Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Early stage techno-economic and environmental analysis of aluminium batteries†
Niklas
Lindahl
ab and
Patrik
Johansson
 *cd
*cd
aDepartment of Physics, Gothenburg University, SE-412 96 Göteborg, Sweden
bEnergy Conversion, Research Institutes of Sweden, SE-504 62, Borås, Sweden
cDepartment of Physics, Chalmers University of Technology, SE-412 96 Göteborg, Sweden. E-mail: patrik.johansson@chalmers.se
dALISTORE-European Research Institute, CNRS FR 3104, Hub de l’Energie, Rue Baudelocque, 80039 Amiens Cedex, France
First published on 1st February 2023
Abstract
For any proper evaluation of next generation energy storage systems technological, economic, and environmental performance metrics should be considered. Here conceptual cells and systems are designed for different aluminium battery (AlB) concepts, including both active and passive materials. Despite the fact that all AlBs use high-capacity metal anodes and materials with low cost and environmental impact, their energy densities differ vastly and only a few concepts become competitive taking all aspects into account. Notably, AlBs with high-performance inorganic cathodes have the potential to exhibit superior technological and environmental performance, should they be more reversible and energy efficient, while at the system level costs become comparable or slightly higher than for both AlBs with organic cathodes and lithium-ion batteries (LIBs). Overall, with continued development, AlBs should be able to complement LIBs, especially in light of their significantly lower demand for scarce materials.
Introduction
The ongoing transition to low-carbon energy systems is characterized by increasing amounts of renewable wind and solar power, leading to variability in sourcing, and thus requires flexible solutions.1,2 Energy storage provides flexibility, not least rechargeable battery energy storage systems (BESSs) with reversible conversion of electrical to chemical energy at very high roundtrip efficiency, most often ca. 90% at the system level.3 Today, rechargeable batteries, in particular lithium-ion batteries (LIBs), are applied in a large range of applications, from portable electronics and electric vehicles (EVs) to utility-scale BESSs.4,5 For mobile applications, energy density and charging time are the most important key performance indicators (KPIs) (in addition to the omni-important safety aspects), while for stationary applications, storage lifetime and cost are more important. For stationary BESSs used in energy arbitrage this translates e.g. to the KPIs for 2030 of a specific energy density of 250 W h kg−1 for 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at capital expenditures (CAPEX) of 0.03 € per kW h per cycle (system level).6 These are in stark contrast to the KPIs for EV application of 450 W h kg−1 for 2000 cycles and a CAPEX of 85 € per kW h at the pack level – rendering 0.04 € per kW h per cycle.6 Furthermore, most stakeholders in utility energy storage consider the environmental impact to be of equal importance to the economic performance and more important than the technological performance.3
000 cycles at capital expenditures (CAPEX) of 0.03 € per kW h per cycle (system level).6 These are in stark contrast to the KPIs for EV application of 450 W h kg−1 for 2000 cycles and a CAPEX of 85 € per kW h at the pack level – rendering 0.04 € per kW h per cycle.6 Furthermore, most stakeholders in utility energy storage consider the environmental impact to be of equal importance to the economic performance and more important than the technological performance.3
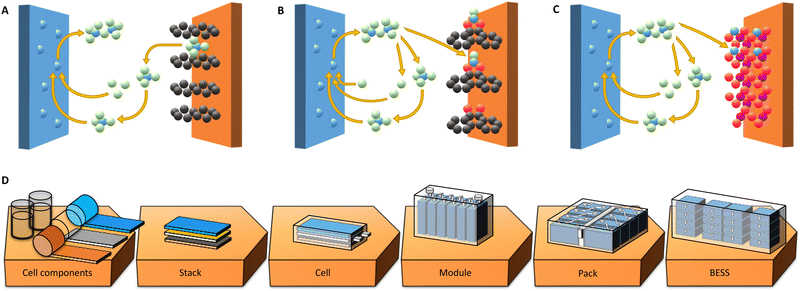
One battery chemistry, amongst the many next generation battery (NGB) chemistries being put forward at present to replace or complement today's LIBs,7,8 that is suitable to reach the KPIs for BESSs is aluminium batteries (AlBs).8–12 The main reasons are their fundamental promise of high capacities and energy densities, from the use of multivalent Al3+ ions and thus up to 3-electron transfer per cation; the very high capacity (2981 mA h g−1) of Al metal anodes; and, especially important for BESS applications, the base being abundant and low-cost materials. On the drawback side, the large amount of energy required to make Al metal naturally comes in, but the well-established highly efficient recycling schemes can largely compensate.9 Also, as for more battery specific properties, all AlBs are significantly limited by the rather high reduction potential of Al (−1.66 V vs. SHE), thus ca. 1.4 V above that of Li (−3.04 V vs. SHE), making it more difficult to create high-voltage cells. Yet, there are many conceptually different designs of AlBs originating from this common baseline, foremost with respect to the different types of cathodes employed e.g. graphitic, organic, or inorganic (Fig. 1(A)–(C)).
One intrinsic and common problem is the high charge/radius ratio of the Al3+ ion, which raises problems in finding electrolyte and cathode combinations allowing for both reversibility and high energy density. The most common electrolytes for AlBs are based on ionic liquids (ILs) that create Al2Cl7− and AlCl4− anions.10,12 The latter anions are intercalated when employing graphitic cathodes (Fig. 1(A)) and extracted from the electrolyte.13,14 Thus, using such cathodes the AlB energy density is fundamentally limited by the volume of electrolyte needed, which in turn depends on the density of ions in the electrolyte, rendering specific cell energy densities of no more than ca. 70 W h kg−1.14
Another approach is to employ the same type of electrolytes and organic cathodes, with redox centers of for example carbonyl groups, such as in quinones, where typically either AlCl2+ or AlCl2+ cations, rather than Al3+ cations, are made to coordinate (Fig. 1(B)).15,16 None of these three cations, however, are thermodynamically stable in the bulk of the electrolyte; they are formed by the dissociation of Al2Cl7− close to the cathode surface.7,17 Since the coordinated cations are both Cl-containing, the AlB concept using organic cathodes also derives its capacity partly from, and is likewise limited by, the electrolyte. A notable difference is that when employing AlCl2+ cations the amount of electrolyte required to store the same amount of charge as when using AlCl4− anions is fundamentally only 1/8.18,19 Indeed, higher specific energy densities have already been demonstrated and are envisaged to be able to reach ca. 200 W h kg−1.19,20
Finally, cathodes able to truly intercalate Al3+ cations, in a similar fashion to traditional inorganic cathodes employed in LIBs (Fig. 1(C)), would in principle endow AlBs with even higher energy densities. This is because they would not depend on the electrolyte in at all the same way. As of today, cathodes based on e.g. Mo, V, or Ti as the active transition metals have together with IL based electrolytes provided specific cell energy densities of 60–120 W h kg−1,21–23 while inorganic cathodes based on V or Mn and aqueous electrolytes have yielded ca. 22524 and 37025–27 W h kg−1, respectively. Although this may sound remarkable, the (very) strong interactions between the Al3+ ions and the cathode hosts result in high charging over-potentials and hence low energy efficiencies of ca. 40% and 70% for TiO2 and MnO2,23,27 respectively, compared to ca. 90% for AlBs with graphite or organic cathodes.14,20 Furthermore, these kinds of cells employing aqueous electrolytes have not yet demonstrated fully reversible stripping/plating of Al.28,29
For any NGB technology, prospective analyses of practical performance can reveal benefits and limitations, especially if the full chain from cell components to assembly for application and subsequent integration can be covered (Fig. 1(D)) and each level explicitly considered. While AlBs are at a very early stage of development and therefore practical cells are still lacking, they can be expected to be similar to LIBs at the manufacturing and assembly stages and levels.7,30,31 Hence, conceptual cells, taking into account the specific possibilities and limitations of the different concepts (Fig. 1(A)–(C)), can be modelled and provide essential information. This has indeed been done before for both LIBs and NGBs, although not for AlBs, through creating balanced cell stacks by a bottom-up approach including properties of both active and passive materials.32,33 One particularly significant difference as compared to LIBs is clearly the importance of the electrolyte and separator properties for those AlB concepts (Fig. 1(A) and (B)) where the electrolyte contributes actively to the cell energy density.33,34
Herein the generic path (Fig. 1(D)) from a single cell and its components to multiple-layer stacked cells, via putting modules into a battery pack, and finally to a BESS integrated in a container is followed.5,35 This is made not only for performance related KPIs, but also for monetary KPIs – hence enabling techno-economic modelling and analysis. The BatPaC software36 has been instrumental for this kind of analysis of cells and packs of commercial LIBs for EV application and has recently also been applied to NGBs.4,8 Here we apply this procedure to the three different AlB concepts, taking care to properly design and balance the cells, and benchmark them vs. several different LIB cells and storage solutions, that are all constructed in the same way.
For the corresponding environmental performance, life cycle analyses (LCAs) have been made bottom-up from materials at the cell level for several LIB chemistries applied in EVs,37 but indeed also for AlBs based on graphite cathodes.38 At the utility-scale, i.e. the BESS level, however, most studies of environmental impact have been based on commercially available modules, and this limits the possibilities to evaluate NGBs, as much of the input needed is not available. Therefore, we here choose a more accessible alternative; the long-term availability in the Earth's crust of the elements used, via the crustal scarcity potential (CSP).39 While this does not take into account any specific routes of production etc., it is at the same time not laden with the large uncertainties as large assumptions of such routes would introduce. To this we also add a minor global warming potential (GWP) analysis.
All of the above, from materials and conceptual cell design to utility-scale BESSs, is made as transparent as possible and all relevant data are outlined in a spreadsheet.
Results
Technical performance
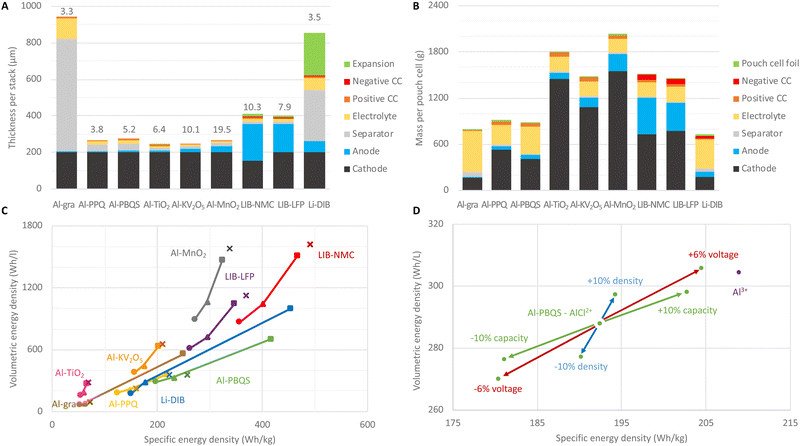
Systematic calculations of the amounts of various materials employed and the practically achievable energy densities were performed for several AlBs with cathodes of graphite (Al-gra); organic compounds, poly(phenanthrenequinone) (Al-PPQ) and poly(benzoquinonyl sulfide) (Al-PBQS); and inorganic hosts, titanium dioxide (Al-TiO2), potassium-vanadium pentoxide (Al-KV2O5), and manganese dioxide (Al-MnO2). In addition, LIBs with anodes of graphite and cathodes of lithium nickel-manganese-cobalt oxide (LIB-NMC), more specifically NMC811-based, and lithium iron phosphate (LIB-LFP), were created as well as a dual-ion battery based on two graphite electrodes (Li-DIB).Starting at the material level and cell designs, we first consider only the properties of the active materials (AM) used in the different concepts, wherefrom some very ideal and theoretical “cell” energy densities can be obtained (Table 1). Please note that for the cells based on graphitic (Al-gra and Li-DIB) and organic (Al-PPQ and Al-PBQS) cathodes the electrolyte needed must be taken into account. Hence, the exceptional capacity of the Al metal anode cannot be used single-handedly as a measure for the promise of all AlB cells, as should be clear by comparing columns 1 and 2 vs. columns 3 and 4 in Table 1. This process overall follows that often used to promote various NGBs (as well as novel materials for LIBs), but which is often also laden with many problems, not least transparency with respect to comparisons with commercial batteries.30,33,40
| Concept | Anode | Anode and electrolyte | Cathode | Cell | |||||
|---|---|---|---|---|---|---|---|---|---|
| Specific capacity | Volumetric capacity | Specific capacity | Volumetric capacity | Specific capacity | Volumetric capacity | Average voltage | Specific energy density | Volumetric energy density | |
| [mA h g−1] | [mA h cm−3] | [mA h g−1] | [mA h cm−3] | [mA hg −1] | [mA h cm−3] | [V] | [W h kg−1] | [W h L−1] | |
| Al-gra | 2981 | 8049 | 49 | 58 | 135 | 297 | 2.00 | 72 | 98 |
| Al-PPQ | 2981 | 8049 | 344 | 413 | 170 | 272 | 1.40 | 159 | 230 |
| Al-PBQS | 2981 | 8049 | 344 | 413 | 300 | 480 | 1.60 | 257 | 355 |
| Al-TiO2 | 2981 | 8049 | 2981 | 8049 | 110 | 465 | 0.64 | 68 | 282 |
| Al-KV2O5 | 2981 | 8049 | 2981 | 8049 | 225 | 716 | 1.00 | 209 | 657 |
| Al-MnO2 | 2981 | 8049 | 2981 | 8049 | 285 | 1428 | 1.30 | 338 | 1577 |
| LIB-NMC | 372 | 818 | 372 | 818 | 210 | 977 | 3.65 | 490 | 1625 |
| LIB-LFP | 372 | 818 | 372 | 818 | 160 | 584 | 3.30 | 369 | 1125 |
| Li-DIB | 372 | 818 | 77 | 107 | 140 | 308 | 4.50 | 223 | 358 |
The Al-PPQ and Al-PBQS cells are based on cathode materials with fair and high specific capacities, but as these have comparatively low volumetric capacities, due to the common feature of low density of organic compounds, the cells have rather low volumetric energy densities. The Al-gra cell, due to the larger electrolyte demand, is less than half as energy dense both gravimetrically and volumetrically. In contrast, the lower specific capacity and cell voltage of TiO2 are “compensated” for by the Al metal anode utilization and the higher densities of inorganic materials provide the Al-TiO2 cells almost similar volumetric energy densities to the Al-PBQS cells, which is ca. half that of Al-KV2O5 and 4 times lower than that for the Al-MnO2 cells. If the latter AlB concept can overcome its energy efficiency problems, all other things being equal, it would be outstanding and even match LIB-NMC in terms of energy density, albeit at a much lower cell voltage.
Thus, when estimating the cell energy densities that are practically obtainable, including electrolyte and passive materials, a slightly different picture emerges than when only looking at the anode and cathode materials. The cell design also deeply depends on the intended application, not least the energy/power performance needed. Here, the intended BESS application is energy arbitrage with daily charge and discharge for 4 h and therefore relatively thick electrodes of up to 200 μm are used.36,41 From this, the thicknesses of all other components are deduced (Fig. 1(D), Methods).
The high volumetric capacity of Al, and that it is used both as an anode and a current collector (CC) in combination, gives ca. 30% thinner single-cell stacks for AlBs than for LIBs (Fig. 2(A)). However, for Al-gra this advantage is lost by the very thick separator needed, and the same is true, to a somewhat lesser extent, for Li-DIBs.34 Furthermore, the graphite expansion requires extra volume in the stack design for both LIBs and Li-DIBs, but for Al-gra the 80% cathode expansion42 is more than compensated for by the 33% electrolyte volume decrease.43 The areal capacity is dependent upon and limited by the volumetric capacity and, hence, cells with inorganic cathodes come out better.
The material masses in a pouch cell (Fig. 2(B)) depend on the layer thicknesses in the stack, the number of stacks that fit into the cell, and the materials’ densities. The high densities of the inorganic cathode active materials lead to both larger masses per cell for these AlBs and a larger percentage of the total cell masses (ca. 75%) than for both AlBs with organic cathodes and LIBs (ca. 50%) as well as for Al-gra and Li-DIBs (ca. 25%). Thus, the relative reduction from theoretical to practical specific capacity is less prominent for the former AlB concept.
Overall, the evolution of the energy densities (Fig. 2(C)) from only electrode active materials (step 1, squares), via the making of electrodes and introducing the electrolyte (step 2, triangles), to practical cells (step 3, circles) shows that the losses between steps 1 and 2 are more pronounced for the cell concepts requiring larger electrolyte amounts. The loss in volumetric energy density between steps 1 and 3 is e.g. >80% for the Al-gra and Li-DIB cell designs. However, the loss of ca. 50% is similar for Al-PPQ, while only slightly higher for Al-PBQS (60%) and correspondingly slightly lower for AlBs and LIBs with inorganic cathodes (ca. 40%). This is largely due to the fact that the volume of electrolyte, needed in practical cells to fill the porous electrodes, is similar. Hence, the theoretical estimates (Fig. 2(C), crosses, and Table 1, columns 8 and 9) are closer to step 2 for the concepts using graphitic or organic cathodes, but closer to step 1 for those with inorganic cathodes.
A comparison between the best performing AlBs reveals that, at the cell level, Al-PBQS has ca. 75% of the specific energy density but only ca. 1/3 of the volumetric energy density of Al-MnO2. For the Al-PBQS cell (Fig. 2(D)) an increase in density or capacity of the organic cathode by 10% only increases the volumetric energy density by ca. 3%. This is because the increased capacities also require larger volumes of electrolyte. If the reaction mechanism could be changed to involve Al3+ instead of AlCl2+ the increase in volumetric energy density would still only be ca. 6%, as an electrolyte is needed to fill the pores. However, if it employed AlCl2+ the volumetric energy density would be reduced by more than 60% (not shown), which stresses the importance of an accurate determination of the reaction mechanism.12
Economic performance
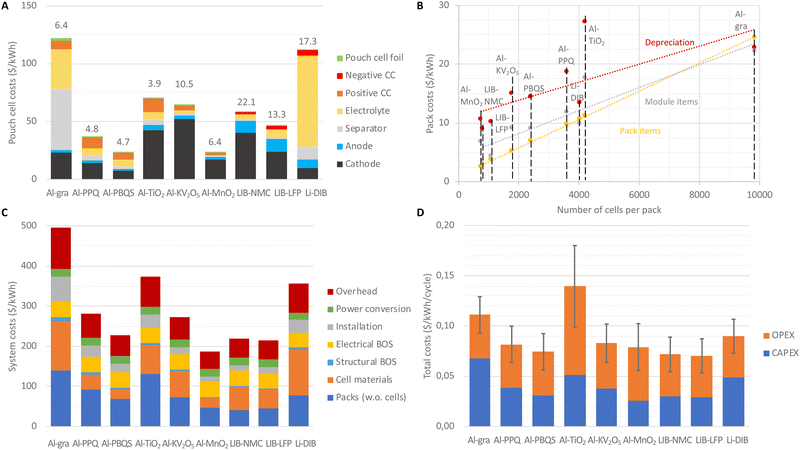
The use of low-cost materials often gives rise to claims of AlBs being inherently low cost, but the relevant metric is cost per unit of (useable) energy stored. Applying this measure, first at the cell level, the Al-PBQS and Al-MnO2 cells achieve ca. 25 $ per kW h, and LIB-NMC and LIB-LFP have ca. 150 and 100% higher cost, respectively, whereas Al-gra has a vast cost of ca. 120 $ per kW h (Fig. 3(A)). Both of the other inorganic cathode-based AlBs come out as relatively expensive, despite the low material costs for Al-TiO2. As compared to the literature, our costs are similar for Al-gra11 and slightly lower for LIB-NMC, much due to our cell being designed with a thicker cathode.5,41 Breaking down the costs (Fig. 3(A)), the inorganic cathodes make up a large majority (70–90%) of the material costs for those AlB cells (with a notable exception for Al-TiO2), but the cathode cost is much less for other AlB cells, e.g. 30% for Al-PBQS. The latter renders the cost of the passive materials relatively more important. Admittedly, there is a need for a caveat here; whereas the costs for materials in LIBs are based on commercial cell costs,36 and therefore rather trustworthy even if volatile, some materials used for the AlBs, e.g. the IL based electrolyte and the organic cathodes, are rather unique and their costs in large quantities are therefore laden with much larger uncertainties. Although these are relatively important at the cell level, where they contribute ca. half of the costs, as described below, at the system level their contribution is significantly smaller. Hence, any deviations, both positive and negative, e.g. as compared to cathode material costs for inorganic LIBs, would have a relatively limited impact overall.Moving to battery-packs and utility-scale BESSs (Fig. 3(B)–(D)) renders another set of pictures compared to that solely based on the cells. The higher volumetric energy densities of cells based on inorganic cathodes provide cost benefits; as in the pack-design the cost in general decreases with fewer cells needed (Fig. 3(B)). In more detail, for pack items the costs are mainly due to the battery jacket, which scales with the outer area of the pack, while for module items, the cost is dominated by state-of-charge-controllers needed for each group of cells connected in parallel. Overall the lower cell voltages of any AlB, as compared to LIBs, result in more cells in series in order to reach the desired pack-voltage and thus more module items are needed.
Looking at different AlB designs and production aspects, the lower areal capacity of organic cathodes as compared to the inorganic cathodes (Fig. 2(A)) means that a larger number of electrodes are required to reach the same pack-energy. For a fixed rate of electrode production, a larger plant is needed and therefore also increased costs for depreciation of manufacturing equipment. Similarly, the low specific energies of Al-gra, Al-TiO2, and Al-V2C require larger masses of battery materials and, hence, more equipment and higher cost for depreciation.
Finally, the volumetric energy density at the pack-level determines the amount of energy stored in each container and thereby the number of containers needed to create the BESSs for a given energy. Again, cells based on inorganic cathodes obtain cost advantages, as the costs for the structural balance of system (BOS) and installation and thereby overheads are reduced (Fig. 3(C)). In the end, the complete BESS costs become ca. 220 $ per kW h for both the Al-PBQS and the LIB concepts, despite the cell materials making up 11% for the former and 27% for LIB-NMC.
For the application of daily energy arbitrage, over the assumed lifetime (20 years and ca. 7300 cycles), the capital expenditure (CAPEX) for the BESSs reaches the KPI for the Al-PBQS, Al-MnO2, and LIB based solutions (Fig. 3(D)). However, the lower efficiencies of the AlB concepts based on inorganic cathodes lead to higher operational expenditures (OPEX), mainly governed by the higher cost associated with the electricity used to charge them, which thus depends on the average electricity price – here set to a modest value of 0.03 ± 0.01 $ per kW h. Therefore total costs (CAPEX + OPEX) are the lowest for the Al-PBQS and LIB BESSs at ca. 0.07 $ per kW h per cycle, closely followed by the Al-PPQ, Al-KV2O5, and Al-MnO2 BESSs at ca. 0.08 $ per kW h per cycle. This small difference can certainly be turned around depending on the exact chemistry employed and this is especially true for the less mature AlB technology and concepts.
Environmental performance
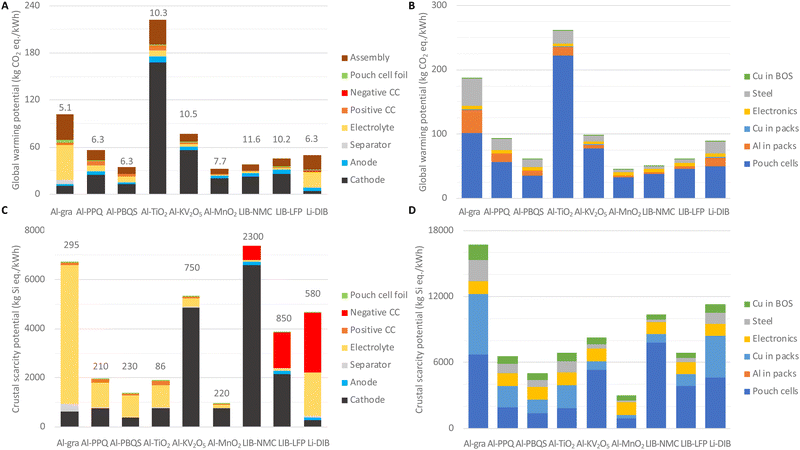
Similar to the economic performance analysis above, all environmental performance analysis is performed in relation to the useable energy storage. Both the GWPs and the CSPs can show whether using abundant materials, often a claim made for AlBs (and other NGBs), is truly beneficial.It is shown that at the cell level Al-gra, Al-TiO2, and Al-KV2O5 have high GWPs, due to lower specific energies and/or high GWPs per mass (Fig. 4(A)). Overall, the cathodes contribute more than half of the GWP for the concepts based on inorganic cathodes, whereas for AlBs based on organic cathodes the electrolyte and the cell assembly add up to ca. 40% of the total GWP, and for Al-gra and Li-DIBs this even makes up 75%. Compared to the literature, our result for LIB-NMC is slightly lower than for similar cells for EVs produced in gigafactories from low carbon energy,44 again mostly due to our high-energy cell design.
At the BESS level (Fig. 4(B)), the main contributions to the GWPs do come from the cell materials. However, the concepts with lower volumetric energy densities require more housing materials, mainly Al and steel in the larger packs and for the BESS containers. For these concepts the use of recycled Al would provide only marginal reductions of the total GWP at the cell level, but together with the use of low-carbon a reduction of ca. 35% at the BESS level can be obtained. Recycling of cell materials could clearly reduce the net GWPs, but yet the benefits are most likely larger for concepts based on more precious materials, e.g.LIB-NMC, than for concepts based on more abundant materials.45 Although the minor GWP analysis here enables basic comparisons, detailed LCAs are required for more complete evaluations of the AlB-concepts.
The CSPs present clear advantages for most AlBs (except for Al-gra and Al-KV2O5) at the cell level (Fig. 4(C)). For the AlBs based on organic cathodes and even more so for Al-gra, the majority of the CSP comes from the Cl in the electrolyte. For the concepts based on inorganic cathodes, some of the metals used (V, Ni, Co, and Li) have an effect that cannot even be offset by the high specific energy of the LIB-NMC cells, or for Al-KV2O5. However, the low CSP of Ti renders a sufficiently low CSP per mass for the Al-TiO2 design (Fig. 4(C)) to counter its low specific energy density, and the resulting CSP is almost as low as that for the organic AlBs. Although Li contributes a significant and similar (ca. 1800 kg Si eq. per kW h) amount to the CSPs in both LIBs and Li-DIB, Ni contributes even more with ca. 54% of the CSP for the NMC cathode. Furthermore, for LIBs the negative CC that has to be made from Cu has a large impact on the total CSP, notably >50% for Li-DIB (that anyhow has a rather average total CSP due to the low contribution from graphite electrodes).
At the BESS level (Fig. 4(D)), the concepts with lower volumetric energy densities, thus the same problematic cases as in the economic analysis and for the GWP above, require more wires connecting the larger number of cells inside the pack and BESS containers. Hence, the relative difference in CSPs between AlBs and LIBs becomes smaller when electronics and Cu-wires are also considered. These in fact add up to ca. 60% of the CSP for Al-PPQ, Al-PBQS, Al-TiO2 and Al-MnO2, but nonetheless, BESSs based on Al-PBQS and in particular Al-MnO2 have significantly lower CSPs than any BESS built from LIB cells and packs. As a side-note, the latter LIB-LFP reassuringly comes out to be significantly better than LIB-NMC and Li-DIB.
Conclusions
In this article, we have evaluated the performance of various AlB concepts based on demonstrated material properties and designs similar to current LIBs. We find that while the low volumetric energy densities hamper Al-gra, next generation organic and inorganic AlBs remain promising. The added value of including performance in all most important aspects is stressed; e.g. for Al-TiO2 the technical performance is relatively poor, the cell level material cost and CSP are fair, and the system lifetime costs and GWP become prohibitive. Overall, the development of novel materials for AlBs, and for NGBs in general, would thus benefit from including estimates of performance in a broad sense, and if not at a complete system level at least at a cell level looking at cell energy density, materials costs and CSP.Yet, most experimental AlB-studies use comparatively low areal capacity cathodes and combine these with a large excess of Al anodes and electrolytes, while optimised electrolyte and electrode loadings are ultimately needed. Lean electrolyte conditions could, however, lead to severely reduced power performance, as observed for Al-gra,46 and the latter could give rise to current densities (for Al-MnO2ca. 5 mA cm−2) higher than optimal for the 2D-foil Al-anodes, which could be mitigated by introducing 3D-anodes.18,47 Furthermore, the cycle-life we assume here has so far only been achieved for Al-gra,13 and fully reversible cell reactions for Al-MnO2 likely require the development of completely novel electrolytes.28 Overall, e.g. Cl-free electrolytes48 could reduce the CSP by more than half at the cell level, all other things being equal.
Even for the most promising AlB concepts, the excellent performance at the cell level still provides relatively small improvements at the BESS level as compared to LIBs, both in terms of costs and GWPs. Modern cell to pack designs have improved the energy densities at the pack level,49 and alternative BESS designs optimized for AlBs could similarly provide relative benefits. Combining these there are thus both significant promise and challenges for the various AlB concepts.
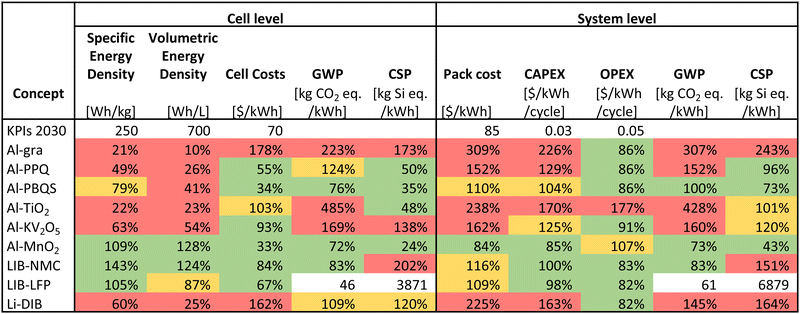
The efforts needed to tackle the above challenges are motivated by the promise of AlBs to reach the KPIs for stationary storage by 2030 (Table 2). Although all AlB concepts are based on materials of relatively low cost and environmental impact, these are preferably also to be combined with sufficiently high energy densities, as obtained e.g. for Al-PBQS and Al-MnO2. Table 2 shows that while the here considered AlB concepts based on organic cathodes do not meet the KPIs for the energy densities at the cell level, they perform (very) well for the economic KPIs, while the Al-MnO2 AlB both reaches the technological KPIs and has the best CAPEX at the BESS level (but still, at present, falls behind in OPEX due to its lower energy efficiency).
Yet, the main benefit of the AlB concepts will anyhow likely be their low CSPs, lacking appropriate LCAs, indicating not only that AlBs could be more sustainable in long-term than LIBs, but also that they will be less susceptible to supply shortages during the ongoing and increasing fast growth in demand for energy storage.4,31 All in all, this implies that AlBs, with either organic or inorganic cathodes, could become a complementary technology to LIBs and readily enable a faster transition to a more sustainable global low-carbon energy system.
Methods
Technical performance
The material properties and costs were mainly obtained from BatPaC (version 5.0).36 The properties of materials needed for the AlBs that are not included in BatPaC should be considered as optimistic, but anyhow realistic estimates, with some continued advancement. The capacity we use for PBQS is based on a similar utilization of the theoretical capacity to that observed for Al-PPQ (ca. 70%),20 and demonstrated in LIBs,50 but not yet for AlBs.16 In the cells using inorganic cathode active materials, the electrolyte volume was set by the volume of the pores in electrodes and separator combined, while for AlBs with graphitic or organic cathodes, and for Li-DIB, the electrolyte was set to balance the capacity. For all of the latter cells, the separator thickness was set to provide sufficient pore volume to incorporate the electrolyte needed.33,34 For each stack, current collectors and the volume needed for electrode expansion are added.The intended application, daily energy arbitrage with charge (and discharge) in 4 h, means a cell design optimized with respect to energy density, but with lower power density demand, i.e. a high E/P number. Here we assume an electrode thickness of 200 μm, which is challenging but possible.36,41 For the AlBs the anode capacity is at least in 50% surplus, due to the need for the Al metal combined anode and CC to keep its mechanical integrity also when the cell is fully discharged. The AlBs use a cathode CC of 14 μm Al foil coated on both sides with 500 nm of dichromium nitride (Cr2N) to prevent corrosion in the Cl-containing IL-based electrolyte.51 All LIBs use an anode CC of 10 μm Cu foil and a cathode CC of 15 μm Al foil.36 In the stack assembly, double-coated electrodes are assumed and hence the CC thickness is shared.32 The pouch cells are 2 cm thick with the anodes set to be 30 cm long and 10 cm wide (and the cathodes 2 mm less in each lateral dimension).
Also at the pack level, BatPaC provided properties and costs of the components.36 For each cell chemistry, the material properties and module configuration were adjusted to give similar pouch cell sizes and energy densities in the BatPaC-model to those in the conceptual cells. The packs were set to provide ca. 960 V and ca. 350 kW h usable energy (85% of the installed energy). The BESS modelled is to provide 60 MW during 4 h, i.e. 240 MWh usable capacity, and was based on modular 40 ft ISO shipping containers with packs of battery modules together with structural and electronic balance of system (BOS) in addition to the power conversion system (PCS).52,53 The volume of the battery packs was set to be 35% of the container volume.
Economic performance
From the materials contents, the materials costs were calculated at the cell-level. The costs of materials in AlBs not included in BatPaC were obtained from other techno-economic analyses: graphite and separators from DIBs;34 organic cathode materials from disulfonic acid, as a proxy for both PPQ and PBQS;54 and inorganic cathode materials from various literature sources: TiO2,55 V2O5 as a proxy for K0.5V2O5,56 and MnO2.57 The cost of the IL-based electrolyte was obtained from a cost analysis of Al-gra.11 The cost of the CC coating was obtained from costs of the thin films of Cr,58 adjusted for the sputtering conditions for Cr2N.51BatPaC provides costs for purchased items and non-materials. The latter include costs of depreciation (for a “Gigafactory” producing 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 packs i.e. ca. 40 GWh per year), direct labor, and overheads.36 Purchased items include e.g. cell-interconnects and SOC-controllers in module enclosures assembled into a pack with interconnects and a battery management system (BMS) enclosed in a battery jacket. From the complete costs for the construction of a BESS of 60 MW up to 4 h duration, the costs per MW and per container could be obtained for structural BOS, electrical BOS, installation, PCS, and overheads, respectively.59 The OPEX was obtained from the costs for daily full charging with electricity at an average cost of 0.03 $ per kW h, over a life-time of 20 years, for operation and maintenance.3
000 packs i.e. ca. 40 GWh per year), direct labor, and overheads.36 Purchased items include e.g. cell-interconnects and SOC-controllers in module enclosures assembled into a pack with interconnects and a battery management system (BMS) enclosed in a battery jacket. From the complete costs for the construction of a BESS of 60 MW up to 4 h duration, the costs per MW and per container could be obtained for structural BOS, electrical BOS, installation, PCS, and overheads, respectively.59 The OPEX was obtained from the costs for daily full charging with electricity at an average cost of 0.03 $ per kW h, over a life-time of 20 years, for operation and maintenance.3
Environmental performance
From the material contents, the environmental impacts were calculated. The GWPs for materials in LIBs and packs were obtained from LCAs performed for LIB-NMC and LIB-LFP.37,60 The specific GWPs for materials exclusively found in AlBs and cell assembly (for both AlBs and LIBs based on renewable electricity only) were obtained from a LCA made for Al-gra.38 The GWPs of cathode materials in AlBs were calculated from LCAs for calix[4]quinone as a proxy for PPQ and PBQS,61 and for TiO2,62 V2O5 as a proxy for K0.5V2O556 and Mn2O.63 The GWP for the AlB IL-based electrolyte was obtained by combining the GWPs for the AlCl3 salt and the [EMIM]Cl IL.64 The GWP of the CC-coating was calculated from LCA for sputtered Cr.58The CSPs for virgin crust elements were used, except for Cl and Cu for which the CSPs were weighted to also account for supplies from other sources, sea-water and recycling, respectively.39 For the CSP of the electrical BOS and PCS, the electronics were assumed to have the compositions as inverters in EVs.65
Author contributions
Niklas Lindahl: conceptualization, funding acquisition, investigation, methodology, project administration, visualization, writing – original draft; Patrik Johansson: conceptualization, funding acquisition, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research reported herein and the report itself have been supported by the Swedish Energy Agency through grants #50121-1 and #43525-1. Our work was significantly improved by the informative discussions with Dr Richard Arvidsson, Chalmers, on environmental impacts; Dr Johan Fridner, Norsk Hydro, on pack level properties; and Dr Jan Eriksson, Hitachi Energy, on system level properties.References
- P. D. Lund, J. Lindgren, J. Mikkola and J. Salpakari, Renewable Sustainable Energy Rev., 2015, 45, 785–807 CrossRef.
- M. S. Ziegler, J. M. Mueller, G. D. Pereira, J. Song, M. Ferrara, Y. M. Chiang and J. E. Trancik, Joule, 2019, 3, 2134–2153 CrossRef.
- M. Baumann, J. Peters and M. Weil, Energy Technol., 2020, 8, 1901019 CrossRef.
- C. Vaalma, D. Buchholz, M. Weil and S. Passerini, Nat. Rev. Mater., 2018, 3, 18013 CrossRef.
- R. Schmuch, R. Wagner, G. Hörpel, T. Placke and M. Winter, Nat. Energy, 2018, 3, 267–278 CrossRef CAS.
- Batteries Europe – Strategic Research Agenda for batteries, 2020.
- Y. Liang, H. Dong, D. Aurbach and Y. Yao, Nat. Energy, 2020, 5, 646–656 CrossRef CAS.
- A. Ponrouch, J. Bitenc, R. Dominko, N. Lindahl, P. Johansson and M. R. Palacin, Energy Storage Mater., 2019, 20, 253–262 CrossRef.
- T. Leisegang, F. Meutzner, M. Zschornak, W. Münchgesang, R. Schmid, T. Nestler, R. A. Eremin, A. A. Kabanov, V. A. Blatov and D. C. Meyer, Front. Chem., 2019, 7, 268 CrossRef CAS PubMed.
- B. Craig, T. Schoetz, A. Cruden and C. Ponce de Leon, Renewable Sustainable Energy Rev., 2020, 133, 110100 CrossRef CAS.
- G. A. Elia, K. V. Kravchyk, M. V. Kovalenko, J. Chacón, A. Holland and R. G. A. Wills, J. Power Sources, 2021, 481, 228870 CrossRef CAS.
- K. L. Ng, B. Amrithraj and G. Azimi, Joule, 2022, 6, 134–170 CrossRef CAS.
- M.-C. Lin, M. Gong, B. Lu, Y. Wu, D.-Y. Wang, M. Guan, M. Angell, C. Chen, J. Yang, B.-J. Hwang and H. Dai, Nature, 2015, 520, 324–328 CrossRef CAS PubMed.
- K. V. Kravchyk, S. Wang, L. Piveteau and M. V. Kovalenko, Chem. Mater., 2017, 29, 4484–4492 CrossRef CAS.
- D. J. Kim, D. J. Yoo, M. T. Otley, A. Prokofjevs, C. Pezzato, M. Owczarek, S. J. Lee, J. W. Choi and J. F. Stoddart, Nat. Energy, 2019, 4, 51–59 CrossRef CAS.
- J. Bitenc, N. Lindahl, A. Vizintin, M. E. Abdelhamid, R. Dominko and P. Johansson, Energy Storage Mater., 2020, 24, 379–383 CrossRef.
- D. J. Yoo and J. W. Choi, J. Phys. Chem. Lett., 2020, 11, 2384–2392 CrossRef CAS PubMed.
- N. Lindahl, J. Bitenc, R. Dominko and P. Johansson, Adv. Funct. Mater., 2020, 2004573, 3–9 Search PubMed.
- D. Yoo, M. Heeney, F. Glöcklhofer and J. W. Choi, Nat. Commun., 2021, 12, 1–9 CrossRef PubMed.
- J. Bitenc, U. Košir, A. Vizintin, N. Lindahl, A. Krajnc, K. Pirnat, I. Jerman and R. Dominko, Energy Mater. Adv., 2021, 2021, 1–9 Search PubMed.
- W. Yang, H. Lu, Y. Cao, B. Xu, Y. Deng and W. Cai, ACS Sustainable Chem. Eng., 2019, 7, 4861–4867 CrossRef CAS.
- A. Vahidmohammadi, A. Hadjikhani, S. Shahbazmohamadi and M. Beidaghi, ACS Nano, 2017, 11, 11135–11144 CrossRef CAS PubMed.
- S. Wang, K. V. Kravchyk, S. Pigeot-Rémy, W. Tang, F. Krumeich, M. Wörle, M. I. Bodnarchuk, S. Cassaignon, O. Durupthy, S. Zhao, C. Sanchez and M. V. Kovalenko, ACS Appl. Nano Mater., 2019, 2, 6428–6435 CrossRef CAS.
- X. Li, Y. Tang, C. Li, H. Lv, H. Fan, W. Wang, T. Cai, Y. Cui, W. Xing, Z. Yan, C. Zhi and H. Li, J. Mater. Chem. A, 2022, 10, 4739–4748 RSC.
- C. Wu, S. Gu, Q. Zhang, Y. Bai, M. Li, Y. Yuan, H. Wang, X. Liu, Y. Yuan, N. Zhu, F. Wu, H. Li, L. Gu and J. Lu, Nat. Commun., 2019, 10, 73 CrossRef CAS PubMed.
- S. He, J. Wang, X. Zhang, J. Chen, Z. Wang, T. Yang, Z. Liu, Y. Liang, B. Wang, S. Liu, L. Zhang, J. Huang, J. Huang, L. A. O’Dell and H. Yu, Adv. Funct. Mater., 2019, 29, 1905228 CrossRef CAS.
- W. Pan, J. Mao, Y. Wang, X. Zhao, K. W. Leong, S. Luo, Y. Chen and D. Y. C. Leung, Small Methods, 2021, 5, 2100491 CrossRef CAS PubMed.
- T. Dong, K. L. Ng, Y. Wang, O. Voznyy and G. Azimi, Adv. Energy Mater., 2021, 11, 2100077 CrossRef CAS.
- G. Pastel, Y. Chen, T. P. Pollard, M. A. Schroeder, M. E. Bowden, A. Zheng, N. T. Hahn, L. Ma, V. Murugesan, J. Ho, M. Garaga, O. A. Borodin, K. T. Mueller, S. G. Greenbaum and K. Xu, Energy Environ. Sci., 2022, 15, 2460–2469 RSC.
- E. Faegh, B. Ng, D. Hayman and W. E. Mustain, Nat. Energy, 2021, 6, 21–29 CrossRef CAS.
- F. Duffner, N. Kronemeyer, J. Tübke, J. Leker, M. Winter and R. Schmuch, Nat. Energy, 2021, 6, 123–134 CrossRef CAS.
- E. J. Berg, C. Villevieille, D. Streich, S. Trabesinger and P. Novák, J. Electrochem. Soc., 2015, 162, A2468–A2475 CrossRef CAS.
- J. Betz, G. Bieker, P. Meister, T. Placke, M. Winter and R. Schmuch, Adv. Energy Mater., 2019, 9, 1803170 CrossRef.
- T. Placke, A. Heckmann, R. Schmuch, P. Meister, K. Beltrop and M. Winter, Joule, 2018, 2, 2528–2550 CrossRef CAS.
- A. Kwade, W. Haselrieder, R. Leithoff, A. Modlinger, F. Dietrich and K. Droeder, Nat. Energy, 2018, 3, 290–300 CrossRef.
- P. Nelson, K. Gallagher, I. Bloom and D. Dees, BatPac v5.0, 2022, https://www.anl.gov/cse/batpac-model-software.
- Q. Dai, J. C. Kelly, L. Gaines and M. Wang, Batteries, 2019, 5, 48 CrossRef CAS.
- M. A. S. Delgado, L. Usai, L. A. W. Ellingsen, Q. Pan and A. H. Strømman, Materials, 2019, 12, 1–14 CrossRef PubMed.
- R. Arvidsson, M. L. Söderman, B. A. Sandén, A. Nordelöf, H. André and A. M. Tillman, Int. J. Life Cycle Assess., 2020, 25, 1805–1817 CrossRef CAS.
- P. Johansson, S. Alvi, P. Ghorbanzade, M. Karlsmo, L. Loaiza, V. Thangavel, K. Westman and F. Årén, Batteries Supercaps, 2021, 4, 1785–1788 CrossRef.
- R. E. Ciez and D. Steingart, Joule, 2020, 4, 597–614 CrossRef CAS.
- G. A. Elia, G. Greco, P. H. Kamm, F. García-Moreno, S. Raoux and R. Hahn, Adv. Funct. Mater., 2020, 30, 2003913 CrossRef CAS.
- K. V. Kravchyk and M. V. Kovalenko, Adv. Energy Mater., 2019, 9, 1901749 CrossRef.
- M. Chordia, A. Nordelöf and L. A. W. Ellingsen, Int. J. Life Cycle Assess., 2021, 26, 2024–2039 CrossRef CAS.
- J. F. Peters, M. Baumann, J. R. Binder and M. Weil, Sustainable Energy Fuels, 2021, 6414–6429 RSC.
- K. V. Kravchyk, C. Seno and M. V. Kovalenko, ACS Energy Lett., 2020, 545–549 CrossRef CAS.
- J. Zheng, D. C. Bock, T. Tang, Q. Zhao, J. Yin, K. R. Tallman, G. Wheeler, X. Liu, Y. Deng, S. Jin, A. C. Marschilok, E. S. Takeuchi, K. J. Takeuchi and L. A. Archer, Nat. Energy, 2021, 6, 398–406 CrossRef CAS.
- T. Mandai and P. Johansson, J. Mater. Chem. A, 2015, 3, 12230–12239 RSC.
- X. G. Yang, T. Liu and C. Y. Wang, Nat. Energy, 2021, 6, 176–185 CrossRef CAS.
- Z. Song, Y. Qian, T. Zhang, M. Otani and H. Zhou, Adv. Sci., 2015, 2, 1–9 Search PubMed.
- S. Wang, K. V. Kravchyk, A. N. Filippin, U. Müller, A. N. Tiwari, S. Buecheler, M. I. Bodnarchuk and M. V. Kovalenko, Adv. Sci., 2018, 5, 1700712 CrossRef PubMed.
- B. Westlake, Recycling and Disposal of Battery-Based Grid Energy Storage Systems: A Preliminary Investigation, EPRI, Palo Alto, CA, 2017, 3002006911.
- ABB White Paper: Utility-scale battery energy storage system (BESS) - BESS 4.0 MWh system design, p. 2021.
- V. Dieterich, J. D. Milshtein, J. L. Barton, T. J. Carney, R. M. Darling and F. R. Brushett, Transl. Mater. Res., 2018, 5, 034001 CrossRef.
- D. T. Cuskelly, E. H. Kisi and H. O. Sugo, J. Solid State Chem., 2016, 233, 150–157 CrossRef CAS.
- S. Weber, J. F. Peters, M. Baumann and M. Weil, Environ. Sci. Technol., 2018, 52, 10864–10873 CrossRef CAS PubMed.
- N. Susarla and S. Ahmed, Estimating the cost and energy demand of producing lithium manganese oxide for Li-ion batteries, United States: N. p., 2020, DOI:10.2172/1607686.
- A. Merlo and G. Léonard, Materials, 2021, 14, 3823 CrossRef CAS PubMed.
- V. Ramasamy, D. Feldman, J. Desai and R. Margolis, U.S. Solar Photovoltaic System and Energy Storage Cost Benchmarks: Q1 2021, National Renewable Energy Laboratory, NREL/TP-7A40-80694, 2021, https://www.nrel.gov/docs/fy22osti/80694.pdf.
- L. V. Thomas, O. Schmidt, A. Gambhir, S. Few and I. Staffell, J. Energy Storage, 2020, 28, 101230 CrossRef.
- M. Iturrondobeitia, O. Akizu-Gardoki, O. Amondarain, R. Minguez and E. Lizundia, Adv. Sustainable Syst., 2022, 6, 2100308 CrossRef CAS.
- S. Middlemas, Z. Fang and P. Fan, J. Cleaner Prod., 2014, 1–11 Search PubMed.
- C. Spanos, D. E. Turney and V. Fthenakis, Renewable Sustainable Energy Rev., 2015, 43, 478–494 CrossRef CAS.
- S. Righi, A. Morfino, P. Galletti, C. Samorì, A. Tugnoli and C. Stramigioli, Green Chem., 2011, 13, 367–375 RSC.
- A. Nordelöf, M. Alatalo and M. L. Söderman, Int. J. Life Cycle Assess., 2019, 24, 78–92 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Spreadsheets with calculations made. See DOI: https://doi.org/10.1039/d2ya00253a |
| This journal is © The Royal Society of Chemistry 2023 |