Open Access Article
Open Access ArticleA promising electrochemical sensor based on PVP-induced shape control of a hydrothermally synthesized layered structured vanadium disulfide for the sensitive detection of a sulfamethoxazole antibiotic†
Mingjiao
Shi
ab,
Peizheng
Shi
b,
Xinxin
Yang
a,
Ningbin
Zhao
b,
Mengfan
Wu
b,
Jing
Li
c,
Chen
Ye
bde,
He
Li
bde,
Nan
Jiang
bde,
Xiufen
Li
 f,
Guosong
Lai
f,
Guosong
Lai
 g,
Wan-Feng
Xie
g,
Wan-Feng
Xie
 h,
Li
Fu
h,
Li
Fu
 i,
Gang
Wang
i,
Gang
Wang
 j,
Yangguang
Zhu
*bf,
Hsu-Sheng
Tsai
*ck and
Cheng-Te
Lin
j,
Yangguang
Zhu
*bf,
Hsu-Sheng
Tsai
*ck and
Cheng-Te
Lin
 *bde
*bde
aSchool of Materials Science and Engineering, Shanghai University, Shanghai, 200072, P.R. China
bQianwan Institute, Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences, Ningbo 315201, China
cSchool of Physics, Harbin Institute of Technology, 150001, Harbin, China. E-mail: hstsai@hit.edu.cn
dCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
eKey Laboratory of Marine Materials and Related Technologies, Zhejiang Key Laboratory of Marine Materials and Protective Technologies, Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences, Ningbo 315201, China. E-mail: linzhengde@nimte.ac.cn
fLaboratory of Environmental Biotechnology, School of Environmental and Civil Engineering, Jiangnan University, Wuxi, 214122, China. E-mail: zhuyangguang@nimte.ac.cn
gHubei Key Laboratory of Pollutant Analysis & Reuse Technology, College of Chemistry and Chemical Engineering, Hubei Normal University, Huangshi, 435002, China
hCollege of Electronics and Information, University-Industry Joint Center for Ocean Observation and Broadband Communication, Qingdao University, Qingdao, 266071, China
iCollege of Materials and Environmental Engineering, Hangzhou Dianzi University, Hangzhou 310018, China
jDepartment of Microelectronic Science and Engineering, School of Physical Science and Technology, Ningbo University, Ningbo, 315211, China
kLaboratory for Space Environment and Physical Sciences, Harbin Institute of Technology, 150001, Harbin, China
First published on 7th November 2023
Abstract
The presence of sulfamethoxazole (SMX) in natural waters has become a significant concern recently because of its detrimental effects on human health and the ecological environment. To address this issue, it is of utmost urgency to develop a reliable method that can determine SMX at ultra-low levels. In our research, we utilized PVP-induced shape control of a hydrothermal synthesis method to fabricate layer-like structured VS2, and employed it as an electrode modification material to prepare an electrochemical sensor for the sensitive determination of SMX. Thus, our prepared VS2 electrodes exhibited a linear range of 0.06–10.0 μM and a limit of detection (LOD) as low as 47.0 nM (S/N = 3) towards SMX detection. Additionally, the electrochemical sensor presented good agreement with the HPLC method, and afforded perfect recovery results (97.4–106.8%) in the practical analysis. The results validated the detection accuracy of VS2 electrodes, and demonstrated their successful applicability toward the sensitive determination of SMX in natural waters. In conclusion, this research provides a promising approach for the development of electrochemical sensors based on VS2 composite materials.
Introduction
Sulfamethoxazole (SMX) is a synthetic antibiotic with a broad antibacterial spectrum of activity.1,2 It is particularly, effective against Staphylococcus aureus and Escherichia coli. It finds widespread application in the medical, agricultural, and animal husbandry industries, making it one of the most utilized sulfonamide medications.3 However, according to the World Health Organization's International Agency for Research on Cancer (IARC), sulfamethoxazole has been classified as a Group 3 carcinogen in their 2017 list of carcinogens.4 The reason behind this classification lies in the fact that sulfamethoxazole is a large molecular organic compound that exhibits limited degradation capabilities. Living organisms in a sulfamethoxazole-contaminated environment might experience compromised growth and reproductive capabilities.5 It is true that these antibiotics can enter the human body through the enrichment effect of the food chain, thereby posing a risk to human health. Therefore, ensuring the sensitive detection of sulfamethoxazole is of utmost importance.Presently, the detection methods for sulfamethoxazole mainly consist of spectrophotometry, high-performance liquid chromatography (HPLC),6,7 enzyme-linked immunosorbent assay (ELISA),8 capillary electrophoresis,9,10 and several other techniques. Nevertheless, the application of traditional detection methods in antibiotic detection technology is significantly impeded by the need for costly equipment, intricate sample preparation, and time-intensive detection procedures.11 On the other hand, electrochemical detection techniques have gained significant attention due to their inherent advantages of being fast, straightforward, portable, and cost-effective.12 The careful selection of appropriate electrode modification materials is a critical step in the electrochemical detection process.
In the past few years, the field of inorganic nanomaterials has witnessed a remarkable surge in interest, largely attributed to their exceptional physicochemical properties.13 In particular, transition metal dichalcogenides (TMDCs) like MoS2, WS2, and VS2 have gained considerable attention ascribed to their distinct morphology and graphene-like properties.14,15 These materials demonstrate excellent chemical, physical, optical, mechanical, magnetic, and electrical characteristics.16 Layered transition metal dichalcogenide (TMD) crystals are composed of interconnected layers connected by strong in-plane covalent bonds.17 Meanwhile, the S–M–S interlayer structure, which consists of sulfur molecules (S) and transition metals (M), is held together by comparatively weaker out-of-plane van der Waals forces.18,19 The distinctive structural characteristics of TMD crystals contribute to their extensive range of physicochemical properties, notably a significant specific surface area and impressive conductivity.20 These attributes have facilitated their exceptional performance in various fields, including electrocatalysis, lithium-ion batteries, optoelectronic devices, and energy storage.21 Consequently, TMDs have become a focal point of intense research and development in the past few decades.22 Among different TMDs, vanadium disulfide (VS2) stands out as an exemplary material. Recent advancements in first-principles theoretical calculations and experimental research have demonstrated the remarkable properties of two-dimensional layered VS2, which include excellent conductivity, a high aspect ratio, ultrathin edges, and favorable mechanical characteristics.23,24 Consequently, VS2 fulfills the essential criteria for an effective electrochemical sensor. Karthik et al. developed a promising non-enzymatic electrochemical sensor for detecting hydrogen peroxide based on a simple sonochemical synthesis of novel grass-like vanadium disulfide.16 Vilian et al. employed a facile hydrothermal method to synthesize electrodes comprising gold nanoparticles decorating VS2-reduced graphene oxide sheets, achieving a calibration dynamic range of 10–340 nM and a limit of detection (LOD) as low as 0.44 nM towards sulfadiazine detection.25 Therefore, the incorporation of two-dimensional VS2 nanocrystals holds great potential in the manufacturing of electrochemical sensors.26 However, to propose a promising VS2-based electrochemical platform, comprehensive studies focusing on the structural regulation and electrochemical optimization of VS2 are still deficient and necessary.
In this work, we utilized the PVP-induced shape control of a hydrothermal synthesis method to fabricate layered structured VS2, and employed it for the first time as an electrode modification material to prepare an electrochemical sensor for sensitive SMX detection in natural waters. The features and properties of the synthesized material were studied using various characterization methods. Various electrochemical techniques were further applied for evaluating the detection performance of the electrochemical sensor. In particular, the differential pulse voltammetry (DPV) analysis demonstrated that the sensor exhibited high sensitivity and a wide linear range, indicating its substantial potential for environmental water analysis applications.
Experimental
Chemicals
Phosphate buffer solution (10× PBS, containing 1.37 M NaCl, 26.8 mM KCl, 81.0 mM Na2HPO4, and 17.6 mM KH2PO4) was purchased from Sangon Biotech (Shanghai) Co., Ltd (Shanghai, China). H2SO4, potassium ferricyanide (K3[Fe(CN)6]), potassium hexacyanoferrate(II) (K4[Fe(CN)6]), and ammonium hydroxide (NH3·H2O) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Polyvinylpyrrolidone (PVP), ammonium metavanadate (NH4VO3), and thioacetamide (TAA) were purchased from Sigma Aldrich (Shanghai) Trading Co., Ltd (Shanghai, China). Sulfamethoxazole, trimethoprim (TMP), furazolidone (FRZ), erythromycin (ERY), chloramphenicol (CPL), and glucose (Glu) were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd (Shanghai, China). All chemical reagents used in our experiments were of analytical grade and used without further purification. Milli-Q deionized water was used throughout our tests.Fabrication of VS2 electrodes
The preparation method of VS2 nanosheets is presented as follows. 1.0 g PVP was dissolved in a mixture comprising 30 mL of deionized water and 2 mL of NH3·H2O. Then 0.234 g of NH4VO3 and 1.5 g of TAA were added into the mixture sequentially. After being subjected to magnetic stirring at room temperature for 1 hour, the mixture was transferred into a Teflon-lined stainless-steel autoclave with a volume of 50 mL, and heated at 180 °C for 20 h. The product was collected by employing centrifugation and washed repeatedly with anhydrous ethanol. Subsequently, the washed product was vacuum-dried at 60 °C for 24 h. Finally, VS2 nanosheets were obtained by annealing for 2 h under a N2 atmosphere at 300 °C at a rate of 2 °C min−1.27 Finally, a 1 mg mL−1 dispersion of tVS2 nanosheets was prepared and preserved for the following experiments.Before the electrode modification, glassy carbon electrodes (GCEs) with a 3 mm diameter were first polished using a 0.05 μm alumina slurry. Subsequently, the electrodes were subjected to ultrasonication in deionized water and ethanol to ensure thorough cleaning. Following the thorough cleaning, GCEs were scanned via repetitive potential range scanning from −1 to 1 V in 0.5 M sulfuric acid at a scan rate of 100 mV s−1 to activate the electrodes. Finally, a 8 μL droplet of aqueous VS2 dispersion was drop-coated on GCE and dried at 60 °C for 10 min to obtain VS2 electrodes.
Analytical tests
For the experiment, 1× PBS obtained after 10 times dilution of 10× PBS was used as the electrolyte. The VS2 electrodes were used as working electrodes, while a saturated calomel electrode (SCE) and a Pt electrode were employed as the reference electrode and counter electrode, respectively. Cyclic voltammetry (CV), DPV and electrochemical impedance spectroscopy (EIS) tests were performed to analyse the voltammetric responses of various modified electrodes. CV curves were conducted from −0.2 to 0.6 V in five cycles at a scan rate of 0.2 V s−1, while DPV tests were performed from 0.7 to 1.1 V with the parameters as: pulse period of 0.5 s, step potential of 0.004 V and amplitude of 0.005 V. EIS was performed in the range of 0.1–100 kHz with a 10 mV amplitude of AC voltage. All our electrochemical tests were conducted using a CHI660e electrochemical workstation (Shanghai Chenhua Co., LTD, China). The zeta potential of the VS2 dispersion and SMX aqueous solution were determined using a dynamic light scattering-zeta instrument (Zeta potential, Zetasizer Ultra, UK). HPLC (e2695-2998, Waters, Ireland) was applied for the SMX determination in real samples.Characterization
Field emission scanning electron microscopy (FE-SEM QUANTA 250 FEG, FEI, Hills-boro, OR, USA), energy dispersive spectroscopy (EDS), and transmission electron microscopy (TEM, JEM-2100F, JEOL, Japan) were employed to observe the morphology of the modified material. Raman spectroscopy (Renishaw inVia Reflex, Renishaw plc, Wotton-under-Edge, London, UK) with a laser wavelength of 532 nm and X-ray diffraction (XRD, D8 Advance, Germany) with Cu Kα radiation (λ: 1.54 Å) were applied for the elemental analysis and crystalline structure analysis of the synthesized material. X-ray photoelectron spectroscopy (XPS, Axis SUPRA+, Shimadzu, Japan) was used to indicate the surface compositions and chemical states.Results and discussion
PVP-induced shape control of the preparation of layer-by-layer stacked VS2 nanosheets
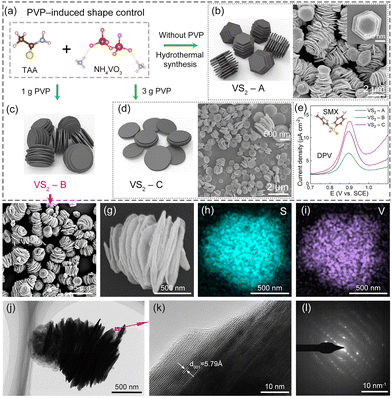
The schematic diagram shown in Fig. 1 depicts the preparation process of layer-by-layer stacked VS2 nanosheets by a PVP-induced shape control method. After thoroughly combining 0.234 g of NH4VO3 with 1.500 g of TAA, the mixture was dissolved in an alkaline solution by adding 0, 1.000, and 3.000 g PVP, respectively. The resulting mixture underwent a hydrothermal process, where it was kept at 180 °C for 20 h. Following that, annealing was carried out at 300 °C for 2 h to synthesize layer-by-layer stacked VS2 nanosheets, named VS2 – A, VS2 – B and VS2 – C. The morphology evolution of VS2 nanosheets induced by PVP is illustrated in Fig. 2a. The morphology of VS2 – A prepared without PVP showed an elongated and hexagonal structure, as seen in Fig. 2b. Interestingly, the morphology of VS2 transformed from a hexagonal to a circular structure and its stacked layers were found to be exfoliated into a structure like an accordion with the addition of PVP, as presented in Fig. 2c. PVP, as an anionic surfactant, possesses hydroxyl groups at the ends of its molecules and can be adsorbed onto VS2 layers through electrostatic interactions.21 With the aid of PVP, the stacked layers of VS2 were inclined to be exfoliated through sonication in the dispersion. By increasing the amount of PVP to 3.000 g, stacked VS2 nanosheets were further exfoliated into fewer layers, as shown in Fig. 2d. The purity of the prepared VS2 samples was confirmed by TEM elemental mapping and elemental composition, as presented in Fig. S1 and Table S1.†To determine the optimal structure of VS2 induced by PVP, the DPV curvesof VS2 – A, VS2 – B and VS2 – C electrodes were constructed for 10 μM SMX, as shown in Fig. 2e. The results demonstrated that the VS2 – B electrodes exhibited the best electrochemical response toward SMX, which was chosen as the optimized VS2 nanosheet for the following experiments. Compared to the stacked layers of VS2 – A, the VS2 – B layers tended to be exfoliated by the induced regulation of PVP. A higher specific surface area of VS2 – B electrodes enhanced the adsorption capability of SMX, presentinga better electrochemical response.28 When regulated with excess PVP, the VS2 – C electrodes showed a dispersed structure with fewer layers, decreasing its adsorption capability towards SMX. Thus, VS2 – B indicates the priority towards SMX detection.
Characterization of layer-by-layer stacked VS2 nanosheets
The SEM images in Fig. 2f and g depict the morphology of VS2. VS2 was composed of vertically stacked nanosheets, exhibiting a diameter and thickness of up to 500–1000 nm and 20–30 nm approximately. Elemental analysis using an energy-dispersive spectrometer (EDS) confirmed the presence and uniform distribution of V and S elements, as shown in Fig. 2h and i. TEM and high-resolution TEM (HRTEM) images revealed the crystal structure of VS2, as presented in Fig. 2j and k. TEM images revealed that the VS2 nanosheets were stacked in the [001] direction, with an interlayer spacing of approximately 5.79 Å.21 This value closely matches the normal interplanar spacing (5.76 Å) of the original VS2 (001) plane.29 Electron diffraction was employed to demonstrate the polycrystalline nature of the synthesized sample, as indicated in Fig. 2l.Fig. 3a presents the crystal structure (the unit cell and molecules) of VS2, referenced from the PDF card number 89-1640. From the observation in Fig. 3a, each layer of vanadium disulfide consists of a vanadium layer sandwiched between two sulfur layers, and the sandwiched structures are connected by van der Waals forces.18
From the XRD patterns (Fig. 3b), all the observed diffraction peaks in the graph can be assigned to VS2 (JCPDS#89-1640), with lattice constants of a = b = 3.22 Å and c = 5.76 Å. These findings provide further confirmation of the successful formation of VS2 nanosheets with regular interlayer spacing. The chemical structure of the synthesized VS2 was investigated using Raman spectroscopy, as shown in Fig. 3c. It displayed characteristic vibration bands at nearly 281 and 405 cm−1, corresponding to E1g and A1g modes, respectively.30 These modes represent the in-plane vibration (E1g) and out-of-plane vibration (A1g) of the S–V–S bonds,31 as depicted in Fig. 3d. In fact, the Raman signals of VS2 demonstrate an analogy to other transition metal sulfides within the vanadium group.32,33
In Fig. 3e, the high-resolution XPS spectrum of V 2p unveiled two prominent peaks centered at approximately 525.0 and 517.5 eV. These peaks are assigned to V 2p1/2 and V 2p3/2, respectively, affirming the existence of the V4+ oxidation state.34 Additionally, the XPS spectrum also exhibits two smaller peaks located at approximately 522.2 and 514.2 eV. These minor peaks suggested the presence of a small quantity of V2+ ions, which can be attributed to the reducing properties of organic amines and the strong reducing nature of hydrogen sulfide generated from the decomposition of thioacetamide in the preparation.35 The XPS spectrum of S 2p shown in Fig. 3f showed distinct peaks at around 162.7 and 161.5 eV, corresponding to S 2p1/2 and S 2p3/2 of S2− species.36 Peaks located at around 163.7 and 164.5 eV are attributed to the S0 species, while the peak at around 169.6 eV can be assigned to the sulfate species.37 These results indicated that slight oxidation and contamination with elemental sulfur and sulfates occurred in the VS2 sample due to its exposure to air.
Performance optimization of VS2/GCE electrodes toward SMX detection
To investigate the electrochemical performance of VS2 towards SMX, the electron transfer ability of VS2 electrodes needs to be understood. The oxidation peak with potential 0.90 V was designated as the characteristic peak for SMX electrochemical analysis. To assess the interfacial charge transfer ability, EIS was performed on GCE and VS2 electrodes in 10 mM [Fe(CN)6]3−/4− (Fig. 4a). Through the model simulation according to its Nyquist curves, the equivalent circuit model of VS2 electrodes is equivalent to Rs(Qdl(Rct(Qc(Rc·ZW)))). In particular, Rct denotes the interfacial electron transfer resistance and determines the electron transfer ability on various electrodes.38 The Rct values of VS2 electrodes and GCE were 567.2 and 5079.0 Ω, respectively. The Rct value of GCE was higher than that for VS2 electrodes, demonstrating that VS2 enhances the charge transfer ability of electrodes. The fitted values of various parameters in the model are presented in Table S2.†To further enhance the detection performance of VS2 electrodes, analytical parameters such as the modified mass of VS2 on the electrode, scan rates, and electrolyte pH were treated in optimization. To determine the appropriate mass of VS2 during electrode preparation, the DPV curves of VS2 electrodes modified with various masses of VS2 as 2, 4, 6, 8, 10 and 12 μg were constructed for PBS containing 10 μM SMX. As shown in Fig. 4b, 8 μg of VS2 was selected for further experiments. To evaluate the electrochemical behaviors on the VS2 electrode surface, CV responses were obtained in 10 mM [Fe(CN)6]3−/4− at scan rates ranging from 20 to 200 m Vs−1 (Fig. 4c). The peak current density of Iox and Ire presented a linear increment with the square root of scan rate v1/2 (Fig. 4d), indicating that the redox reaction on VS2 electrodes was diffusion-controlled.39 The influence of electrolyte pH on the voltammetric response of VS2 electrodes was examined using the DPV curves with the pH ranging from 5 to 9, as shown in Fig. 4e. With an increase in the electrolyte pH, the peak potential shifted negatively, indicating that the redox reaction on the electrode is determined by a proton transfer process.40 The oxidation of the amine group in SMX proceeded via a pH-dependent reaction.41 The maximum peak current was achieved at pH = 6 and was selected as the optimal pH. According to the results presented in Fig. 4f, the linear relationship of potential Epcversus pH was fitted as Epc (V) = 1.108 − 0.032 pH (R2 = 0.990). The obtained slope value is smaller than the Nernstian value (59 mV pH−1), demonstrating the same number of protons and electrons involved in the redox reaction.42
Electrochemical determination of SMX using the VS2 electrode and its sensing mechanism
The DPV method was employed for the quantitative electrochemical detection of SMX on the VS2 electrodes, as depicted in Fig. 5a and Fig. S2.† The peak current values on VS2 electrodes exhibited an upward trend as the SMX concentration increased. A calibration curve for SMX on the VS2 electrodes was acquired within the linear range of 0.06–10 μM, as illustrated in Fig. 5b. The calibration equation is Ipc (μA cm−2) = 1.10c(SMX) (μM) + 2.850 (R2 = 0.995). Based on the equation, the LOD for SMX on VS2 electrodes is determined to be 0.047 μM (S/N = 3). DPV tests were performed for the SMX detection on three individual electrodes. Compared to other electrodes used for SMX detection, as presented in Table 1, our SMX electrode achieved a comparatively low LOD for SMX detection and has prospects for application in a wider range for SMX detection.| Electrode material | Techniques | Linear range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|
| CNTs | DPV | 50–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10.0 | 43 |
| CNTs/PPy | DPV | 1.99–10.9 | 0.413 | 44 |
| CNTs/N,N-dimethylfomamide | Amperometry | 0.50–110 | 0.094 | 5 |
| CNTs/Ti-3 | DPV | 0.2–100 | 0.060 | 45 |
| CNTs/Prussian blue nanocubes | DPV | 1.0–10.0 | 0.038 | 46 |
| Graphene | DPV | 1.0–10.0 | 0.090 | 47 |
| Graphene/ZnO | DPV | 1.0–220 | 0.400 | 48 |
| GO/ITO | DPV | 0.1–50 | 0.060 | 41 |
| GO/NiO | CV | 0.08–550 | 0.040 | 49 |
| GO/ZnO | DPV | 0.1–1.5 | 0.029 | 50 |
| VS2 | DPV | 0.06–10 | 0.047 | This work |
The sensing mechanism model of VS2 electrodes is proposed to better illustrate the reason why VS2 electrodes can be a good candidate for the electrochemical platform towards the sensitive detection of SMX, as presented in Fig. 5c. The voltammetric technique was applied for the determination of SMX on VS2 electrodes in river water samples. During the electrochemical oxidation of SMX on the VS2 electrode interface, two electrons and protons are involved in the reaction process. Herein, on behalf of carbon-based nanomaterials with good electrical conductivity, CNTs and rGO were used to compare their charge transfer ability with VS2. DPV curves of VS2, graphene and CNT electrodes with 10 μM SMX are presented in Fig. 5d, demonstrating the superior catalytic response of VS2 toward SMX. Based on the fitting of Nyquist curves of CNTs, rGO and VS2 electrodes presented in Fig. 5e, the VS2 electrodes showed the lowest Rct values. These results demonstrated the charge transfer ability of VS2 to be more than those of CNTs and rGO at the electrode interface, validating the prospect of VS2 as a promising base material for modifying electrodes for sensitive SMX detection. The result is also consistent with the results presented in Table 1. Furthermore, CNT or rGO based nanocomposite modified electrodes presented good detection performance towards SMX as presented in Table 1, supporting the great potential of VS2 based nanocomposite modified electrodes for the sensitive detection of SMX in the future.
Repeatability, anti-interference, and real sample analysis
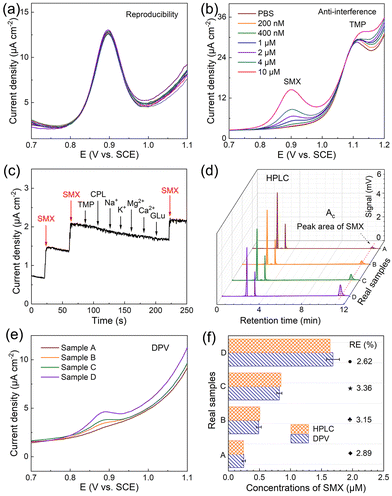
To evaluate the reproducibility of VS2 electrodes for SMX detection (10 μM), DPV tests were conducted on 10 different electrodes in the potential range of 0.7–1.1 V. The DPV curves remained stable at a potential of 0.90 V approximately and maintained good overlapping of the current density, as shown in Fig. 6a. The relative standard deviation (RSD) of the peak current was approximately 2.6%, demonstrating the good repeatability of VS2 electrodes. Interference experiments were performed using the DPV curves(Fig. 6b), where 10 μM of TMP was used as a typical interfering substance within a concentration range of 0.1–10 μM. Notably, to achieve a continuous synergistic effect of antibacterial activity, the combination of TMP and SMX was usually utilized in the clinical treatment.51 The interference of TMP in SMX detection in real samples is necessary.The result demonstrated that TMP did not affect the SMX detection, supporting the potential practical applicability of VS2 electrodes. Besides, the i–t technique was performed on VS2 electrodes in electrolyte containing 10 μM SMX and other interfering substances such as 50 μM CPL, 50 μM FRZ, 50 μM ERY, 50 μM Glu, 100 μM Na±, 100 μM K±, 100 μM Mg2±, and 100 μM Ca2±, as shown in Fig. 6c and Fig. S3.† The results revealed the exceptional resistance of VS2 electrodes to interference from other molecules during electrochemical detection.
To validate the detection accuracy of VS2 electrodes by the DPV method, HPLC was employed to detect SMX in the same real samples.
The relative error (RE) is used as the evaluation index calculated using the equation RE = |(a − b)/b| × 100%, where a and b represent the average values of DPV and HPLC, respectively (μM). Real water samples were prepared by adding a certain mass of SMX to tap water, designated as sample A, B, C, and D. HPLC results were obtained by the computation of the calibration equation, which was determined as Ac = 8.58 × 103C − 206 (R2 = 0.999). Herein, C is the concentration of SMX (μM), and Ac denotes the peak area at a retention time of 11.3 minutes (μV s), as presented in Fig. 6d. The DPV curves of VS2 electrodes were constructed for the same real samples, as shown in Fig. 6e. Based on the data comparison of the two methods presented in Fig. 6f, the REs for samples A, B, C and D were determined as 2.89%, 3.15%, 3.36%, and 2.62%, respectively, validating the good accuracy of our electrodes. To further demonstrate the practical applicability of VS2 electrodes, the recovery performance in river water samples was determined using the standard addition method.52 As shown in Table S3,† the prepared SMX sensor exhibited good recovery rates (97.4–106.8%) and low RSD values (1.16–1.79%), demonstrating its significant capability for real sample analysis.
Conclusions
This study constructed a sensitive SMX sensor based on layered structured VS2 nanosheets prepared by the PVP-induced shape control of a hydrothermal synthesis method. Compared to the bare GCE, the VS2 electrode exhibited higher sensitivity towards SMX oxidation, demonstrating excellent SMX detection performance within a detection range of 0.06–10 μM and a LOD as low as 0.047 μM. The good recovery rates (97.4–106.8%) and practicality of SMX detection in natural waters were validated using the VS2 electrode. Furthermore, the interference resistance of the VS2/GCE electrode was examined by adding other potential interfering substances. In conclusion, this research provides a promising approach for the development of electrochemical sensors based on VS2 composite materials.Author contributions
Mingjiao Shi: data curation, methodology, formal analysis, and writing – original draft. Peizheng Shi: software, investigation, and data curation. Xinxin Yang: methodology, data curation, and supervision. Ningbin Zhao: investigation and data curation. Mengfan Wu: software and data curation. Jing Li: investigation and data curation. Chen Ye: data curation and methodology. He Li: methodology, supervision, and data curation. Nan Jiang: methodology, supervision, writing – review and editing. Xiufen Li: methodology, data curation, writing – review and editing. Guosong Lai: resources, validation, and software. Wan-Feng Xie: data curation, supervision, writing – review and editing. Li Fu: methodology, supervision, and data curation. Gang Wang: visualization, methodology, and supervision. Yangguang Zhu: methodology, data curation, writing – review and editing. Hsu-Sheng Tsai: visualization, supervision, writing – review and editing. Cheng-Te Lin: conceptualization, writing – review and editing, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (52272053, 52075527 and 52102055), the National Key R&D Program of China (2022YFA1203100, 2022YFB3706602 and 2021YFB3701801), the Ningbo Key Scientific and Technological Project (2021Z120, 2021Z115, 2022Z084 and 2022Z191), the Yongjiang Talent Introduction Program of Ningbo (2021A-037-C and 2021A-108-G), the Youth Fund of Chinese Academy of Sciences (JCPYJ-22030), the China Postdoctoral Science Foundation (2020M681965 and 2022M713243), the CAS Youth Innovation Promotion Association (2020301), Science and Technology Major Project of Ningbo (2021ZDYF020196 and 2021ZDYF020198), the Project of Chinese Academy of Science (XDA22020602 and ZDKYYQ2020001), and the Ningbo 3315 Innovation Team (2019A-18-C).References
- P. S. Kumar, G. Padmalaya, N. Elavarasan and B. Sreeja, Chemosphere, 2023, 332, 138814 CrossRef PubMed.
- P. Balasubramanian, R. Settu, S.-M. Chen and T.-W. Chen, Microchim. Acta, 2018, 185, 396 CrossRef PubMed.
- A. Lamaoui, A. Karrat and A. Amine, Sens. Actuators, B, 2022, 368, 132122 CrossRef CAS.
- M. Ramya, P. S. Kumar, G. Rangasamy, V. U. Shankar, G. Rajesh and K. Nirmala, Environ. Res., 2023, 216, 114463 CrossRef CAS PubMed.
- B.-S. He and W.-B. Chen, J. Braz. Chem. Soc., 2016, 27, 2216–2225 CAS.
- E. Karageorgou, N. Manousi, V. Samanidou, A. Kabir and K. G. Furton, Food Chem., 2016, 196, 428–436 CrossRef CAS PubMed.
- N. Sun, S. Wu, H. Chen, D. Zheng, J. Xu and Y. Ye, Microchim. Acta, 2012, 179, 33–40 CrossRef CAS.
- A. L. Krall, S. M. Elliott, M. L. Erickson and B. A. Adams, Environ. Pollut., 2018, 234, 420–428 CrossRef CAS.
- H. Sun, H. Qi and H. Li, Food Anal. Methods, 2013, 6, 1049–1055 CrossRef.
- L. Liu, Q. Wan, X. Xu, S. Duan and C. Yang, Food Chem., 2017, 219, 7–12 CrossRef CAS PubMed.
- Y. Wang, G. Zhu, D. Wang, M. Huang, J. Yang and J. Liu, Electrochim. Acta, 2022, 436, 141434 CrossRef CAS.
- R. Chokkareddy, S. Kanchi and G. G. Redhi, J. Mol. Liq., 2022, 359, 119232 CrossRef CAS.
- G. Fiori, F. Bonaccorso, G. Iannaccone, T. Palacios, D. Neumaier, A. Seabaugh, S. K. Banerjee and L. Colombo, Nat. Nanotechnol., 2014, 9, 768–779 CrossRef CAS PubMed.
- P. He, M. Yan, G. Zhang, R. Sun, L. Chen, Q. An and L. Mai, Adv. Energy Mater., 2017, 7, 1601920 CrossRef.
- J. Li, Y. Zhang, M. Huo, S.-H. Ho and H.-S. Tsai, Mater. Today Chem., 2022, 26, 101241 CrossRef CAS.
- R. Karthik, J. V. Kumar, S.-M. Chen, P. Sundaresan, B. Mutharani, Y. C. Chen and V. Muthuraj, Ultrason. Sonochem., 2018, 48, 473–481 CrossRef CAS PubMed.
- A. Sharma, P. Mane, B. Chakraborty and C. S. Rout, ACS Appl. Energy Mater., 2021, 4, 14198–14209 CrossRef CAS.
- J. Zhou, L. Wang, M. Yang, J. Wu, F. Chen, W. Huang, N. Han, H. Ye, F. Zhao and Y. Li, Adv. Mater., 2017, 29, 1702061 CrossRef PubMed.
- Z. Shi, H. Huang, C. Wang, M. Huo, S.-H. Ho and H.-S. Tsai, Chem. Eng. J., 2022, 447, 137469 CrossRef.
- J. Zhang, C. Zhang, Z. Wang, J. Zhu, Z. Wen, X. Zhao, X. Zhang, J. Xu and Z. Lu, Small, 2018, 14, 1703098 CrossRef.
- R. Sun, Q. Wei, J. Sheng, C. Shi, Q. An, S. Liu and L. Mai, Nano Energy, 2017, 35, 396–404 CrossRef.
- J. Kang, F. Xu, C. Zhang, F. Li, O. A. Al-Hartomy, A. Al-Ghamdi, S. Wageh, G. Zhao, T. Yang and H. Zhang, Adv. Electron. Mater., 2022, 8, 2100567 CrossRef.
- J. Feng, L. Peng, C. Wu, X. Sun, S. Hu, C. Lin, J. Dai, J. Yang and Y. Xie, Adv. Mater., 2012, 24, 1969–1974 CrossRef PubMed.
- M. Mulazzi, A. Chainani, N. Katayama, R. Eguchi, M. Matsunami, H. Ohashi, Y. Senba, M. Nohara, M. Uchida and H. Takagi, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 075130 CrossRef.
- A. E. Vilian, S.-K. Hwang, M. J. Lee, B. Park, Y. S. Huh and Y.-K. Han, Chem. Eng. J., 2022, 439, 135782 CrossRef.
- A. Sarkar, A. B. Ghosh, N. Saha, G. R. Bhadu and B. Adhikary, ACS Appl. Nano Mater., 2018, 1, 1339–1347 CrossRef.
- Y. Zhao, D. Yang, T. He, J. Li, L. Wei, D. Wang, Y. Wang, X. Wang, G. Chen and Y. Wei, Chem. Eng. J., 2021, 421, 129715 CrossRef.
- V. M. Varsha and G. Nageswaran, J. Electrochem. Soc., 2020, 167, 136502 CrossRef.
- X. Xue, R. Chen, C. Yan, P. Zhao, Y. Hu, W. Kong, H. Lin, L. Wang and Z. Jin, Adv. Energy Mater., 2019, 9, 1900145 CrossRef.
- H. Liang, H. Shi, D. Zhang, F. Ming, R. Wang, J. Zhuo and Z. Wang, Chem. Mater., 2016, 28, 5587–5591 CrossRef CAS.
- X. Liu, H.-L. Shuai and K.-J. Huang, Anal. Methods, 2015, 7, 8277–8284 RSC.
- S. Sugai, K. Murase, S. Uchida and S. Tanaka, Solid State Commun., 1981, 40, 399–401 CrossRef CAS.
- W. G. McMullan and J. C. Irwin, Solid State Commun., 1983, 45, 557–560 CrossRef CAS.
- X. Chia, A. Ambrosi, P. Lazar, Z. Sofer and M. Pumera, J. Mater. Chem. A, 2016, 4, 14241–14253 RSC.
- N. Alov, D. Kutsko, I. Spirovová and Z. Bastl, Surf. Sci., 2006, 600, 1628–1631 CrossRef CAS.
- Y.-J. Tang, Y. Wang, X.-L. Wang, S.-L. Li, W. Huang, L.-Z. Dong, C.-H. Liu, Y.-F. Li and Y.-Q. Lan, Adv. Energy Mater., 2016, 6, 1600116 CrossRef.
- Y. Xue, Z. Zuo, Y. Li, H. Liu and Y. Li, Small, 2017, 13, 1700936 CrossRef.
- Y. Zhu, X. Li, M. Wu, M. Shi, Q. Tian, L. Fu, H.-S. Tsai, W.-F. Xie, G. Lai, G. Wang, N. Jiang, C. Ye and C.-T. Lin, Anal. Chim. Acta, 2023, 1275, 341607 CrossRef CAS PubMed.
- Y. Zhu, Q. Tian, X. Li, L. Wu, A. Yu, G. Lai, L. Fu, Q. Wei, D. Dai and N. Jiang, Biosensors, 2021, 11, 462 CrossRef CAS PubMed.
- C. Chen, Y.-C. Chen, Y.-T. Hong, T.-W. Lee and J.-F. Huang, Chem. Eng. J., 2018, 352, 188–197 CrossRef CAS.
- S.-H. Yeh, M.-S. Huang and C.-H. Huang, J. Taiwan Inst. Chem. Eng., 2022, 131, 104155 CrossRef CAS.
- Y. Zhu, X. Li, Y. Xu, L. Wu, A. Yu, G. Lai, Q. Wei, H. Chi, N. Jiang and L. Fu, Sensors, 2021, 21, 1220 CrossRef CAS PubMed.
- S. Issac and K. G. Kumar, Drug Test. Anal., 2009, 1, 350–354 CrossRef CAS PubMed.
- A. Turco, S. Corvaglia, P. P. Pompa and C. Malitesta, J. Colloid Interface Sci., 2021, 599, 676–685 CrossRef CAS PubMed.
- L. V. de Souza, O. Tkachenko, B. N. Cardoso, T. M. Pizzolato, S. L. Dias, M. A. Vasconcellos, L. T. Arenas, T. M. Costa, C. C. Moro and E. V. Benvenutti, Microporous Mesoporous Mater., 2019, 287, 203–210 CrossRef CAS.
- L. F. Sgobbi, C. A. Razzino and S. A. S. Machado, Electrochim. Acta, 2016, 191, 1010–1017 CrossRef CAS.
- T. S. Martins, J. L. Bott-Neto, O. N. Oliveira Jr. and S. A. S. Machado, J. Electroanal. Chem., 2021, 882, 114985 CrossRef CAS.
- X. Yue, Z. Li and S. Zhao, Microchem. J., 2020, 159, 105440 CrossRef CAS.
- M. Shabani-Nooshabadi and M. Roostaee, J. Mol. Liq., 2016, 220, 329–333 CrossRef CAS.
- P. S. Kumar, B. Sreeja, K. K. Kumar and G. Padmalaya, Chemosphere, 2022, 302, 134926 CrossRef PubMed.
- L. Ren, M. Chen, J. Zheng, Z. Li, C. Tian, Q. Wang and Z. Wang, Bioresour. Technol., 2021, 338, 125527 CrossRef CAS.
- Q. Tian, Y. She, Y. Zhu, D. Dai, M. Shi, W. Chu, T. Cai, H.-S. Tsai, H. Li and N. Jiang, Sensors, 2023, 23, 2870 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3an01355c |
| This journal is © The Royal Society of Chemistry 2024 |