All solutions added in the circular zone contained 4% ethanol to simulate the diluted whiskey sample.Distance-based μPAD design and fabrication
A wax printer (Colorqube 8870, Xerox, Norwalk, CT, USA) was used to fabricate the microfluidic distance paper-based devices on Whatman™ chromatographic paper type 1. The specific layout was designed using Corel Draw X8 software and printed using the wax printer. The device design was projected with a circular zone (6 mm diameter) connected to a rectangular channel (40 mm × 1.5 mm). Parallel to the channel, a ruler with marks every 10 and 5 mm was included for distance measurement. Afterward, the devices were heated using an oven model 402 (Ethik Technology, Vargem Grande Paulista, SP, BR) for 2 minutes at 130 °C (ref. 43) to ensure efficient wax penetration into the paper. Then, the back of the device was sealed with the help of adhesive tape to prevent solution leakage. The chromogenic reagents were directly applied to the channels, sealing the sample application zone with insulating tape. Distance results were measured using a digital calliper model 316119 (MTX – Tools world, Russia).Optimization studies
To investigate the optimal conditions for colorimetric distance analysis, first, determining the optimal volume required to saturate the μPAD channel was performed introducing 3, 5, 7, or 10 μL of a red dye mixture containing 4% ethanol. To ensure complete fill of the entire device in the analysis, a sequential addition of volumes of the colored solution (8, 10, 12, or 15 μL) was introduced at the circular zone. The potassium ferricyanide and iron chloride concentrations were systematically adjusted within the channel to ascertain the most conductive reaction conditions. The time needed for the solution to dry was investigated by capturing consecutive images over a span of 20 to 60 min using an office scanner and software (model G4050, Hewlett-Packard, Palo Alto, CA, USA) with a resolution of 600 dpi. The images were then restored to their original color and converted to TIFF format. Pixel intensities were analysed in two regions of the channel (with and without PAR) using Corel PhotoPaint X8 software in the CMYK color system, specifically in the cyan color channel.Detection of PAR in whisky samples
The viability of the proposed device for forensic screening was assessed using one original and three adulterated samples of whiskey. The three simulated samples were created by adding different proportions of liquid PAR (200 mg mL−1) (CIMED Industria de Medicina LTDA, SP, Brazil), sourced from a local vendor. These samples were diluted tenfold in ultrapure water before being applied to the device. The analyses conducted through the distance assay were compared with a reference method, carried out following the procedure outlined by Pourkarim et al.44 with some modifications, utilizing a spectrophotometer (UV-M51 – BEL Engineering®, Monza, Italy). Briefly, the reference method consisted of adding 2.0 mL of the standard solution to construct the curve in the range of 0.2–10.0 mg L−1 or of the diluted whiskey sample into a test tube. Then 397.6 μL of 20 mmol L−1 iron chloride and 39.48 μL of 20 mmol L−1 potassium hexacyanoferrate(III) were added. Finally, ultrapure water was added to complete a volume of 2.8 mL. After 3 minutes at room temperature (25 °C), the solution was transferred to a quartz cuvette, and absorbance was measured in a UV-vis spectrophotometer at 715 nm.To evaluate the device's performance, a recovery test was conducted using three distinct concentrations of PAR. Repeatability studies, both intra-day (1 day) and inter-day (three days), were performed to assess accuracy and precision, respectively. Furthermore, the colorimetric sensor's specificity for PAR was validated by introducing potential adulterant drugs commonly found in alcoholic beverages, including midazolam maleate, (−)-scopolamine N-butyl bromide, and ibuprofen. Each drug was incorporated at a concentration of 50 mg L−1 in samples containing 4% ethanol, and the assessment was carried out in quadruplicate.
Evaluation of the μPAD with distance-based detection regarding the principles of green analytical chemistry (GAC)
To evaluate the proposed analytical methodology and the reference method about the GAC Principles, an Analytical GREEnness (AGREE) calculator was used.45 The results were shown as coloured pictograms with a numerical scale from 0 to 1.Results and discussion
Principle of paracetamol detection in distance-based device
The reaction described in this protocol is based on an adaptation of methodology first utilized by Abed et al.46 and subsequently modified by Pourkarim et al.44 In accordance with the oxidation potential of paracetamol, the color-forming reaction relies on the production of Prussian blue, KFe[Fe(CN)6]. Paracetamol reduces Fe(III) (reaction (I)), which later reacts with potassium hexacyanoferrate(III) – K3[Fe(CN)6], – to produce Prussian blue (reaction (II)). The product quantity directly depends on the amount of paracetamol used.| C8H9NO2 + 2Fe3+ → C8H7NO2 + 2Fe2+ | (I) |
| Fe2+ + K3[Fe(CN)6] → KFe[Fe(CN)6] + 2K+ | (II) |
To perform the reaction on μPAD, the reagents iron chloride (Fe(III)) and potassium hexacyanoferrate(III) were incorporated into the channel according to the optimizations realized. The sample containing paracetamol was added into the application zone located at the channel extremity and as flowed by capillary action, reacted with the other components to form Prussian blue, as exemplified in Fig. 1.
Optimization studies
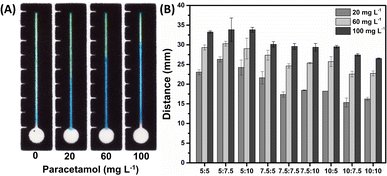
The volumes applied in the rectangular channel and circular sample zone of the μPAD were optimized. Fig. S2A† shows that the 5 μL was sufficient to fill the channel but for 7 and 10 μL the solution filled the sample zone. In Fig. S2B,† it is possible to appreciate that solution volume of 12 μL applied to the sample zone was sufficient to fill the entire device. Therefore, 5 μL and 12 μL volumes were selected to be added to the channel and circular sample zone, respectively. Notably, this device significantly reduces the consumption of reagents and samples, being of considerable potential for a more sustainable analytical method.Then, optimizations were performed to assess the ideal concentration of chromogens applied in the channel to form Prussian blue. The concentrations of Fe(III) and potassium hexacyanoferrate(III) were evaluated by measuring the color formation response through distance in the channel. To achieve this, standard solutions of PAR were established with concentrations of 20, 60, and 100 mg L−1, varying in predefined ratios within a range of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–10
5–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mmol L−1 for both Fe(III) and potassium hexacyanoferrate(III) concentrations, respectively.
10 mmol L−1 for both Fe(III) and potassium hexacyanoferrate(III) concentrations, respectively.
As depicted in Fig. 2A, the solution containing a ratio of 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 mmol L−1 demonstrated a distinct transition from the green color, which is the result of a slower kinetic reaction between the Fe(III) species, to the formation of Prussian blue in the presence of paracetamol and potassium hexacyanoferrate(III). This correlation can also be observed quantitatively in the graph in Fig. 2B for concentration of 20 mg L−1. Additionally, this concentration minimized the washing effect when compared to other concentrations, as observed in ESI Fig. S2A.† Therefore, a mixture containing 7.5 mmol L−1 of Fe(III) and 7.5 mmol L−1 of potassium hexacyanoferrate(III) was maintained as the optimal condition for the concentration of reagents in the subsequent steps, since a greater distance difference indicates a sensor with higher sensitivity (Fig. S2B†).
7.5 mmol L−1 demonstrated a distinct transition from the green color, which is the result of a slower kinetic reaction between the Fe(III) species, to the formation of Prussian blue in the presence of paracetamol and potassium hexacyanoferrate(III). This correlation can also be observed quantitatively in the graph in Fig. 2B for concentration of 20 mg L−1. Additionally, this concentration minimized the washing effect when compared to other concentrations, as observed in ESI Fig. S2A.† Therefore, a mixture containing 7.5 mmol L−1 of Fe(III) and 7.5 mmol L−1 of potassium hexacyanoferrate(III) was maintained as the optimal condition for the concentration of reagents in the subsequent steps, since a greater distance difference indicates a sensor with higher sensitivity (Fig. S2B†).
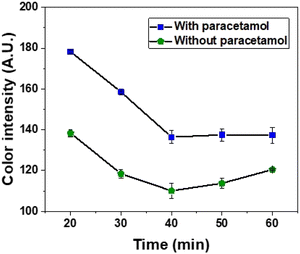
The kinetic extent of Prussian blue formation is rapid, necessitating an investigation into the optimal time frame for extracting distance information from the device. For this study, an assay was conducted using a paracetamol standard with concentrations ranging from 20 to 100 mg L−1. The solution was applied to the zone, and fluidic transport occurred nearly instantaneously. Due to the contrast effect with the chromogenic compounds, which inherently produce a greenish color, the color intensity at the green/blue boundary was evaluated. Here, the blue color corresponds to the effective reaction response with PAR.
The drying time of the sample solution was assessed between 20 and 60 min by interrogating pixel intensities in both the blue color (positive reaction for PAR), and green color (negative reaction for PAR). Time intervals shorter than 20 min were not evaluated, as the sample had not completely traveled the channel, resulting in a humid region that posed challenges for accurate distance readings. As shown in Fig. 3, it is evident that after 20 min, the pixel intensity in both regions decreases, indicating a fading of color within the channel. After 40 min, the blue region maintained a constant pixel intensity, whereas in the green region, the pixel intensity increased, bringing the negative values closer to the positive values, indicating the presence of PAR. One hypothesis is that this increase may be attributed to the presence of oxygen in the air, which can facilitate the reduction of Fe(III) to Fe(II), a common state for iron species.44 Consequently, it can be stated that beyond 40 min, the response may undergo fluctuations, making it challenging to accurately determine the distance traveled in the presence of PAR. Therefore, 20 min was deemed the optimal drying time, enabling a clearer differentiation between the blue and green hues.
Analytical performance
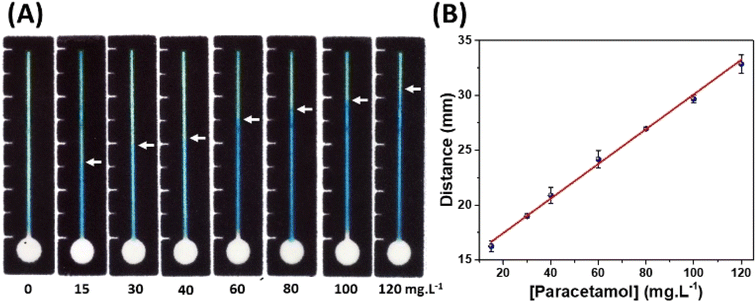
After optimizing the μPAD conditions, the linearity of the method was evaluated in the concentration range between 15 and 120 mg L−1. This range was selected to identify adulteration of alcoholic beverages.14Fig. 4A and B show the recorded optical images and the resulting analytical curve. As it can be seen in Fig. 4B, the proposed distance-based measurement for determining PAR revealed a satisfactory linear behavior, with a correlation coefficient of 0.998. The relative standard deviation (RSD) values ranged from 0.3 to 3.3% (n = 3), thus indicating acceptable precision since the traveled distances were measured with a digital calliper. In addition, it does not require complicated instrumentation, demonstrating great potential for forensic applications on field.The sensitivity obtained in the distance-based device for PAR was 0.16 (mm/mg L−1), and the limit of quantification (LOQ) was calculated based on the ratio between ten times the ordinate intercept deviation and the slope of the analytical curve obtaining a value of 13.1 mg L−1. This value indicates that the method can quantify ca. 1 drop of a commercial formulation of paracetamol diluted to 1 L of solution. In addition, the limit of detection (LOD) obtained was 3.9 mg L−1, calculated by a ratio between three times the ordinate intercept deviation and the slope of the analytical curve. Thus, the method can detect the addition of less than half of 1 drop (0.3 drop) in 1 L. Therefore, the proposed method is promising for forensic application, identifying drink adulteration.
Although the LOD value of the proposed method is higher than the ones using paper-based devices with electrochemical detection,14,20,47–49 it presents simplicity and practicality in its use. Also, this method does not require complicated equipment to perform the analysis, since the measurements can be realized with the naked eye or using a commercial ruler, allowing the use in the point-of-care/need. Additionally, even presenting a higher LOD, the achieved detectability level is enough to identify the presence of PAR in beverages, once that the adulteration certainly will contain more than one drop per dose (ca. 200 mL).
Concerning the studies reported in the literature for colorimetric detection of PAR (Table 1), the detectability is higher than the proposed. However, most methods require a spectrophotometer50–52 or smartphone,53 which represent an additional cost. Furthermore, the previously reported tests require larger amount of sample and reagents. For example, Shaban and collaborators50 used a total volume of 1000 μL for analysis, while the proposed device consumes 17 μL. In this way, a reduced among of waste is generated, thus making it promise to be a greener analytical method.
| Detection method | LOD (mg L−1) | Device material | Sample | Reference |
|---|---|---|---|---|
| a NR – not reported. | ||||
| Spectrometer | 0.048 | Cuvette | Human urine and panadol tablets | 50 |
| Smartphone | 0.0084 | Microcentrifuge tubes | Pharmaceutical formulations and human urine | 53 |
| Spectrometer | 0.9 | Tube | Saliva | 51 |
| Naked eyes | NR | PMMA-based analytical device | Real herbal medicine | 54 |
| Scanner | NR | Paper-based analytical device | NR | 60 |
| Spectrometer | NR | Cuvette | Pharmaceutical formulation | 52 |
| Distance measurements | 3.9 | Paper-based analytical device | Whisky | This work |
Notably, the colorimetric methods present in the literature were applied to analyse urine50,53 and saliva samples,51 from pharmaceutical formulation tables52,53 and real herbal medicine.54 However, to the best of our knowledge, this is the first study applied in the evaluation of adulterated whiskies samples with PAR that uses distance-based colorimetric detection. According to the Federal Police and Civil Police, counterfeit whiskeys consist of cachaça, caramel colouring, flavourings, and painkillers.55,56 One of the most sold painkillers in Brazil is PAR, which is easily accessible and low cost. Thus, the proposed device can be used in the forensic field and can be explored for quality control of pharmaceutical formulations.
Recovery tests and the repeatability for the investigation of the method's accuracy and precision were performed. The recovery values found ranged from 91.0 to 99.7% (Table S1†). Intra-day (n = 10) and inter-day (three days, n = 30) repeatability revealed the RSD values were lower than 4.0% and 6.0%, respectively. These values demonstrate that the proposed method presents good accuracy and precision.57
Device specificity for paracetamol detection
In the test of interferences, whiskey samples were enriched with substances used as adulterants in alcoholic beverages.58,59 Thus, each sample was doped with 50 mg L−1 of midazolam maleate, (−)-scopolamine N-butyl bromide, and ibuprofen. As seen in Fig. S3,† there is no well-defined formation of the blue color, indicating that these substances do not interfere with the method for detecting PAR.Analysis of real samples
A quantitative detection of PAR was performed in four whisky samples to demonstrate the method's viability for forensic applications. One original whiskey sample and three adulterated with random volumes of commercial PAR were analyzed. The paracetamol concentrations found using the proposed method were compared with the reference method44 and revealed an experimental error ranging from 1.0 to 4.4% (Table S2†). Also, the results were not significantly different at 95% confidence level, demonstrating no statistical difference between the two methodologies. Therefore, distance-based μPADS for paracetamol detection demonstrated outstanding potential for identifying adulteration in whisky samples, which can be promising for another study with alcoholic beverages. This system provides fast and portable analysis, which can be useful to perform analysis on customs, police operations, and crime scenes.Evaluation of the μPAD with distance-based detection regarding the principles of green analytical chemistry (GAC)
The evaluation of the proposed analytical methodology about the GAC Principles using AGREE classifies the analytical method conformed 12 principles on a scale of 0 to 1, with 0 indicating that the method is in disagreement and 1 in agreement with GAC. Furthermore, agreement with each of the principles is presented in the pictogram through colours, with green being the greenest method and red being the least ecological method.45 As shown in Fig. 5A and B, the proposed distance-based μPADs with paracetamol detection proved to be more environmentally friendly, with a GAC value of 0.76, when compared to the reference method, which obtained a value of 0.61. Therefore, μPADs with distance-based detection present, in addition to reliable analytical performance, practicality, and simplicity in their application, which is desirable for application in the field, is a method that cooperates with the principles of GAC.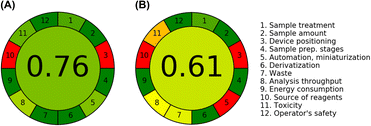 | ||
| Fig. 5 Evaluation and comparison of μPADs (A) and reference method (B) regarding GAC principles using the AGRESS software. | ||
Conclusions
This study described the use of a μPAD based on distance measurements for paracetamol analysis. The μPAD is easy and low-cost to fabricate, and to obtain the distance just a ruler or a calliper is necessary. The proposed method was successfully applied to original e adulterated whisky samples analysis demonstrating the efficiency on real samples analysis. Paracetamol was detected and quantified, and the results were compared with a spectrophotometer observing no statistical difference between both methodologies. The proposed method not only is a promising tool to perform field analysis for forensic applications, but it is also in agreement with the GAC parameters. Besides that, this study can be applied to detect and quantify the presence of PAR in other beverages to evaluate adulterations.Author contributions
Nikaele S. Moreira: formal analysis, investigation, methodology, software, validation, visualization, writing – original draft, writing – review & editing; Kemilly M. P. Pinheiro: conceptualization, investigation, methodology, validation, visualization, writing – review & editing; Lucas R. de Sousa: investigation, visualization, writing – original draft, writing – review & editing; Gabriel D. S. Garcia: methodology; Federico Figueredo: resources, writing – review & editing; Wendell K. T. Coltro: conceptualization, funding acquisition, project administration, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support from CNPq (grants 307554/2020-1, 405620/2021-7 and 382604/2022-9), CAPES (finance code 001), FAPEG (grant 202310267000258) and INCTBio (grant 465389/2014-7). CNPq and CAPES are also thanked for the scholarships and researcher fellowships granted to the authors.References
- S. P. Clissold, Drugs, 1986, 32, 46–59 CrossRef PubMed.
- U. Freo, C. Ruocco, A. Valerio, I. Scagnol and E. Nisoli, J. Clin. Med., 2021, 10, 3420 CrossRef PubMed.
- K. Brune, B. Renner and G. Tiegs, Eur. J. Pain, 2015, 19, 953–965 CrossRef PubMed.
- S. H. Youssef, D. Mohamed, M. A. M. Hegazy and A. Badawey, BMC Chem., 2019, 13, 1–15 CrossRef PubMed.
- B. Ward and J. M. Alexander-Williams, Acute Pain, 1999, 2, 139–149 CrossRef.
- D. R. B. S. Novi, C. B. Vidigal, K. F. Moura, D. G. Da Silva, A. F. L. Serafim, R. M. Klein, E. G. Moreira, D. C. C. Gerardin and G. S. Ceravolo, J. Cardiovasc. Pharmacol., 2021, 78, 858–866 CrossRef CAS PubMed.
- G. W. Przybyła, K. A. Szychowski and J. Gmiński, Clin. Exp. Pharmacol. Physiol., 2021, 48, 3–19 CrossRef PubMed.
- M. C. Frost and M. E. Meyerhoff, Curr. Opin. Chem. Biol., 2002, 6, 633–641 CrossRef CAS.
- L. F. Prescott, Br. J. Clin. Pharmacol., 2023, 1–8 Search PubMed.
- G. Mannocchi, R. Tittarelli, F. Pantano, F. Vernich, M. Pallocci, P. Passalacqua, M. Treglia and L. T. Marsella, Toxics, 2022, 10, 486 CrossRef PubMed.
- C. Cole, L. Jones, J. McVeigh, A. Kicman, Q. Syed and M. Bellis, Drug Test. Anal., 2011, 3, 89–96 CrossRef CAS PubMed.
- D. P. Rocha, R. M. Dornellas, E. Nossol, E. M. Richter, S. G. Silva, M. H. P. Santana and R. A. A. Munoz, Electroanalysis, 2017, 29, 2418–2422 CrossRef CAS.
- M. C. Marra, B. M. De Castro Costa, R. A. A. Munoz, M. H. P. Santana, A. O. Maldaner, É. D. Botelho, W. K. T. Coltro and E. M. Richter, Anal. Methods, 2018, 10, 2875–2880 RSC.
- A. A. Dias, T. M. G. Cardoso, C. L. S. Chagas, V. X. G. Oliveira, R. A. A. Munoz, C. S. Henry, M. H. P. Santana, T. R. L. C. Paixão and W. K. T. Coltro, Electroanalysis, 2018, 30, 2250–2257 CrossRef CAS.
- J. Yao, X. Xu, L. Liu, H. Kuang, Z. Wang and C. Xu, Analyst, 2021, 146, 6228–6238 RSC.
- G. Poulladofonou, C. Freris, A. Economou and C. Kokkinos, Anal. Chem., 2022, 94, 4087–4094 CrossRef.
- United States Pharmacopeia – Brazil | USP, https://www.usp.org/usp-brazil, (accessed 25 September 2023) Search PubMed.
- M. Idris, C. John, P. Ghosh, S. K. Shukla and T. R. R. Baggi, J. Anal. Sci. Technol., 2013, 4, 1–6 CrossRef.
- J. T. Franeta, D. Agbaba, S. Eric, S. Pavkov, M. Aleksic and S. Vladimirov, Farm, 2002, 57, 709–713 CrossRef PubMed.
- L. Y. Shiroma, M. Santhiago, A. L. Gobbi and L. T. Kubota, Anal. Chim. Acta, 2012, 725, 44–50 CrossRef.
- M. S. Kavai, L. F. de Lima and W. R. de Araujo, Mater. Lett., 2023, 330, 133211 CrossRef.
- H. Guo, L. Sun, M. Yang, M. Wang, N. Wu, T. Zhang, J. Zhang, F. Yang and W. Yang, Anal. Methods, 2021, 13, 4994–5002 RSC.
- N. İslamoğlu, İ. E. Mülazımoğlu and A. Demir Mülazımoğlu, Anal. Methods, 2023, 15, 4149–4158 RSC.
- G. G. Morbioli, T. Mazzu-Nascimento, A. M. Stockton and E. Carrilho, Anal. Chim. Acta, 2017, 970, 1–22 CrossRef PubMed.
- A. W. Martinez, S. T. Phillips, M. J. Butte and G. M. Whitesides, Angew. Chem., Int. Ed., 2007, 46, 1318–1320 CrossRef PubMed.
- E. Noviana, T. Ozer, C. S. Carrell, J. S. Link, C. McMahon, I. Jang and C. S. Henry, Chem. Rev., 2021, 121, 11835–11885 CrossRef PubMed.
- N. Nuchtavorn, T. Rypar, L. Nejdl, M. Vaculovicova and M. Macka, TrAC, Trends Anal. Chem., 2022, 150, 116581 CrossRef CAS.
- H. Li, D. Han, G. M. Pauletti and A. J. Steckl, Lab Chip, 2014, 14, 4035–4041 RSC.
- D. M. Cate, S. D. Noblitt, J. Volckens and C. S. Henry, Lab Chip, 2015, 15, 2808–2818 RSC.
- D. M. Cate, W. Dungchai, J. C. Cunningham, J. Volckens and C. S. Henry, Lab Chip, 2013, 13, 2397–2404 RSC.
- K. Katelakha, V. Nopponpunth, W. Boonlue and W. Laiwattanapaisal, Biosensors, 2021, 11, 90 CrossRef CAS.
- W. Dungchai, Y. Sameenoi, O. Chailapakul, J. Volckens and C. S. Henry, Analyst, 2013, 138, 6766–6773 RSC.
- M. P. Nguyen, S. P. Kelly, J. B. Wydallis and C. S. Henry, Anal. Chim. Acta, 2020, 1100, 156–162 CrossRef CAS PubMed.
- C. T. Gerold, E. Bakker and C. S. Henry, Anal. Chem., 2018, 90, 4894–4900 CrossRef CAS.
- H. Shibata, Y. Hiruta and D. Citterio, Analyst, 2019, 144, 1178–1186 RSC.
- K. Phoonsawat, N. Ratnarathorn, C. S. Henry and W. Dungchai, Analyst, 2018, 143, 3867–3873 RSC.
- M. Rahbar, B. Paull and M. Macka, Anal. Chim. Acta, 2019, 1063, 1–8 CrossRef CAS PubMed.
- X. Liu, W. Hou, J. Zhao, L. Zhang, A. Li and R. Ma, New J. Chem., 2023, 47, 16735–16740 RSC.
- A. Apilux, W. Siangproh, N. Praphairaksit and O. Chailapakul, Talanta, 2012, 97, 388–394 CrossRef.
- L. G. A. Dias, L. C. Duarte, K. M. P. Pinheiro, N. S. Moreira and W. K. T. Coltro, Talanta Open, 2023, 7, 100216 CrossRef.
- L. Cai, Z. Ouyang, J. Song and L. Yang, ACS Omega, 2020, 5, 18935–18940 CrossRef PubMed.
- M. Granica and Ł. Tymecki, Anal. Chim. Acta, 2020, 1136, 125–133 CrossRef.
- E. Carrilho, A. W. Martinez and G. M. Whitesides, Anal. Chem., 2009, 81, 7091–7095 CrossRef PubMed.
- F. Pourkarim, E. Rahimpour, M. Khoubnasabjafari, V. Jouyban-Gharamaleki, A. Gharakhani and A. Jouyban, Chem. Pap., 2021, 75, 2901–2906 CrossRef.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, Anal. Chem., 2020, 92, 10076–10082 CrossRef CAS.
- S. S. Abed, J. Al-Nahrain Univ. Sci., 2009, 12, 46–53 CrossRef.
- L. Y. Shiroma, M. Santhiago, A. L. Gobbi and L. T. Kubota, Anal. Chim. Acta, 2012, 725, 44–50 CrossRef CAS.
- J. R. Camargo, I. A. A. Andreotti, C. Kalinke, J. M. Henrique, J. A. Bonacin and B. C. Janegitz, Talanta, 2020, 208, 120458 CrossRef CAS.
- T. R. de Oliveira, W. T. Fonseca, G. de Oliveira Setti and R. C. Faria, Talanta, 2019, 195, 480–489 CrossRef CAS.
- S. M. Shaban, B. S. Moon and D. H. Kim, Environ. Technol. Innovation, 2021, 22, 101517 CrossRef CAS.
- J. Ryan, C. Mandelt, H. Wade and S. D. Vasikaran, Ann. Clin. Biochem., 2009, 46, 149–151 CrossRef CAS.
- P. Nagaraja, K. C. Srinivasa Murthy and K. S. Rangappa, J. Pharm. Biomed. Anal., 1998, 17, 501–506 CrossRef CAS PubMed.
- R. Jain, R. R. Jha, A. Kumari and I. Khatri, Microchem. J., 2021, 162, 105870 CrossRef CAS.
- R. Pratiwi, S. E. Suherman, R. A. L. Poongan, M. Mutakin and A. N. Hasanah, Int. J. Anal. Chem., 2018, 2018, 8 Search PubMed.
- G1 – Brasil, https://g1.globo.com/Noticias/Brasil/0,MUL347785-5598,00-PESSOAS+SAO+PRESAS+POR+FALSIFICACAO+DE+BEBIDA+NO+DF.html, (accessed 13 November 2023) Search PubMed.
- F. Seligman and F. de S. Paulo, https://www1.folha.uol.com.br/fsp/cotidian/ff2111201009.htm, (accessed 13 November 2023).
- Elemental Analysis Manual (EAM) for Food and Related Products | FDA, https://www.fda.gov/food/laboratory-methods-food/elemental-analysis-manual-eam-food-and-related-products, (accessed 25 September 2023) Search PubMed.
- M. V. Paschoarelli, M. S. Kavai, L. F. de Lima and W. R. de Araujo, Talanta, 2023, 255, 124214 CrossRef PubMed.
- H. I. Santos, K. M. P. Pinheiro, E. M. Richter and W. K. T. Coltro, Talanta, 2024, 266, 124960 CrossRef.
- T. L. Mako and M. Levine, J. Chem. Educ., 2019, 96, 1719–1726 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental optimization and comparison with reference technique. See DOI: https://doi.org/10.1039/d3ay01739g |
| This journal is © The Royal Society of Chemistry 2024 |