Open Access Article
Open Access ArticleProbing the effect of the Si/Al ratio in Cu-CHA zeolite catalysts on SO2 exposure: in situ DR UV-vis spectroscopy and deactivation measurements†
Reza K.
Abasabadi
 ab,
Ton V. W.
Janssens
ab,
Ton V. W.
Janssens
 *a,
Silvia
Bordiga
*a,
Silvia
Bordiga
 b and
Gloria
Berlier
b and
Gloria
Berlier
 *b
*b
aUmicore Denmark ApS, Kogle Allé 1, 2970 Hørsholm, Denmark. E-mail: TonV.W.Janssens@eu.umicore.com
bDepartment of Chemistry and NIS Centre, University of Turin, Via Pietro Giuria 7, 10125 Turin, Italy. E-mail: gloria.berlier@unito.it
First published on 29th April 2024
Abstract
Cu-exchanged chabazite zeolite (Cu-HA) is one of the most effective catalysts for ammonia-assisted selective catalytic reduction (NH3-SCR) in diesel exhaust systems. However, this catalyst is sensitive to small amounts of SO2 in the exhaust gases, causing deactivation after prolonged exposure. To have a better understanding of the effect of the Si/Al ratio of zeolite on the SO2 exposure of Cu-CHA catalysts, we measured in situ diffuse reflectance UV-vis NIR spectroscopy, SO2 uptake, and deactivation of SO2 poisoned Cu-CHA catalysts with the same Cu loading (3.2 wt%) and different Si/Al ratios (6.7, 11 and 15) at 200 °C. SO2 selectively reacts with an oxygen-bridged diamine dicopper(II) complex [CuII2(NH3)4O2]2+, resulting in 50% deactivation in all catalysts, with an SO2 uptake which varies from a 0.2 S/Cu ratio for the catalyst with Si/Al = 6.7, to S/Cu = 0.12 for Si/Al = 15. For the fresh catalysts, the NH3-SCR activity decreases as the Si/Al ratio increases from 6.7 to 15, as also indicated by the amount of [CuII2(NH3)4O2]2+ complexes. After exposure of the [CuII2(NH3)4O2]2+ complex to SO2, the change in UV-vis spectra correlates well with the SO2 uptake and the expected Cu-species formed for all three Si/Al ratios. This suggests that, under the applied conditions, the SO2 reaction with the [CuII2(NH3)4O2]2+ complex in Cu-CHA does not depend on the Si/Al ratio.
Introduction
Nitrogen oxides (NOx) are harmful by-products of diesel fuel combustion in automotive engines. The release of nitrogen oxides from diesel exhaust systems into the environment is regulated by various authorities, making it crucial to abate them before they are dispersed.1 By employing the NH3-SCR (selective catalytic reduction) reaction, NOX emissions from diesel exhaust systems can be effectively reduced. In the standard NH3-SCR reaction, NO is reduced with NH3 in the presence of O2 to produce N2 and H2O with a stoichiometry of 4NO + 4NH3 + O2 → 4N2 + 6H2O.2,3 Cu-Chabazite (CHA) small pore zeolites are commercialized catalysts for the NH3-SCR reaction. Cu-CHA catalysts are highly active in the NH3-SCR reaction, particularly at low temperatures, and demonstrate excellent thermal stability.4,5The catalytic cycle for the NH3-SCR reaction on the Cu-CHA catalyst at low temperatures (<250 °C) is based on an oxidation and a reduction part that involve CuI and CuII species with different ligands.6,7 During the reduction part, the [CuI(NH3)2]+ complex is an important intermediate with a certain mobility in the zeolite cage.8 The oxidation step is crucial, as it involves the activation of O2 by two [CuI(NH3)2]+ complexes to form the [CuII2(NH3)4O2]2+ complex.9,10 The formation of [CuII2(NH3)4O2]2+ is favored at high Cu density, with a fraction of unoxidized CuI that depends on the Cu loading and Si/Al ratio.11,12 UV-vis spectroscopy can be used to monitor changes in the oxidation state of Cu during the formation of [CuII2(NH3)4O2]2+, particularly in the d–d region (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–8000 cm−1 interval), where CuII ions in Cu-CHA have a specific fingerprint.13–15 No absorption is observed in this region by CuI ions, since their d shell is filled.16 Furthermore, the NIR (near-infrared) region of spectra allows for specific tracking of the ligands interacting with the Cu ions and the zeolite surface.17
000–8000 cm−1 interval), where CuII ions in Cu-CHA have a specific fingerprint.13–15 No absorption is observed in this region by CuI ions, since their d shell is filled.16 Furthermore, the NIR (near-infrared) region of spectra allows for specific tracking of the ligands interacting with the Cu ions and the zeolite surface.17
The Cu-CHA catalyst is sensitive to SO2 produced by the combustion of S-compounds in diesel fuel.18–20 This causes a loss of activity below 350 °C, which is in the normal operation temperature range for NH3-SCR catalysts.21–23 Different studies have focused on the nature of the sulfur-based compounds formed during SO2 exposure, which could be responsible for the deactivation. Namely, Cu bisulfite, Cu sulfate or bisulfate, ammonium sulfate or bisulfate, Al sulfate, sulfuric acid and ammonium sulfate attached to Cu ions have been observed depending on the composition and treatments of catalysts.21,22,24–29 These compounds are characterized by different thermal stabilities,22,26,27,30 meaning that the activity of catalysts can be partially recovered by thermal treatments, typically above 350–400 °C.19,22,27,29 X-ray absorption spectroscopy (XAS) measured during SO2 exposure of Cu-CHA at low temperature after different pretreatments indicates that the [CuII2(NH3)4O2]2+ complex is the most sensitive to SO2. In contrast, CuI and CuII ions stabilized by the zeolite framework (fw-CuI and fw-CuII) are hardly affected by SO2 exposure at 200 °C.30 A two-step mechanism was proposed, where one SO2 molecule reacts with two [CuII2(NH3)4O2]2+ complexes, resulting in the formation of two [CuI(NH3)2]+, one fw-CuI, and one CuII-sulfated compound.31
The Si/Al ratio of zeolite affects the formation of the [CuII2(NH3)4O2]2+ complex,11 which is an important intermediate in the NH3-SCR reaction. A high Si/Al ratio resulted in a lower amount of [CuII2(NH3)4O2]2+ complexes and a higher residual amount of [CuI(NH3)2]+ after the oxidation part,11 while catalysts with a low Si/Al ratio showed a higher yield of [CuII2(NH3)4O2]2+ formation and higher catalytic activity.32 These observations point to an influence of the Si/Al ratio on the NH3-SCR reaction.32
In this study, the impact of SO2 on the Cu-CHA catalyst in the NH3-SCR reaction at 200 °C was investigated by employing in situ diffuse reflectance UV-vis-NIR spectroscopy, SO2 uptake and deactivation measurements for three Cu-CHA catalysts with Si/Al ratios of 6.7, 11 and 15 and a Cu loading of 3.2 wt%. By examining the UV-vis spectra in the visible region (CuII d–d transitions) and the first-order rate constant measured at 200 °C, we investigated the effect of the Si/Al ratio on the formation of the [CuII2(NH3)4O2]2+ complex and its reactivity to SO2. We measured the deactivation and quantified the UV-vis absorption in the CuII d–d region to gain a better understanding of the impact of the Si/Al ratio on the SO2 reaction with the [CuII2(NH3)4O2]2+ complex.
Experimental
Three H-CHA (also known as H-SSZ-13) zeolites with Si/Al = 6.7, 11 and 15 were used to prepare Cu ion-exchanged chabazite zeolite catalysts by incipient wetness impregnation with the appropriate amount of an aqueous solution of Cu-nitrate to obtain a Cu loading of 3.2 wt%. The samples were impregnated by spraying the solution under stirring of the powder to obtain a more even distribution of Cu. Then, the samples were dried for 2 h at 90 °C, followed by 2 h of calcination at 600 °C in air to decompose the nitrates.To determine the effect of SO2 on the Cu-CHA catalysts, four well-defined Cu-species, namely framework-bound CuI and CuII (fw-CuI and fw-CuII) and [CuI(NH3)2]+ and [CuII2(NH3)4O2]2+ complexes, were prepared in situ, before exposure to SO2 (see Fig. S1† for details). In short, fw-CuII is produced by oxidation in 10% O2 at 400 °C,17,30,33,34 fw-CuI is produced by reduction of fw-CuII in 1% H2 at 400 °C,30 the [CuI(NH3)2]+ complex is formed by exposure of the fw-CuII species to a mixture of 500 ppm NO and 600 ppm NH3 at 200 °C,12,35–37 and finally the [CuII2(NH3)4O2]2+ complex is formed by exposure of [CuI(NH3)2]+ to 10% O2 at 200 °C.11,12,30,37,38 The effect of exposure to 100 ppm SO2 in N2 on these four Cu-species was then determined by in situ diffuse reflectance (DR) UV-vis-NIR, SO2 uptake and activity measurements.
For the DR UV-vis-NIR measurements, the spectra were recorded with a Varian Cary 5000 spectrophotometer, equipped with an R928 PMT UV-vis detector and a cooled PbS photocell NIR detector. The spectra were collected with a Praying Mantis® element, coupled with a low temperature reaction chamber, connected to a gas manifold system. For all UV-vis measurements, a 40 mg sample of the catalyst (sieve fraction 150–300 μm) was placed in the reaction chamber. Before each specific procedure described above and in Fig. S1,† the catalyst was first heated at 400 °C in 10% O2 in N2 with a total flow of 6 NL h−1 and left at this temperature for 1 hour, before cooling to the desired temperature. Teflon powder was used to measure the reference spectrum needed to determine the relative reflectance (R%):
 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, characteristic of d–d transitions of CuII ions. The reflectance of the samples during the measurements was adjusted in the range of 20–50% in order to compare the calculated areas without artefacts due to a different vertical offset.39
000 cm−1, characteristic of d–d transitions of CuII ions. The reflectance of the samples during the measurements was adjusted in the range of 20–50% in order to compare the calculated areas without artefacts due to a different vertical offset.39
The effect of SO2 on the catalytic activity was determined using a powder flow reactor setup. The deactivation of the catalyst was determined as the ratio of the activities before and after exposure to 100 ppm SO2 in N2 at 200 °C. The entire procedure for the activity measurements is as follows: a sample of 10 mg of the fresh catalyst was diluted with 150 mg silicon carbide and added to a quartz U-tube reactor with an inner diameter of 4 mm; quartz wool was used to keep the catalysts as a fixed catalytic bed. The compositions of the feed and outlet gases were measured with a Gasmet CX4000 FTIR analyzer. First, the catalyst was subjected to heating at 550 °C in 10% O2 in N2 with a total flow of 12 NL h−1 and maintained at this temperature for 1 hour. To determine the activities, the catalyst was exposed to 500 ppm NO, 600 ppm NH3, 10% O2 and 5% H2O (SCR gases) at 200 °C. Then, each Cu species was formed by the mentioned procedures and exposed to 100 ppm SO2 in N2. Subsequently, the catalyst was exposed to SCR gases to measure the activity of the SO2 exposed catalyst. The activities were evaluated by determining the first-order rate constants, k (mol gcat−1 h−1), for the fresh and SO2 exposed catalysts with the following equations:
 | (2) |
The ratio of the rate constants after and before the SO2 exposure is then the measured for the deactivation:
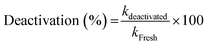 | (3) |
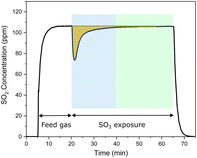 | ||
| Fig. 1 The SO2 uptake of Cu-CHA with a Si/Al ratio of 6.7 during exposure to 100 ppm SO2 in N2 at 200 °C. | ||
Results
Effect of SO2 exposure on different Cu species
To determine the oxidation state of Cu for different Cu species, the d–d transitions in the 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–8000 cm−1 interval in the DR UV-vis spectra were investigated. The light coloured lines in Fig. 2 are the spectra obtained for the different CuI and CuII species in Cu-CHA with a Si/Al ratio of 6.7 before exposure to SO2. The spectra for the CuII species show a clear absorption in the d–d transition region (panels a and d), while those for the CuI species do not (panels b and c). The [CuII2(NH3)4O2]2+ complex exhibits an absorption in the d–d region, centered at 13
000–8000 cm−1 interval in the DR UV-vis spectra were investigated. The light coloured lines in Fig. 2 are the spectra obtained for the different CuI and CuII species in Cu-CHA with a Si/Al ratio of 6.7 before exposure to SO2. The spectra for the CuII species show a clear absorption in the d–d transition region (panels a and d), while those for the CuI species do not (panels b and c). The [CuII2(NH3)4O2]2+ complex exhibits an absorption in the d–d region, centered at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1, with slight variations in the position and shape compared to fw-CuII (Fig. 2a and d, respectively).37 In summary, these spectra confirm the formation of well-defined CuII and CuI species, similar to what was observed by XAS after similar pre-treatments.30
500 cm−1, with slight variations in the position and shape compared to fw-CuII (Fig. 2a and d, respectively).37 In summary, these spectra confirm the formation of well-defined CuII and CuI species, similar to what was observed by XAS after similar pre-treatments.30
 | ||
| Fig. 2 Effect of SO2 on different Cu species monitored by in situ DR UV-vis spectroscopy on Cu-CHA with a Si/Al ratio of 6.7 before (light lines) and after SO2 exposure (dark lines) at 200 °C. | ||
The changes in the d–d transition upon SO2 exposure reveal the different interactions of these four Cu species with SO2, (dark coloured lines in Fig. 2). For the [CuII2(NH3)4O2]2+ complex, the fingerprint of CuII ions is affected, indicating an interaction of SO2. The decrease in intensity indicates that some reduction of Cu has taken place, in good agreement with earlier results obtained by X-ray absorption spectroscopy.30 On the other hand, the spectra for the framework-bound species are not affected by the exposure to SO2. Notice that the spectra of fw-CuII and [CuII2(NH3)4O2]2+ are hardly distinguishable in the d–d region, but their response to SO2 is clearly different. The spectrum of the [CuI(NH3)2]+ complex exhibits small changes, with a small portion of CuII being formed (Fig. 2c, dark coloured line). This change will be addressed in the Discussion section.
The interaction of NH3 ligands on Cu species was investigated in the near-infrared (NIR) region (Fig. 3c and d). After exposing the sample to NO/NH3 to obtain [CuI(NH3)2]+ and [CuII2(NH3)4O2]2+ complexes, the near-infrared (NIR) region shows the vibrational modes of NH3 coordinated to Cu ions and NH4+ formed by protonation from residual Brønsted sites.40 More in detail, the band at 6510 cm−1 can be related to the overtone of the NH stretching modes of NH3 and NH4+, with the corresponding stretching + bending combination modes at 4960 and 4730 cm−1, respectively. These bands are absent in the NIR spectra of fw-CuII and fw-CuI (panels a and b, respectively) showing only the vibrational modes of silanols and Brønsted sites at 7050 and 4600 cm−1.17 After exposure to SO2, all bands in the NIR region were unchanged, except for the NH4+ band, which shows a slight increase in the [CuII2(NH3)4O2]2+ complex (Fig. 3d). This indicates that the presence of the [CuII2(NH3)4O2]2+ complex leads to NH4+ formation after SO2 exposure.
 | ||
| Fig. 3 Effect of SO2 on different Cu species in the NIR region on sample Cu-CHA with a Si/Al ratio of 6.7 before (light lines) and after SO2 exposure (dark lines) at 200 °C. | ||
Having verified the higher sensitivity of the [CuII2(NH3)4O2]2+ complex to SO2, we have repeated the same protocols to measure the corresponding SO2 uptakes and deactivation. The S/Cu ratio of the catalysts was determined by measuring SO2 uptakes after each pretreatment. The SO2 uptake for the [CuII2(NH3)4O2]2+ complex is higher (S/Cu = 0.2), compared to those of the other Cu species, in agreement with the changes observed in the UV-vis spectra. The amount of SO2 uptakes in μmol per gram of catalyst and the S/Cu ratio are in good agreement with what was reported in ref. 30 for the same catalyst (Table 1).
| Cu state | fw-CuII | fw-CuI | [CuI(NH3)2]+ | [CuII2(NH3)4O2]2+ |
|---|---|---|---|---|
| SO2 uptake (μmol SO2 per gCatalyst) | 30 | 7 | 27 | 101 |
| S/Cu ratio | 0.02 | 0.06 | 0.05 | 0.2 |
The NH3-SCR activity of the same Cu-CHA catalyst was measured at 200 °C before and after SO2 exposure in the four states described above. Fig. 4 reports the calculated deactivation (eqn (3)) as a function of SO2 uptake, expressed as the S/Cu ratio. The data show that the deactivation increases linearly with the sulfur uptake. More in detail, the lowest deactivation (7%) corresponds to the catalyst pretreated to obtain fw-CuI, followed by [CuI(NH3)2]+ (15%) and fw-CuII (20%). The catalyst measured after formation of the [CuII2(NH3)4O2]2+ complex exhibits the highest SO2 uptake and deactivation (50%) compared to the other species. This observation fits well with the measured UV-vis spectra, which indicates a significant reduction of CuII to CuI when SO2 interacts with the [CuII2(NH3)4O2]2+ complex. In summary, the catalyst exposing only fw-CuI sites can be exposed to SO2 without loss of activity, while the activity decreases more when the Cu species are divalent and coordinated by NH3 ligands.
Reducibility of the [CuII2(NH3)4O2]2+ complex after SO2 exposure followed by in situ DR UV-vis spectroscopy
The results discussed so far show that the Cu-CHA catalyst takes up more SO2 after a pretreatment forming the [CuII2(NH3)4O2]2+ complex, and this corresponds to the highest deactivation measured in this study. The deactivation is caused by a less efficient reduction of the [CuII2(NH3)4O2]2+ complex by NO/NH3 after SO2 exposure. Because this reduction represents the reduction part of the NH3-SCR cycle, a less effective reduction of the [CuII2(NH3)4O2]2+ complex leads to a slower NH3-SCR reaction.6,33 The change in reducibility of the [CuII2(NH3)4O2]2+ complex species by SO2 was measured by exposing this species to 500 ppm NO/600 ppm NH3 at 200 °C, without and with SO2 exposure (see Fig. S2† for details about the procedure).The complete [CuI(NH3)2]+/[CuII2(NH3)4O2]2+/[CuI(NH3)2]+ redox cycle was followed by DR UV-vis spectroscopy on Cu-CHA with a Si/Al ratio of 6.7 (Fig. 5a). The data show that after the reaction with NO/NH3, the [CuII2(NH3)4O2]2+ complex is completely reduced to [CuI(NH3)2]+ (red and blue spectra in Fig. 5a), in agreement with previous reports.37 A reoxidation reproduced the original spectrum for the [CuII2(NH3)4O2]2+ complex (green curve in Fig. 5a), indicating that all Cu can be reversibly reduced and oxidized. If the [CuII2(NH3)4O2]2+ state is exposed to SO2, some of the Cu is reduced (green and yellow curve in Fig. 5b), in agreement with previous XAS measurements and Fig. 2d.30,31 The subsequent reduction with NO/NH3 (red curve in Fig. 5b) does not restore the initial [CuI(NH3)2]+ state, indicating that some of the Cu remains in a CuII state, and therefore, the Cu-CHA cannot be fully reduced to [CuI(NH3)2]+ after exposure of [CuII2(NH3)4O2]2+ to SO2. This provides evidence for the less efficient reduction of Cu after exposure to SO2; the reaction of SO2 with the [CuII2(NH3)4O2]2+ complex seems to remove a fraction of CuII sites from the redox CuII/CuI cycle.
Effect of the Si/Al ratio on the reactivity of the [CuII2(NH3)4O2]2+ complex with SO2
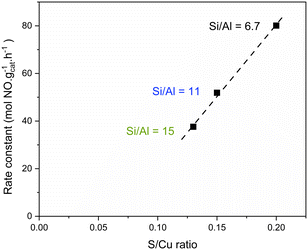
Having verified the higher reactivity of SO2 with the [CuII2(NH3)4O2]2+ complex with respect to the other Cu states in Cu-CHA with a Si/Al ratio of 6.7, we explored the same protocol as in Fig. 2d on other two Cu-CHA catalysts with the same Cu loading (3.2 wt%) and different Si/Al ratios (11 and 15). The UV-vis spectra measured before and after SO2 interaction were converted into the Kubelka–Munk function, in order to make a semiquantitative comparison of the integrated area in the CuII d–d region.Before SO2 exposure (solid lines in Fig. 6a), the measured area should be proportional to the amount of the formed [CuII2(NH3)4O2]2+ complex. It is clear from Fig. 6a and from the area values listed in Table 2 (1st column) that the band intensity follows the Si/Al order 6.7 > 11 > 15. This would imply an important effect of the catalyst Si/Al ratio (keeping constant the Cu loading) on the amount of the formed [CuII2(NH3)4O2]2+ complex. Interestingly, the same trend is followed by the first order kinetic constant measured at 200 °C on the fresh catalysts (2nd column in Table 2) which is consistent with the hypothesis that the [CuII2(NH3)4O2]2+ complex is relevant for the low temperature activity in the reaction (Fig. 7). In summary, a larger fraction of Cu is present as the [CuII2(NH3)4O2]2+ complex at a low Si/Al ratio, and this is reflected in the measured kinetic constant.
| Si/Al ratio | UV-vis area of the [CuII2(NH3)4O2]2+ complex × 10−4 (cm−1) | Rate constant of fresh catalysts at 200 °C (mol NO per gcat h−1) |
|---|---|---|
| 6.7 | 0.58 | 80.1 |
| 11 | 0.43 | 51.9 |
| 15 | 0.315 | 37.6 |
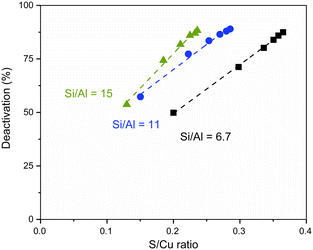
All catalysts were exposed to SO2 to compare changes of UV-vis area and measure SO2 uptakes. As previously discussed, the exposure of the [CuII2(NH3)4O2]2+ complex to SO2 causes a decrease in the intensity of CuII d–d transitions (Fig. 6a, dashed lines). The relative variation in the spectral area (coloured area in Fig. 6) follows again the trend Si/Al 6.7 > 11 > 15. Thus, the catalyst showing the most abundant formation of the [CuII2(NH3)4O2]2+ complex is the most affected by SO2 exposure. This is also reflected in the SO2 uptake measured under the same conditions (reported as the S/Cu ratio in Fig. 6b), which follows a similar trend.
Repeated SO2 exposure and NH3-SCR catalytic tests
Cu-CHA with a Si/Al ratio of 6.7 showed a deactivation of around 50% after SO2 exposure of the [CuII2(NH3)4O2]2+ complex. After the same procedure, the Cu-CHA samples with Si/Al ratios of 6.7, 11 and 15 show very similar deactivation values (50%, 57% and 53%, respectively) but different S/Cu ratios: 0.2, 0.15 and 0.13 for Cu-CHA with Si/Al ratios of 6.7, 11 and 15, respectively. To compare the SO2 uptakes and deactivation for the three catalysts, we thus carried out experiments to take up more SO2 and deactivate more catalysts. According to Fig. 1 (blue part), the catalysts' SO2 uptake takes place in the first 20 min of SO2 exposure. By repeating the cycle of reduction in NO/NH3 followed by oxidation to form the [CuII2(NH3)4O2]2+ complex and SO2 exposure (see Fig. S3† for details about the procedure), the SO2 uptake increases gradually, and the total amount is measured by adding the SO2 uptake during each exposure step. The catalytic activity was measured after each SO2 exposure to calculate the deactivation. Totally, these steps – reduction, oxidation, SO2 exposure and measurement of catalytic activity – were repeated six times.In Fig. 8, the deactivation measured at 200 °C is plotted as a function of the SO2 uptakes, expressed as the S/Cu ratio. A good linearity is observed for each Si/Al ratio. As mentioned, all catalysts lose around 50% of their activity during the first cycle although the amount of SO2 uptake is different. In the second cycle, the deactivation increases around 75%, meaning that a further 50% is lost with respect to the first poisoning cycle for all catalysts. This corresponds to an increase of the S/Cu ratio which is only half of the initial S/Cu ratio for the three catalysts. Both deactivation and the S/Cu ratio change by less than 50% in the following cycles, eventually reaching a stable level after several cycles. This indicates that the deactivation behavior of the Cu-CHA catalysts through SO2 exposure on the sensitive [CuII2(NH3)4O2]2+ complex is different from the beginning to the end, suggesting that complete deactivation of the catalyst is not achievable.
Discussion
SO2 reaction with the [CuII2(NH3)4O2]2+ complex causes CuII-sulfated formation and deactivation
DR UV-vis, SO2 uptake and deactivation measurements confirm the sensitivity of the [CuII2(NH3)4O2]2+ complex to SO2 exposure. The [CuII2(NH3)4O2]2+ complex is formed upon activation of O2 by a pair of [CuI(NH3)]+ complexes, and therefore the reaction of SO2 with [CuII2(NH3)4O2]2+ is a key step in the deactivation mechanism. It has been proposed that the SO2 poisoning is more pronounced upon exposure to a typical NH3-SCR feed gas (NO/NH3/O2/H2O mixture), as compared to an O2/H2O mixture.41 This aligns well with the observation that SO2 primarily interacts with the [CuII2(NH3)4O2]2+ complex, because the formation of the [CuII2(NH3)4O2]2+ complex requires the presence of both NO and NH3, according to the NH3-SCR reaction cycle.6,7,37Moreover, we have shown by DR UV-vis that the catalyst cannot be completely reduced in NO/NH3, closing the catalytic cycle, after the SO2 reaction with the [CuII2(NH3)4O2]2+ complex. According to Molokova et al., the SO2 reaction with the [CuII2(NH3)4O2]2+ complex causes the formation of a mixture of fw-CuI, [CuI(NH3)2]+, a small amount of unreacted [CuII2(NH3)4O2]2+ complex and a ‘CuII-sulfated component’, likely coordinated to NH3 ligands.28,30 This means that the band in the d–d region measured after SO2 interaction is given by a fraction of the (active) [CuII2(NH3)4O2]2+ complex and by this CuII-sulfated component, which does not react efficiently with NO/NH3 to close the catalytic cycle and is thus responsible for the measured deactivation.
Notably, the observation of NH4+ formation in the NIR region only after SO2 exposure of the [CuII2(NH3)4O2]2+ complex could be related to the formation of some ammonium sulphate (NH4)2SO4 or bisulfate (NH4)HSO4 as proposed by different authors.22,25,42 However, the deactivation after the SO2 reaction with the [CuII2(NH3)4O2]2+ complex is not compatible with an accumulation of ammonium bisulfate causing the physical blocking on the zeolite pores. This mechanism is not compatible with the observation that a small amount of SO2 (S/Cu = 0.2) causes a deactivation of 50%.
Formation of the [CuII2(NH3)4O2]2+ complex and SO2 exposure on catalysts with different Si/Al ratios
The first-order rate constant at 200 °C for the three catalysts with identical Cu loading (3.2 wt%) exhibits a correlation with the obtained DR UV-vis spectra and the corresponding calculated Kubelka–Munk area. It is reasonable to consider that the UV-vis area in the CuII d–d region after the described experimental procedure is associated with the capability of the catalysts to form the [CuII2(NH3)4O2]2+ complex. This implies a correlation between the rate constant and the amount of the formed [CuII2(NH3)4O2]2+ complex, which can be monitored by DR UV-vis spectroscopy. This observation is in agreement with the hypothesis that the [CuII2(NH3)4O2]2+ complex is the active site in the low temperature NH3-SCR reaction, so that the kinetic constant depends on its concentration (Fig. 7). The concentration of the [CuII2(NH3)4O2]2+ complex and the kinetic constant are higher for Cu-CHA with a Si/Al ratio of 6.7. We can hypothesize that this is due to the higher negative charge in the framework induced by the presence of Al, which would favour the divalent oxidation state. Thus, maintaining Cu as CuI species within the zeolite cages becomes more challenging.After SO2 exposure of the [CuII2(NH3)4O2]2+ complex, all the catalysts showed a 50% decrease of activity after the first SO2 exposure (Fig. 8), irrespective of the SO2 uptake, which instead shows a linear correlation with the rate constant of the fresh catalysts (Fig. 9).
Following the deactivation mechanism by SO2 on catalysts with different Si/Al ratios
Notably, a small S/Cu ratio (0.2 for Cu-CHA with a Si/Al ratio of 6.7) is capable of reducing the rate constant by 50%. Based on XAS data, coupled to the measurement of SO2 uptake, Molokova et al. proposed a two-step mechanism where one single SO2 molecule reacts with two [CuII2(NH3)4O2]2+ complexes.31 This, coupled to steric hindrance, could explain the high impact on catalytic activity of a small SO2 uptake.42 The stoichiometry resulting from this mechanism is| 2[CuII2(NH3)4O2]2+ + SO2 → 2CuI(NH3)2 + fw-CuI + CuII–SO4Z | (4) |
The sulfated species (CuII–SO4Z) is characterized by a square-planar coordination of Cu with 4 ligands including oxygen and NH3 as Z, as demonstrated by EXAFS and XANES analyses.31 Based on this stoichiometry, we have considered as input for each catalyst the measured S/Cu ratio to calculate the fraction of formed CuII-sulfated and CuI species (2CuI(NH3)2 + fw-CuI), considering the rest of the Cu as unreacted [CuII2(NH3)4O2]2+ (Table 3). For instance, an SO2 uptake corresponding to S/Cu = 0.2 (Cu-CHA with a Si/Al ratio of 6.7, Table 3) would imply 20% CuII-sulfated components, 60% CuI species and 20% [CuII2(NH3)4O2]2+ complexes. Thus, the UV-vis area in the d–d region measured in Cu-CHA with a Si/Al ratio of 6.7 (calibration sample) after SO2 dosage would be due to 20% unreacted [CuII2(NH3)4O2]2+ complexes plus 20% CuII-sulfated components. This assumption was used to estimate the relative absorption coefficient of CuII-sulfated components with respect to that of [CuII2(NH3)4O2]2+. The obtained value (1.8) was then used to calculate the expected variation on Cu-CHA with Si/Al ratios of 11 and 15 based on the corresponding S/Cu ratios (second column of Table 3). The agreement between the calculated area variations and the measured ones (6th and 5th column of Table 3) is striking, supporting the validity of the mechanism proposed in ref. 31 for catalysts with three different Si/Al and S/Cu ratios.
| Si/Al ratio | CuII sulfated (S/Cu ratio) | [CuI(NH3)2]+ and fw-CuI | [CuII2(NH3)4O2]2+ | UV-vis decrease (%) | |
|---|---|---|---|---|---|
| Meas. | Calc. | ||||
| Bold entries were measured values used as input for calculation of the UV-vis decrease in the d–d region after SO2 uptake; Cu-CHA with a Si/Al ratio of 6.7 was used as a calibration sample. | |||||
| 6.7 | 0.2 | 0.60 | 0.20 | 44 | — |
| 11 | 0.15 | 0.45 | 0.40 | 33 | 33 |
| 15 | 0.12 | 0.36 | 0.52 | 25 | 26 |
The deactivation caused by SO2 on the [CuII2(NH3)4O2]2+ complex exhibits similar behavior across the catalysts with different Si/Al ratios, although the SO2 uptakes are different. The rate constant measurements indicate a 50% decrease in activity when SO2 reacts with the [CuII2(NH3)4O2]2+ complex. It is interesting to notice that the activity of all catalysts further decreases by 50% in the second step of SO2 exposure. This would suggest a mechanism where SO2 affects half of the active [CuII2(NH3)4O2]2+ complex in the first step, and half of the remaining [CuII2(NH3)4O2]2+ complex in the second one. This does not happen in the following SO2 exposure steps, which could be explained by the fact that the concentration of the [CuII2(NH3)4O2]2+ complex is too small, and the accessibility to SO2 is limited by the high concentration of the CuII-sulfated compound in the zeolite pores.
As a further comment, from the stoichiometry proposed in eqn (4), one SO2 molecule would decompose two [CuII2(NH3)4O2]2+ complexes, resulting in one inactive CuII-sulfated compound, two [CuI(NH3)2]+ and one fw-CuI. This should result in a 25% decrease in activity for each SO2, since the [CuI(NH3)2]+ and fw-CuI sites should be still available for the NH3-SCR reaction. Clearly, this is not in agreement with the deactivation measured in this work, suggesting that the poisoning mechanism is more complex.
O2 sensitivity of CuI species on Cu-CHA catalysts
A small increase of CuI to CuII during SO2 exposure of the [CuI(NH3)2]+ complex was observed (Fig. 2c). However, the spectrum of fw-CuI does not change after SO2 exposure, showing that SO2 does not react with CuI. Moreover, SO2 has been shown to reduce CuII to CuI, meaning that the observed oxidation of CuI to CuII for the [CuI(NH3)2]+ complex cannot be related to the reaction with SO2 itself, but could be related to the presence of small traces of oxygen stored in the sample. To explore this concept, the sensitivity of fw-CuI and [CuI(NH3)2]+ species to O2 was examined through pulse oxidation steps. Namely, the catalyst was exposed 10 times to a 2% O2/N2 flow for 5 seconds after forming [CuI(NH3)2]+ or fw-CuI species (Fig. 10a and b, respectively). Subsequently, the catalyst was left in O2 for 30 min to achieve complete oxidation (see Fig. S4† for details about the measurement). | ||
| Fig. 10 DR UV-vis experiments: 5 s 2% O2 pulses on a) [CuI(NH3)2]+ and b) fw-CuI on Cu-CHA with a Si/Al ratio of 6.7 at 200 °C. | ||
Fig. 10 illustrates the changes in the spectra after each O2 pulse, leading to the formation of CuII species. It is evident that the mobile [CuI(NH3)2]+ complex is more sensitive compared to the fw-CuI species. Indeed, the formation of the [CuII2(NH3)4O2]2+ complex has been related to the ability of a couple of mobile [CuI(NH3)2]+ complexes to activate O2, while the oxidation of fw-CuI ions is more difficult.43–45 Nevertheless, both CuI species can be oxidized to CuII in fully oxidizing environments at 200 °C. Notably, the rate of oxidation and the final spectral shape differ, indicating the formation of two different CuII species. These results indicate the high reactivity of [CuI(NH3)2]+ to a small contamination or to the amount of oxygen stored in the catalysts in CuxOy species.30 Indeed, this suggests that the small amount of SO2 uptake and deactivation after forming the [CuI(NH3)2]+ complex could be related to the presence of a small amount of the [CuII2(NH3)4O2]2+ complex.
Conclusions
In this study, we investigated the interaction of SO2 with Cu-CHA catalysts with different Si/Al ratios at 200 °C, after specific pre-treatments to obtain well defined CuI and CuII species. DR UV-vis spectroscopy confirms the observation that SO2 preferentially interacts with the [CuII2(NH3)4O2]2+ complex, which is believed to be an important intermediate in the NH3-SCR reaction at low temperature.This interaction results in a reduction of CuII to CuI and in the formation of a CuII-sulfated compound, which cannot be reduced by NO/NH3. This indicates that the CuII-sulfated compound is responsible for the observed loss of activity.
The Si/Al ratio of the zeolite affects the amount of the formed [CuII2(NH3)4O2]2+ complex, as measured by UV-vis spectroscopy, showing a correlation with the reaction rate measured at 200 °C, which is in the order 6.7 > 11 > 15. The same trend is observed for the SO2 uptake measured after SO2 interaction with the [CuII2(NH3)4O2]2+ complex. However, the three catalysts show similar deactivation of around 50% after the first SO2 exposure. Repeated SO2 exposure cycles show a correlation between SO2 accumulation and deactivation for the three catalysts, with both parameters gradually levelling off.
The measured changes in the UV-vis after SO2 interaction with the [CuII2(NH3)4O2]2+ complex is consistent with the formation of the CuII-sulfated compound following a reaction mechanism recently proposed.31 This in turns indicates that the same reaction mechanism takes place in the three catalysts, irrespective of the Si/Al ratio.
Author contributions
Reza K. Abasabadi: measurements, data analysis, interpretation, and writing. Ton V. W. Janssens and Gloria Berlier: conceptualization, interpretation, editing, supervision, and funding. Silvia Bordiga: interpretation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge support from the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 955839 (CHASS) and from the Project CH4.0 under the MUR program “Dipartimenti di Eccellenza 2023-2027” (CUP: D13C22003520001).References
- A. Wang and L. Olsson, Nat. Catal., 2019, 2, 566–570 CrossRef.
- R. Gounder and A. Moini, React. Chem. Eng., 2019, 4, 966–968 RSC.
- I. Nova and E. Tronconi, Urea-SCR technology for deNOx after treatment of diesel exhausts, 2014, vol. 5 Search PubMed.
- C. H. F. Peden, J. Catal., 2019, 373, 384–389 CrossRef CAS.
- E. Borfecchia, K. A. Lomachenko, F. Giordanino, H. Falsig, P. Beato, A. V. Soldatov, S. Bordiga and C. Lamberti, Chem. Sci., 2015, 6, 548–563 RSC.
- Y. Feng, X. Wang, T. V. W. Janssens, P. N. R. Vennestrøm, J. Jansson, M. Skoglundh and H. Grönbeck, ACS Catal., 2021, 11, 14395–14407 CrossRef CAS.
- L. Chen, T. V. W. Janssens, P. N. R. Vennestrøm, J. Jansson, M. Skoglundh and H. Grönbeck, ACS Catal., 2020, 10, 5646–5656 CrossRef CAS.
- R. Millan, P. Cnudde, V. Van Speybroeck and M. Boronat, JACS Au, 2021, 1, 1778–1787 CrossRef CAS PubMed.
- X. Wang, L. Chen, P. N. R. Vennestrøm, T. V. W. Janssens, J. Jansson, H. Grönbeck and M. Skoglundh, ChemCatChem, 2021, 13, 2577–2582 CrossRef CAS.
- F. Gao, D. Mei, Y. Wang, J. Szanyi and C. H. F. Peden, J. Am. Chem. Soc., 2017, 139, 4935–4942 CrossRef CAS PubMed.
- A. Martini, C. Negri, L. Bugarin, G. Deplano, R. K. Abasabadi, K. A. Lomachenko, T. V. W. Janssens, S. Bordiga, G. Berlier and E. Borfecchia, J. Phys. Chem. Lett., 2022, 13, 6164–6170 CrossRef CAS PubMed.
- C. Paolucci, I. Khurana, A. A. Parekh, S. Li, A. J. Shih, H. Li, J. R. Di Iorio, J. D. Albarracin-Caballero, A. Yezerets, J. T. Miller, W. N. Delgass, F. H. Ribeiro, W. F. Schneider and R. Gounder, Science, 2017, 357, 898–903 CrossRef CAS PubMed.
- F. Giordanino, P. N. R. Vennestrøm, L. F. Lundegaard, F. N. Stappen, S. Mossin, P. Beato, S. Bordiga and C. Lamberti, Dalton Trans., 2013, 42, 12741–12761 RSC.
- H. Li, C. Paolucci, I. Khurana, L. N. Wilcox, F. Göltl, J. D. Albarracin-Caballero, A. J. Shih, F. H. Ribeiro, R. Gounder and W. F. Schneider, Chem. Sci., 2019, 10, 2373–2384 RSC.
- C. Tyrsted, E. Borfecchia, G. Berlier, K. A. Lomachenko, C. Lamberti, S. Bordiga, P. N. R. Vennestrøm, T. V. W. Janssens, H. Falsig, P. Beato and A. Puig-Molina, Catal. Sci. Technol., 2016, 6, 8314–8324 RSC.
- B. Centrella, G. Deplano, A. Damin, M. Signorile, M. Tortora, C. Barolo, M. Bonomo and S. Bordiga, Dalton Trans., 2022, 51, 14439–14451 RSC.
- C. Negri, M. Signorile, N. G. Porcaro, E. Borfecchia, G. Berlier, T. V. W. Janssens and S. Bordiga, Appl. Catal., A, 2019, 578, 1–9 CrossRef CAS.
- P. S. Hammershøi, A. D. Jensen and T. V. W. Janssens, Appl. Catal., B, 2018, 238, 104–110 CrossRef.
- P. S. Hammershøi, Y. Jangjou, W. S. Epling, A. D. Jensen and T. V. W. Janssens, Appl. Catal., B, 2018, 226, 38–45 CrossRef.
- L. Ge, A. Wang, X. Hu, J. Zhang, J. He, P. Wang, L. Han and D. Zhang, Catal. Sci. Technol., 2023, 13, 4186–4196 RSC.
- V. V. Mesilov, S. L. Bergman, S. Dahlin, Y. Xiao, S. Xi, M. Zhirui, L. Xu, W. Chen, L. J. Pettersson and S. L. Bernasek, Appl. Catal., B, 2021, 284, 119756 CrossRef CAS.
- J. Du, X. Shi, Y. Shan, G. Xu, Y. Sun, Y. Wang, Y. Yu, W. Shan and H. He, Catal. Sci. Technol., 2020, 10, 1256–1263 RSC.
- J. Ma, S. Chang, F. Yu, H. Lai and Y. Zhao, Catalysts, 2022, 12, 1499–1510 CrossRef CAS.
- Y. Qiu, C. Fan, C. Sun, H. Zhu, W. Yi, J. Chen, L. Guo, X. Niu, J. Chen, Y. Peng, T. Zhang and J. Li, Catalysts, 2020, 10, 1–12 RSC.
- K. Wijayanti, K. Leistner, S. Chand, A. Kumar, K. Kamasamudram, N. W. Currier, A. Yezerets and L. Olsson, Catal. Sci. Technol., 2016, 6, 2565–2579 RSC.
- Y. Jangjou, D. Wang, A. Kumar, J. Li and W. S. Epling, ACS Catal., 2016, 6, 6612–6622 CrossRef CAS.
- Y. Jangjou, Q. Do, Y. Gu, L. G. Lim, H. Sun, D. Wang, A. Kumar, J. Li, L. C. Grabow and W. S. Epling, ACS Catal., 2018, 8, 1325–1337 CrossRef CAS.
- S. L. Bergman, S. Dahlin, V. V. Mesilov, Y. Xiao, J. Englund, S. Xi, C. Tang, M. Skoglundh, L. J. Pettersson and S. L. Bernasek, Appl. Catal., B, 2020, 269, 118722 CrossRef CAS.
- V. Mesilov, S. Dahlin, S. L. Bergman, S. Xi, J. Han, L. Olsson, L. J. Pettersson and S. L. Bernasek, Appl. Catal., B, 2021, 299, 120626–120638 CrossRef CAS.
- A. Yu. Molokova, E. Borfecchia, A. Martini, I. A. Pankin, C. Atzori, O. Mathon, S. Bordiga, F. Wen, P. N. R. Vennestrøm, G. Berlier, T. V. W. Janssens and K. A. Lomachenko, JACS Au, 2022, 2, 787–792 CrossRef CAS PubMed.
- A. Yu. Molokova, R. K. Abasabadi, E. Borfecchia, O. Mathon, S. Bordiga, F. Wen, G. Berlier, T. V. W. Janssens and K. A. Lomachenko, Chem. Sci., 2023, 14, 11521–11531 RSC.
- S. H. Krishna, A. Goswami, Y. Wang, C. B. Jones, D. P. Dean, J. T. Miller, W. F. Schneider and R. Gounder, Nat. Catal., 2023, 6, 276–285 CrossRef CAS.
- C. Negri, A. Martini, G. Deplano, K. A. Lomachenko, T. V. W. Janssens, E. Borfecchia, G. Berlier and S. Bordiga, Phys. Chem. Chem. Phys., 2021, 23, 18322–18337 RSC.
- E. Borfecchia, K. A. Lomachenko, F. Giordanino, H. Falsig, P. Beato, A. V. Soldatov, S. Bordiga and C. Lamberti, Chem. Sci., 2015, 6, 548–563 RSC.
- T. V. W. Janssens, H. Falsig, L. F. Lundegaard, P. N. R. Vennestrøm, S. B. Rasmussen, P. G. Moses, F. Giordanino, E. Borfecchia, K. A. Lomachenko, C. Lamberti, S. Bordiga, A. Godiksen, S. Mossin and P. Beato, ACS Catal., 2015, 5, 2832–2845 CrossRef CAS.
- K. A. Lomachenko, E. Borfecchia, C. Negri, G. Berlier, C. Lamberti, P. Beato, H. Falsig and S. Bordiga, J. Am. Chem. Soc., 2016, 138, 12025–12028 CrossRef CAS PubMed.
- C. Negri, T. Selleri, E. Borfecchia, A. Martini, K. A. Lomachenko, T. V. W. Janssens, M. Cutini, S. Bordiga and G. Berlier, J. Am. Chem. Soc., 2020, 142, 15884–15896 CrossRef CAS PubMed.
- A. Oda, H. Shionoya, Y. Hotta, T. Takewaki, K. Sawabe and A. Satsuma, ACS Catal., 2020, 10, 12333–12339 CrossRef CAS.
- F. C. Meunier, React. Chem. Eng., 2016, 1, 134–141 RSC.
- F. Giordanino, E. Borfecchia, K. A. Lomachenko, A. Lazzarini, G. Agostini, E. Gallo, A. V. Soldatov, P. Beato, S. Bordiga and C. Lamberti, J. Phys. Chem. Lett., 2014, 5, 1552–1559 CrossRef CAS PubMed.
- K. Wijayanti, K. Xie, A. Kumar, K. Kamasamudram and L. Olsson, Appl. Catal., B, 2017, 219, 142–154 CrossRef CAS.
- J. D. Bjerregaard, M. Votsmeier and H. Grönbeck, J. Catal., 2023, 417, 497–506 CrossRef CAS.
- H. Falsig, P. N. R. Vennestrøm, P. G. Moses and T. V. W. Janssens, Top. Catal., 2016, 59, 861–865 CrossRef CAS.
- L. Chen, H. Falsig, T. V. W. Janssens, J. Jansson, M. Skoglundh and H. Grönbeck, Catal. Sci. Technol., 2018, 8, 2131–2136 RSC.
- L. Chen, H. Falsig, T. V. W. Janssens and H. Grönbeck, J. Catal., 2018, 358, 179–186 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cy00129j |
| This journal is © The Royal Society of Chemistry 2024 |