Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A first estimate of blue carbon associated with oil & gas industry marine infrastructure
Abigail J.
Davies
 *a and
Astley
Hastings
b
*a and
Astley
Hastings
b
aThe National Decommissioning Centre, University of Aberdeen, Newburgh, Scotland, UK. E-mail: a.davies.19@abdn.ac.uk
bThe School of Biological Sciences, University of Aberdeen, Aberdeen, Scotland, UK. E-mail: astley.hastings@abdn.ac.uk
First published on 8th November 2023
Abstract
Oil and gas industry manmade structures (OGIMMS) in the marine environment can support thriving and biodiverse ecosystems. ‘Clear seabed’ policies require that all OGIMMS are removed once commercial activity has ceased, thereby removing a large proportion of the ecosystem and leaving the remaining community degraded beyond repair. The environmental impact of this method of decommissioning is huge and wide ranging, but no studies have to date looked at the possible impacts that removing these ecosystems has to climate change. This first of its kind study modelled biomass associated with OGIMMS and the potential Blue Carbon (BC) that these ecosystems may represent. The study found that in the UK North Sea (UKNS) there is currently 1.75 MtC of BC adhered to OGIMMS and globally there is 64 MtC. The study investigated the consequences of removing this BC and found that if it is allowed to degrade in landfill, up to 96 MtCO2e of greenhouse gas (GHG) emissions will be released from UK BC and globally up to 2,730 MtCO2e of GHG emissions will be released. Furthermore, forward modelling techniques were uniquely employed to look at future potential growth of these ecosystem and found that in the UKNS if the ecosystem was allowed to remain in place by decommissioning in situ, at 100 years since installation, UKNS OGIMMS BC could grow to 27 MtC and global BC could grow to 264 MtC. The study demonstrates the vast potential of BC associated with OGIMMS in the marine environment to sequester carbon over the long term and that current clear seabed practises damage these important ecosystems beyond repair, destroying current BC stocks and the vast potential BC stocks that could develop over time, as well as releasing large volumes of GHG emissions from the degradation of the biomass.
Environmental significanceOur paper represents a completely new model that for the first time quantifies carbon sequestration in the marine environment, Blue Carbon (BC) associated with oil & gas industry infrastructure. We use a multi-disciplinary approach that combines ecosystem modelling with forward modelling techniques developed in geophysical basin studies to understand current BC mass and future growth potential of this biomass. The work presented advances our knowledge and understanding of a wide range of topics including ecosystem development, GHG emissions from marine biowaste, GHG emissions from decommissioning oil & gas industry infrastructure and nature-based solutions. Furthermore, the work also has global marine policy implications. |
1 Introduction
The oil and gas industry (OGI) has been active in the marine environment for more than 70 years1 in the exploration, production and transport of hydrocarbons (HC). Globally there are currently around 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 platforms, 180,000 km of pipeline2 and a vast array of other types of infrastructure in place in various marine settings. Once the HC fields are depleted and production of the remaining HCs becomes either technically or financially unfeasible, wells must be plugged and abandoned (P&A) with the intention of securing any remaining HCs in the subsurface. Once P&A is complete, the decommissioning of the structures located above the mudline, on the seabed and in the water column can begin. This study focussed on the decommissioning of these above mudline structures, and did not include P&A.
000 platforms, 180,000 km of pipeline2 and a vast array of other types of infrastructure in place in various marine settings. Once the HC fields are depleted and production of the remaining HCs becomes either technically or financially unfeasible, wells must be plugged and abandoned (P&A) with the intention of securing any remaining HCs in the subsurface. Once P&A is complete, the decommissioning of the structures located above the mudline, on the seabed and in the water column can begin. This study focussed on the decommissioning of these above mudline structures, and did not include P&A.
Most global policies related to decommissioning OGI manmade structures (OGIMMS) assume that a clear seabed is in the best interest of the natural environment (for example the OSPAR convention and decision 98/3), however a growing body of evidence is available to show that OGIMMS sustain and support large assemblages of marine biota3–5 and that thriving opportunistic ecosystems will develop that can sustain many trophic levels. Furthermore, it is not straight forward to define the natural state of the marine environment as there is huge amounts of variability and very little historical data available for comparison.6 In the case of the North Sea industrial and commercial trawling has obliterated the original natural environment7 and therefore no “pristine” baseline exists, in fact current environmental impact assessment procedure is to consider the current environmental situation as the new baseline for any interventions.
Marine growth occurs throughout the life-cycle of the OGIMMS from the moment the OGIMMS is emplaced in the marine environment. OGIMMS form artificial hard substrates which allows opportunistic organisms to settle on the structures (epifauna and epifloral) within a few days. Within five to six years a stable ecosystem forms, with small variations in species composition and abundances.8 At the time of decommissioning, after decades in the marine environment these OGIMMS structures support a diverse range of marine life.9 Guerin et al.10 studied steel jackets in the northern north sea (NS), central NS and southern NS of different ages and in different water depths and found that they all shared the same fouling organisms but not the same patterns of fouling.
According to Burden11 OGI infrastructure and operations have both negative and positive impacts on marine ecosystems. When infrastructure is first put into place, the original habitats (usually sandy or muddy seabed) are degraded.12 The OGIMMS and the operations can interfere with local hydrodynamic processes and sedimentation patterns13 as well as introducing contaminants, nutrients, and artificial noise and light that may alter species behaviours.14–16 However, Watson et al.1 found that the impact on other marine users, possible contaminant releases and liability issues are the main concerns of academics, industry leaders and policy makers.
Current decommissioning practises in the north sea OGI require that a ‘clear seabed’ is achieved17 that requires the complete removal of all infrastructure above the mudline, except under very limited exceptional circumstances. In effect this means that marine growth, the ecosystems that grow adhered to and directly associated with OGIMMS is decommissioned along with the OGIMMS and will be taken onshore and usually disposed of in landfill with the material decomposing and the carbon returning to the atmosphere.18 The entire ecosystem dies off as its habitat has been removed, and this has consequences for the wider food web as nutrients are removed and protection afforded by the OGIMMS has gone, leaving any free-swimming parts of the ecosystem vulnerable to predation and human activity such as trawling.
Significantly, although there are large numbers of studies into the environmental impact of OGIMMS in both academia and industry, they all fail to acknowledge the impact that the consequential GHG emissions from decommissioning has. Those that do mention GHG emissions fail to understand the consequences because they do not recognise the magnitude of the challenge, nor the cumulative impact, primarily due to the lack of published studies to date. Recent work by Davies & Hastings19 show that decommissioning has released 25 MtCO2e of cumulative GHG emissions to date and that due to growth in offshore wind farms (OWF) as well as the continued growth of HC exploration and production, this will increase 200-fold to 5 GtCO2e by 2067. Furthermore, no studies have to date investigated the potential blue carbon (BC) stocks associated with OGIMMS, nor the consequential GHG emissions produced when they are removed and allowed to degrade in landfill.
In decommissioning, biomass has no fiscal value, even if the species found is endangered or protected. Lophelia pertusa for example, is protected but removed along with the OGIMMS it is attached to, with no consideration for preservation as the OGI are not obliged to by the local governing body. But by looking at the ecosystems in terms of carbon sequestration potential, value can be attributed to the biomass in a quantifiable way. This also enables comparisons to be made between different reduction emissions technologies and processes, such as leaving these ecosystems in place and allowing them to grow naturally and ‘negative’ emissions technologies such as carbon capture, utilisation and storage (CCUS).
Originally the term Blue Carbon (BC) was coined to describe how coastal ecosystems including mangroves, salt marshes and seagrass sequester and store carbon from the atmosphere and water column.20 The term now describes any carbon that has been sequestered in the marine environment including both organic carbon and inorganic carbon.21 The IPCC21 definition requires that an element of human management is possible for it to be considered BC, which seems rather limiting when we consider the vast carbon rich rocks deposited for 100 s of millions of years of Earth's history – from limestones to HC source rocks – that contain vast quantities of carbon, extracted through various pathways from the atmosphere22 well before humans - even our most distant relatives – evolved.
Furthermore, there is still much debate about whether the biomineralization process of calcifying organisms in the marine environment is a net emitter of CO2, as some theoretical studies have suggested or if they should be considered carbon sequesters. It is not within the scope of this study to reproduce these arguments, but it is important to acknowledge that this study follows the work of Moore et al.22 who clearly define calcifiers as net sequesters. From a geological perspective, calficiers must be a net store of BC as they produce long lasting carbon rich rock layers – carbon that was extracted from the atmosphere.
Using the geological record to understand past environments, their climates and deposition settings is key to understanding current environments and the necessary settings for depositional or erosional settings and the best methods for natural carbon sequestration. Most OGIMMS are located in shallow seas which in the geological past have been highly productive in sequestering carbon over the long term. The Kimmeridge clay for example – the most prolific source rock in the north sea – was deposited in a shallow sea during the late Jurassic and had high rates of primary production (PP) and organic matter burial through intermittent oxic-euxinic conditions that promoted the preservation of the organic matter.23 This illustrates the vast potential for this type of BC stock.
Another sedimentary rock that shows the vast potential for carbon sequestration in the geological record is that of ancient reefs. Reef structures are built up over thousands of years, with new growth building atop of the dead carbonate frames of ancestors. In the natural environment reefs build their own complex communities and are self-supporting. Deep-water cold coral reefs such as those built by L. pertusa24 and warm water reefs such as the Great Barrier Reef, Australia25 are both calcifying and long lasting with24 estimating the L. pertusa reef to be 1000's of years old. Ancient reefs may not be created by the same species as today, but it does point to the potential significance of OGIMMS as a starting point for the development of ecosystems that could provide vast potential for carbon sequestration, including where reef building corals are found such as L. pertusa. Price et al.26 consider cold-water coral reefs to be hotspots for biodiversity. Henry et al.27 found that the structural complexity of the 3D framework of the coral skeleton contributes to reef assemblage and biodiversity and that this works on multiple scales.28
Cold-water coral reefs are vulnerable to activities such as trawling and as such are classified as a Vulnerable Marine Ecosystem (VME).29 OGIMMS provide a protective hard substrate away from trawling, this protection has allowed L. pertusa to thrive on many OGIMMS, especially in northern north sea. No previous work has looked at the differences between natural cold water coral reefs and those found adhered to OGIMMS, however the structural complexity of the OGIMMS could provide an alternative to natural locations by providing hard substrate with protection from trawling.
The aim of the study was to develop a model to quantify current OGIMMS biomass, BC stocks and potential future growth if the OGIMMS (and thereby the ecosystems) are left in situ and allowed to grow naturally as well as the GHG emissions that may be released as a consequence of marine waste produced when the MMS is decommissioned.
2 Methods and materials
The main aim of the study was to understand current ecosystems, biomass, BC and consequential GHG emissions if the biomass was removed along with the OGIMMS it is adhered to during decommissioning, both for the UK North Sea (UKNS) and globally. This model studied biomass (living and dead organisms) but did not look at the impacts to sediment deposition, burial rates or potential accumulations of sediment (other than the accumulation of dead biomass).However, very little data is available and very little knowledge exists in the public sphere in regard to how much biomass is present when the MMS is in the marine environment nor the mass of biological material decommissioned along with the MMS, nor how this dynamic system changes over time. Accurate accounts of the carbon content of different species are also unavailable, as is any publicly available detailed study of the total and type of biomass present.
Therefore, a top-down approach was deemed most appropriate to estimate BC associated with OGIMMS, and the following steps were taken.
2.1 Step 1. quantifying OGI MMS mass
The total mass of UKNS OGI MMS was found using the data from Table 1 where 20%30 of the total of all OGIMMS is made up of steel jackets, and each jacket has been estimated to be on average 50,000 t in mass.31| Offshore decommissioning statistics | |||
|---|---|---|---|
| Data | Unit | Ref. | |
| UKNS # steel platforms | 320 | 32 | |
| Proportion of platforms to rest of infrastructure | 20 | % | 30 |
| Average mass of single jacket | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
t | 31 |
| Total mass of UKNS OGIMMS | 80 | Mt | |
| GHG emissions for all UKNS OGI decom | 176 | MtCO2e | 19 |
| Global # steel platforms | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1 | |
| Proportion of platforms to rest of infrastructure | 20 | % | 30 |
| Total mass of global OGIMMS | 3000 | Mt | |
The number of steel jackets in the UKNS is 320,32 multiply this by the mass of each = (320 × 50,000 t) = 16 Mt.
As 20% of MMS is jackets then the total mass of MMS is (16 × 5) = 80 Mt.
For global OGI MMS was found by the same method:
Number of steel jackets globally is 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000,1 multiply this by the mass of each = (12
000,1 multiply this by the mass of each = (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × 50,000 t) = 600 Mt as 20% of MMS are jackets, the total mass of global MMS is (600 × 5) = 3,000 Mt.
000 × 50,000 t) = 600 Mt as 20% of MMS are jackets, the total mass of global MMS is (600 × 5) = 3,000 Mt.
For the purposed of modelling, we have assumed the remaining OGIMMS has the same average mass of the steel jackets.
2.2 Step 2. understanding and quantifying the current ecosystem around OGIMMS
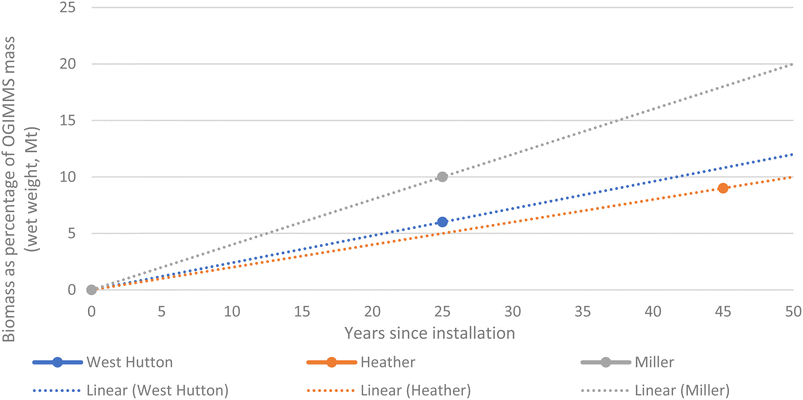
Very little data exists in the public domain that quantifies total biomass on OGIMMS whilst in the marine environment. In the UK north sea, oil & gas UK18 report marine waste from three OGI jackets as a percentage of the mass of the structure it is adhered to and found the following:• 6% for West Hutton (emplaced 1981, decommissioned 2006, age at time 25 years),
• 9% for Heather (emplaced 1978, decommissioned 2021, age at time 43 years), and
• 10% for Miller (emplaced 1991, decommissioned 2017, age at time 26 years).
Fig. 1 illustrates our model for total biomass adhered to OGIMMS and does not include sediment. However, this only represents a proportion of the total biomass present as an unknown amount will be left in place or will fall off due to activities such as cutting and lifting during decommissioning.
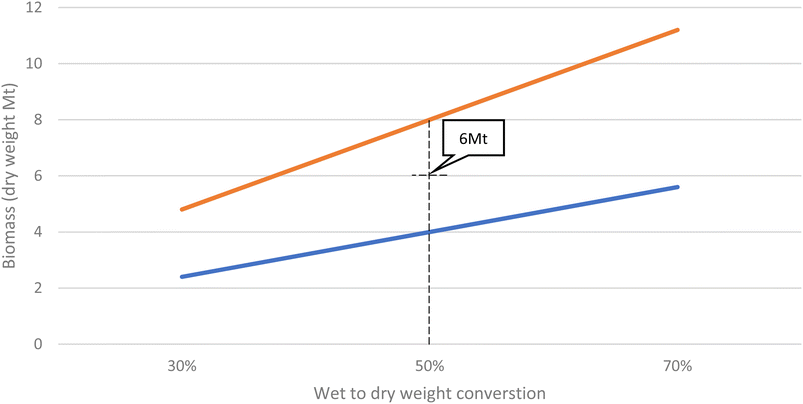
Furthermore, these values are for biomass plus water (from the marine environment), known as the wet weight. It is difficult to quantify the dry weight (the biomass without water) due to the lack of data, not only in terms of masses, but also in terms of the type of species present because each will have a different wet vs. dry weight. Although conversions are available33 these do not include the shell material, which makes up a large proportion of the biomass, use heating methods to speed up the drying process and do not consider GHG emissions produced as a consequence of the biomass degrading, so are not useful in this instance.
Fig. 1 illustrates the trend lines of biomass as a percentage of the mass of the OGIMMS it is adhered over time up to 50 years since installation, based on the three data points from West Hutton, Heather and Miller platform. Fig. 2 illustrates the method used for conversion of wet to dry weights. An average was used, rather than a more conservative figure because it is more representative of the biodiversity within these ecosystems.
The age of the MMS structure at the time of decommissioning will also impact the total biomass present as over time biomass will increase due to natural growth, by births and immigrations as well as the evolution of complexity of ecosystem and organisms (Fig. 3).
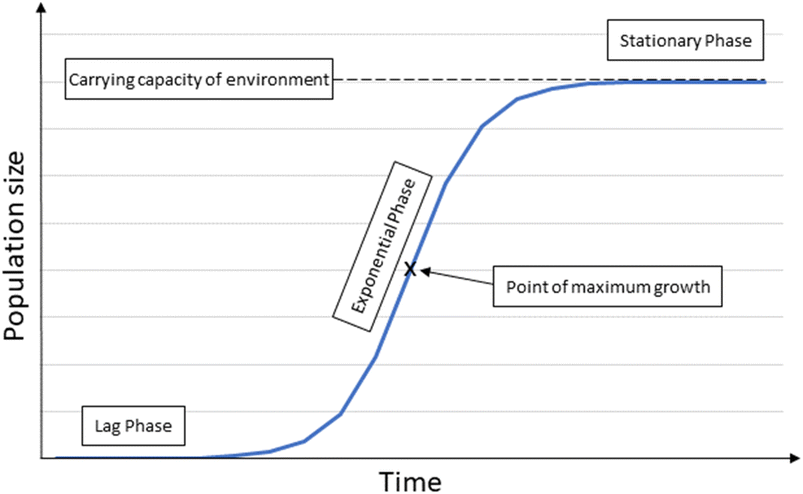 | ||
| Fig. 3 Logistic growth model illustrating the three different phases of growth; the lag phase the initial growth stage, the exponential phase where growth is unconstrained and exponential, and the stational phase where the carrying capacity of the environment has been reached and growth is at a steady state. Adapted from ref. 40 and 41. | ||
6% was selected for global biomass as it conservatively reflects the higher levels of uncertainty, and the huge variation in environmental conditions, geological location, climate conditions and water depth, etc.
2.3 Step 3. understanding dead biomass
Reef and shell rubble (dead biomass) on and around the structures must also be taken into account as it is valuable source of carbon sequestration through calcification as well as providing a valuable ecosystem for microscopic organisms.24 Wolfe et al.24 estimate that dead matter can be as high as 73% of the total mass when L. pertusa are present, however very little data exists to quantify this figure for other types of ecosystem.The mass and rate of growth of the dead matter is currently unknown and very little data exists that quantifies this for other ecosystems. Healthy reefs34 estimate that a typical coral reef is made up of 75% live biomass, 5% of recently dead biomass and 20% of older dead biomass. However, as reefs are known to build atop of the skeletal remains of their ancestors, dead matter accumulates in these environments as vital components of the reef structure, providing habitat for the younger generations. It is likely that due to a lack of data that quantifies the mass of dead matter, the reported percentages above are an underestimate.
For the purposes of this model, it was assumed that at the reference time, the total biomass will be made up of 75% of live biomass and 25% of dead biomass (mainly skeletal remains). The dead biomass accumulates over time as each successive biomass generation dies and adds their mass to the dead mass accumulation.
50 years since installation was chosen as the reference point for the study as there is great variability in the ages of the OGIMMS that are decommissioned, with a large number having been in location for many decades.
2.4 Step 4. quantifying the carbon content of the live biomass
Marine organisms extract calcium and carbonate ions from sea water to form the solid crystals in shells and skeletons.35 Very little data exits that quantifies the carbon content of individual marine species, Fry et al.36 found C content to be 22% in mussel shells and 44% in oyster shells and Baker & Baker37 found that some proteins in mussels contained 35–40% C compared to just 12% C in the shell. This indicates that each species will have a unique average carbon content by mass. This study used an average value for carbon content of 18% for live biomass as this was the only publicly available data.24,38 However, it should be noted that this value will very much depend on biodiversity, biomass, age distribution of biomass, age of ecosystem and many other factors as discussed above.2.5 Step 5. quantifying the carbon content of the dead mass
The vast majority of the dead matter will be the remains of shells and skeletons of calcifying organisms such as mussels, oysters and cold-water coral as this material – calcium carbonate (CaCO3) – is more resistant to degradation than the soft parts of the dead mass in the marine environment. In this model it has been assumed that 100% of the dead mass is calcium carbonate.Marine growth is considered biohazard waste and is usually discarded and sent to landfill. In this instance, the degradation process means that as the dead mass degrades the carbon becomes available to make CO2 and CH4. The exact ratio of each will depend on specific environmental factors but the normal range is 30–50% CO2 and 50–60% CH4, with numerous other gases making up the remainder.39 It is likely that the soft tissue will degrade to 50% methane and the calcium carbonate will degrade to mostly carbon dioxide, however for the purposes of this model, and because data is lacking, an even 50/50% ratio has been used and other gases have been ignored. Furthermore, we have assumed that all the biowaste material will degrade in landfill due to the low Ph of the initial phase of landfill degradation.
In terms of the dead matter left in situ, it is the cumulative impacts that are important as dead matter will not degrade if the ecosystem is left in place.
2.6 Step 6. quantifying blue carbon
The total blue carbon was estimated based on the carbon content of the live biomass and the carbon content of the dead biomass added together.However, it should be noted that this does not include the carbon sequestered in other blue carbon sources such as phytoplankton, viruses and bacteria, free swimming species and the wider food web as well as the carbon sequestered in the sediments around the OGIMMS.
2.7 Step 7. growth rate model
In theory, most ecosystems have a growth pattern that has three stages, the lag phase, the exponential phase and the stationary phase (Fig. 1).40,41 This is due to numerous constraints in the environmental including limited nutrients, light and too much sediment input, competition and predation.The lag phase is the initial early stage of growth where births and immigration are higher than deaths and emigrations,41 but the populations and therefore the biomass is relatively small. In the ecosystems around OGIMMS it is assumed that immigration will be a significant and main contributing factor in this early stage. The exponential phase has unconstrained, exponential growth. As resources become limited, growth begins to slow and at some point, the carrying capacity of the environment is reached. Detailed data is lacking in respect to the carrying capacity of the ecosystems around OGIMMS and this presents as a major unknown in this model.
For the purposes of modelling, it has been assumed that the ecosystem is at the point of maximum growth, after which point growth begins to slow until a steady state has been reached at the carrying capacity. However, it should be noted that the reference point (the point at which decommissioning is about to commence), could be much earlier in the exponential phase, in which case the carrying capacity would be much larger.
By using this model design, the study aimed to:
1. Make predictions about current biomass and BC around OGIMMS.
2. Make predictions about the potential GHG emissions released as a consequence of decommissioning the BC along with the OGIMMS.
3. Make predictions about future growth of BC around OGIMMS if the OGIMMS were to remain in place.
2.8 Step 8. forward modelling
As a consequence of the model design using the sigmoidal growth rate to express ecosystem growth, the model is also able to look forward to what would happen to the ecosystem if the OGIMMS was allowed to remain in situ.Forward modelling is often employed in the Oil & Gas industry (OGI) for different types of geophysical and geological studies on various scales including stratigraphic and reservoir scale.42–44 This type of modelling, where the aim is to understand a system and its behaviour over time is uniquely applied here to understand the ecological system relating to man-made objects in the marine environment, its evolution and how that translates into a quantification of biomass, carbon and GHG emissions if these ecosystems are removed as marine waste, die and degrade.
A sigmoidal wave function was applied to find biomass at various ages of the OGIMMS based on the reference point and mass at 50 years since installation. To do this it was assumed that this point was the maximum point of growth and that the carrying capacity of the environment would be reached in 100 years since installation.
Understanding the mass of dead material was also important because when calcifying organisms die, their calcium carbonate structures remain, providing an important method of carbon sequestration as well as providing their ecosystems with an important source of nutrients and protection. However very few studies have been undertaken into understanding the dead biomass.45 Importantly because calcium carbonate dead biomass remains, it accumulates over time, and this must also be taken into account in the model. Table 2 shows the data used in the model.
| CH4 GWP: | 80 | 21 |
| 1 kg C = | 3.67 | kgCO2 |
| 1 kg C = | 1.33 | kgCH4 |
| C in dead | 44% | |
| Recently dead | 5% | Cumulative |
| Old dead | 20% | |
| Total dead per year | 12% |
After the point of maximum growth, the dead biomass will continue to accumulate at the same rate as the ecosystem replacement rate.
3 Results
3.1 Assessment of ecosystems associated with OGI MMS
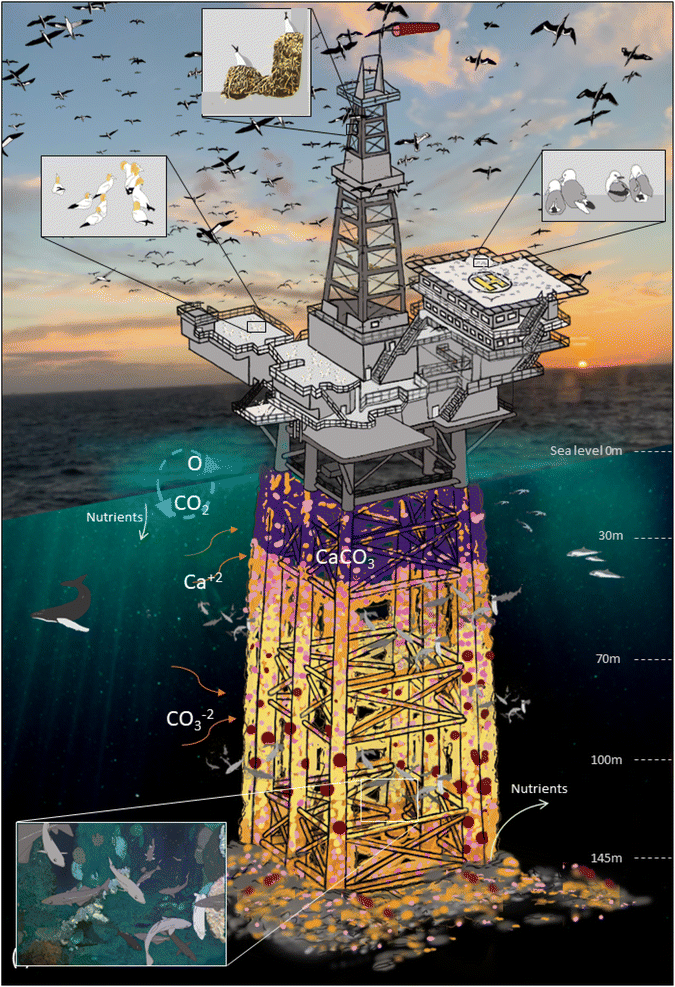
Fig. 4 illustrates the functioning ecosystem associated with an example OGIMMS steel jacket and shows that biomass is present throughout the water column and that birds colonise the topsides. In the water column, different assemblages of species are present at specific depth zones.18 Shallow-water assemblages are present from the sea surface until depths of around 30 m and include mussels, barnacles, solitary tube worms, kelp and other seaweeds.18 Upper mid-assemblages found at depths of between 30–70 m include anemones, soft corals, hydroids, tube worms and barnacles.18 The next depth zone found at between 70–140 m include similar assemblages to the upper-mid assemblages of anemones, soft corals, hydroids, tube worms and barnacles, as well as the hard bodies deep water cold coral, L. pertusa. At greater depths assemblages can become sparser, but this isn't necessarily the case, L. pertusa for example has been found at depths greater than 1000 m.46 Birds including gules, garnets and kittiwakes47 colonise the topsides (structures above sea level) as structures such as drilling derricks provide many places to build nests and raise chicks and deposit guano.Fig. 5 illustrates the evolution of the species assemblages over time from emplacement to the point at which the OGI MMS are ready for decommissioning. Over time the assemblages become more complex, with a number of specific stages recognised18 as (1) the ‘mature muscle stage’, between 3–5 years (Fig. 5(b)), (2) the ‘mature anemone/soft coral stage’, between 5–15 years (Fig. 5(c) and (d)) the early L. pertusa stage a number of decades after emplacement (Fig. 5(e) and (f)).
 | ||
| Fig. 5 Evolution of biomass adhered to OGI MMS. (a) Is 3 months after installation, (b) represents 5–10 years since installation, (c) 10–20 years after installation, (d) 20–30 years after installation. (e) 30–40 years after installation and (f) 50 years after installation. Diagrams (a–e) would be during the operational stage of producing HCs (hot phase) and (f) would be around the time of cold decommissioning, the well will be plugged and abandoned and no more HCs should be present. Data from ref. 8 and 18. | ||
3.2 UK north model of sea biomass, blue carbon stocks and GHG emissions
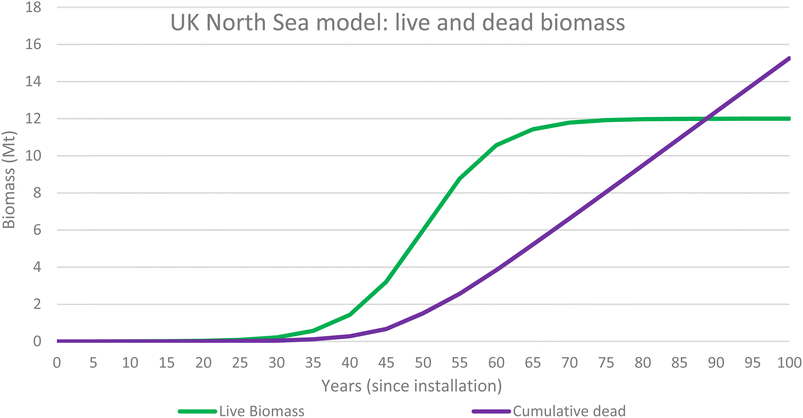
The results were obtained by applying a sigmoidal wave function to determine biomass growth rate over time. The study assumed that the point of maximum growth occurs at 50 years since installation and 6 Mt of biomass is present at this time. The carrying capacity of the ecosystem has therefore been modelled at 100 years since installation when 12 Mt of biomass is present. Table 3 shows the results for the UKNS biomass 100 year model and Table 4 describes the UKNS waste model (Fig. 9).| UK North Sea Model | |||||||
|---|---|---|---|---|---|---|---|
| Function | Time | Live biomass | Dead biomass | Cumulative dead | Dead Bio C content | Live bio C content | Total bio C content |
| (Years since installation) | (Mt) | (Mt) | (Mt) | (MtC) | (MtC) | (MtC) | |
| 4.54 × 10−5 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 0.000123395 | 5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 0.00033535 | 10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 0.000911051 | 15 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 0.002472623 | 20 | 0.03 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 |
| 0.006692851 | 25 | 0.08 | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 |
| 0.01798621 | 30 | 0.22 | 0.03 | 0.04 | 0.02 | 0.04 | 0.06 |
| 0.047425873 | 35 | 0.57 | 0.07 | 0.11 | 0.05 | 0.10 | 0.15 |
| 0.119202922 | 40 | 1.43 | 0.17 | 0.28 | 0.12 | 0.26 | 0.38 |
| 0.268941421 | 45 | 3.23 | 0.39 | 0.67 | 0.29 | 0.58 | 0.87 |
| 0.5 | 50 | 6.00 | 0.85 | 1.52 | 0.67 | 1.08 | 1.75 |
| 0.731058579 | 55 | 8.77 | 1.05 | 2.57 | 1.13 | 1.58 | 2.71 |
| 0.880797078 | 60 | 10.57 | 1.27 | 3.84 | 1.69 | 1.90 | 3.59 |
| 0.952574127 | 65 | 11.43 | 1.37 | 5.21 | 2.29 | 2.06 | 4.35 |
| 0.98201379 | 70 | 11.78 | 1.41 | 6.63 | 2.92 | 2.12 | 5.04 |
| 0.993307149 | 75 | 11.92 | 1.43 | 8.06 | 3.54 | 2.15 | 5.69 |
| 0.997527377 | 80 | 11.97 | 1.44 | 9.49 | 4.18 | 2.15 | 6.33 |
| 0.999088949 | 85 | 11.99 | 1.44 | 10.93 | 4.81 | 2.16 | 6.97 |
| 0.99966465 | 90 | 12.00 | 1.44 | 12.37 | 5.44 | 2.16 | 7.60 |
| 0.999876605 | 95 | 12.00 | 1.44 | 13.81 | 6.08 | 2.16 | 8.24 |
| 0.999954602 | 100 | 12.00 | 1.44 | 15.25 | 6.71 | 2.16 | 8.87 |
| Waste management | ||||||
|---|---|---|---|---|---|---|
| Time | Disposal in ocean or atmosphere | Disposal in landfill GHG emissions: 50% CO2 and 50% CH4 | ||||
| GHG emissions: 100% C to CO2 | 50% C | 50% C to CO2 | 50% C to CH4 | CH4 to CO2e | Landfill GHG Emissions | |
| (Years since installation) | (MtCO2e) | (MtC) | (MtCO2e) | (MtCH4) | (MtCO2e) | (MtCO2e) |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 |
| 5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 |
| 10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.06 |
| 15 | 0.01 | 0.00 | 0.01 | 0.00 | 0.15 | 0.16 |
| 20 | 0.03 | 0.00 | 0.01 | 0.01 | 0.42 | 0.43 |
| 25 | 0.08 | 0.01 | 0.04 | 0.01 | 1.13 | 1.16 |
| 30 | 0.21 | 0.03 | 0.10 | 0.04 | 3.03 | 3.13 |
| 35 | 0.55 | 0.08 | 0.28 | 0.10 | 8.01 | 8.29 |
| 40 | 1.40 | 0.19 | 0.70 | 0.25 | 20.28 | 20.98 |
| 45 | 3.21 | 0.44 | 1.61 | 0.58 | 46.55 | 48.16 |
| 50 | 6.42 | 0.87 | 3.21 | 1.16 | 93.00 | 96.21 |
| 55 | 9.95 | 1.36 | 4.97 | 1.80 | 144.19 | 149.16 |
| 60 | 13.18 | 1.80 | 6.59 | 2.39 | 191.09 | 197.68 |
| 65 | 15.97 | 2.18 | 7.98 | 2.89 | 231.44 | 239.43 |
| 70 | 18.48 | 2.52 | 9.24 | 3.35 | 267.93 | 277.17 |
| 75 | 20.88 | 2.85 | 10.44 | 3.78 | 302.71 | 313.15 |
| 80 | 23.24 | 3.17 | 11.62 | 4.21 | 336.82 | 348.44 |
| 85 | 25.57 | 3.48 | 12.79 | 4.63 | 370.67 | 383.46 |
| 90 | 27.90 | 3.80 | 13.95 | 5.06 | 404.44 | 418.39 |
| 95 | 30.23 | 4.12 | 15.11 | 5.48 | 438.16 | 453.28 |
| 100 | 32.55 | 4.43 | 16.28 | 5.90 | 471.88 | 488.16 |
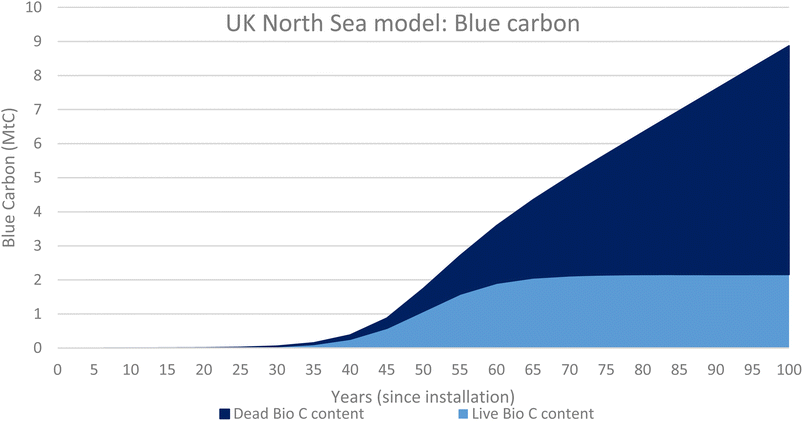
Fig. 6 illustrates the biomass values for both live biomass and dead biomass over time and shows that at 50 years since installation the model predicts that there is 6 Mt of live biomass and 1.5 Mt of dead biomass, 7.5 Mt in total. Dead biomass accumulates over time and the total mass of dead will overtake the live biomass at around 87 years since installation, and it will continue to accumulate for the entire life of the ecosystem.
Fig. 7 shows the total carbon content of both the live biomass (1.08 MtC) and dead biomass (0.67 MtC) at 1.75 MtC. This is the value of blue carbon associated with the OGIMMS in the nsorth sea.
When marine waste is removed from the marine environment, the biomass will begin to decompose immediately and produces GHG emissions as a consequence. In the atmosphere, it is likely that only carbon dioxide is produced, however, if the marine waste is sent to landfill (which most of it is ref. 18) 50% of the carbon content is able to combine with other gases and processes and is available to produce methane. Methane is a GHG gas with a much higher warming impact than carbon dioxide with a global warming potential of 80 compared to carbon dioxide21 for organic methane over a 20 year period. This has been included in the model and is the reason landfill emissions are considerably higher than the emissions produced form decomposition in other environments. The study has assumed that 100% of the marine waste is able to degrade to GHG emissions during landfill decomposition.
Fig. 8 illustrates the possible range of GHG emissions and show that if 100% of the carbon is converted to CO2, 6.5 MtCO2 will be released. If the carbon is converted to 50% CO2 and 50% CH4 the GHG emissions will be significantly higher at 96MtCO2e.
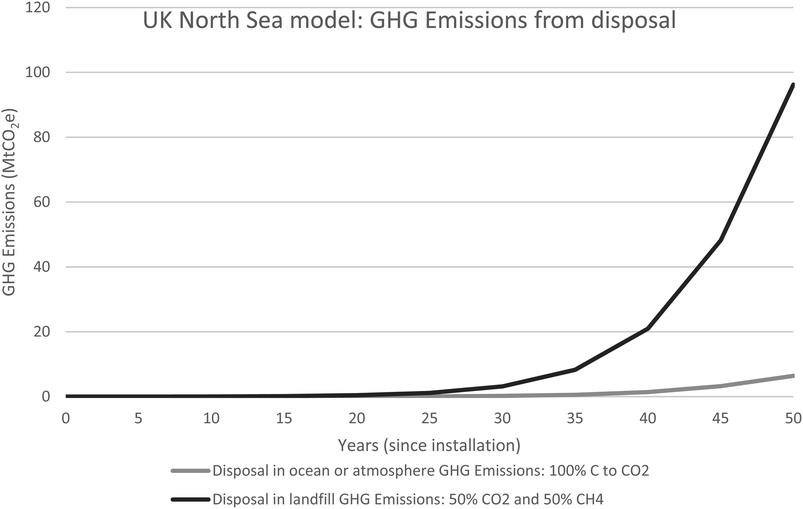 | ||
| Fig. 8 UKNS model illustrating the range of possible GHG emissions if the marine waste degrades in the atmosphere or ocean or in landfill. In the atmosphere or ocean 100% of the carbon content becomes available to produce carbon dioxide whereas if the marine waste is disposed of in landfill, 50% of the carbon content is available to create carbon dioxide and 50% is available to be converted to methane. Data is available in Table 3. | ||
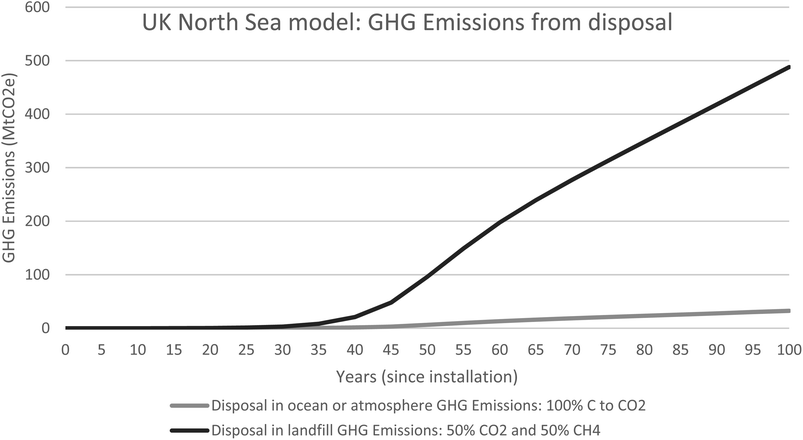 | ||
| Fig. 9 UK north sea model for GHG emissions produced as a consequence of removing the biomass from the marine environment. | ||
3.3 Global model of biomass, blue carbon stocks and GHG emissions
The method was extrapolated to the global scale. The model predicts that there is currently 180 Mt of biomass adhered to OGI MMS at 50 years since installation, at the point of maximum growth (please see Table 5). The carrying capacity of the ecosystem has been modelled to be reached at 100 years since installation with a capacity of 360 Mt of live biomass, as in the UKNS model the dead biomass accumulates over time and as such it continues to build after the carrying capacity as been reached. The carbon content of the live biomass is modelled to be 42 MtC at 50 years since installation, and dead biomass is 22 MtC at 50 years since installation so the total biomass at 50 years since installation is 64 MtC (please refer to Table 5). Table 6 illustrates the waste model.| Global 100 year model | |||||||
|---|---|---|---|---|---|---|---|
| Function | Time | Live biomass | Dead biomass | Dead Bio (cumulative) | Dead bio C content | Live bio C content | Total bio C content |
| (Years since installation) | (Mt) | (Mt) | (Mt) | (MtC) | (MtC) | (MtC) | |
| 4.54 × 10−5 | 0 | 0.02 | 0.00 | 0.00 | 0.00 | 0 | 0 |
| 0.000123395 | 5 | 0.04 | 0.01 | 0.01 | 0.00 | 0 | 0 |
| 0.00033535 | 10 | 0.12 | 0.01 | 0.02 | 0.01 | 0 | 0 |
| 0.000911051 | 15 | 0.33 | 0.04 | 0.06 | 0.03 | 0 | 0 |
| 0.002472623 | 20 | 0.89 | 0.11 | 0.17 | 0.07 | 0 | 0 |
| 0.006692851 | 25 | 2.41 | 0.29 | 0.46 | 0.20 | 0 | 1 |
| 0.01798621 | 30 | 6.48 | 0.78 | 1.23 | 0.54 | 1 | 2 |
| 0.047425873 | 35 | 17.07 | 2.05 | 3.28 | 1.44 | 3 | 5 |
| 0.119202922 | 40 | 42.91 | 5.15 | 8.43 | 3.71 | 8 | 11 |
| 0.268941421 | 45 | 96.82 | 11.62 | 20.05 | 8.82 | 17 | 26 |
| 0.5 | 50 | 180.00 | 21.60 | 41.65 | 18.33 | 32 | 51 |
| 0.731058579 | 55 | 263.18 | 31.58 | 73.23 | 32.22 | 47 | 80 |
| 0.880797078 | 60 | 317.09 | 38.05 | 111.28 | 48.96 | 57 | 106 |
| 0.952574127 | 65 | 342.93 | 41.15 | 152.43 | 67.07 | 62 | 129 |
| 0.98201379 | 70 | 353.52 | 42.42 | 194.86 | 85.74 | 64 | 149 |
| 0.993307149 | 75 | 357.59 | 42.91 | 237.77 | 104.62 | 64 | 169 |
| 0.997527377 | 80 | 359.11 | 43.09 | 280.86 | 123.58 | 65 | 188 |
| 0.999088949 | 85 | 359.67 | 43.16 | 324.02 | 142.57 | 65 | 207 |
| 0.99966465 | 90 | 359.88 | 43.19 | 367.21 | 161.57 | 65 | 226 |
| 0.999876605 | 95 | 359.96 | 43.19 | 410.40 | 180.58 | 65 | 245 |
| 0.999954602 | 100 | 359.98 | 43.20 | 453.60 | 199.58 | 65 | 264 |
| Waste management | ||||||
|---|---|---|---|---|---|---|
| Time | Degradation in ocean or atmosphere | Disposal in landfill GHG emissions: 50% CO2 and 50% CH4 | ||||
| GHG emissions: 100% C to CO2 | 50% C | 50% C to CO2 | 50% C to CH4 | CH4 to CO2e | Landfill GHG emissions | |
| (Years since installation) | (MtCO2e) | (MtC) | (MtCO2e) | (MtCH4) | (MtCO2e) | (MtCO2e) |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 1 | 1 |
| 10 | 0 | 0 | 0 | 0 | 2 | 2 |
| 15 | 0 | 0 | 0 | 0 | 5 | 5 |
| 20 | 1 | 0 | 0 | 0 | 12 | 13 |
| 25 | 2 | 0 | 1 | 0 | 34 | 34 |
| 30 | 6 | 1 | 3 | 1 | 91 | 92 |
| 35 | 17 | 2 | 8 | 3 | 240 | 243 |
| 40 | 42 | 6 | 21 | 8 | 608 | 616 |
| 45 | 96 | 13 | 48 | 17 | 1396 | 1414 |
| 50 | 186 | 25 | 93 | 34 | 2699 | 2732 |
| 55 | 292 | 40 | 146 | 53 | 4234 | 4287 |
| 60 | 389 | 53 | 195 | 71 | 5641 | 5712 |
| 65 | 473 | 64 | 236 | 86 | 6852 | 6938 |
| 70 | 548 | 75 | 274 | 99 | 7947 | 8046 |
| 75 | 620 | 84 | 310 | 112 | 8990 | 9102 |
| 80 | 691 | 94 | 345 | 125 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 013 013 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138 138 |
| 85 | 761 | 104 | 380 | 138 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 029 029 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 167 167 |
| 90 | 831 | 113 | 415 | 151 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042 042 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 192 192 |
| 95 | 901 | 123 | 450 | 163 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 054 054 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 217 217 |
| 100 | 970 | 132 | 485 | 176 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 065 065 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241 241 |
3.4 UK north sea forward model
The added benefit of using the growth rates of the live and dead biomass to model the ecosystems associated with OGIMMS and make predictions about current blue carbon stocks is that also makes predictions about the future. All models look forward from 50 years since installation to 100 years since installation and show that the ecosystem will likely continue to grow and evolve, if the OGIMMS that it is associated with was to remain in situ.For the UK North Sea, the model predicts that by 100 years since installation, there will be 12 Mt of live biomass and 15 Mt of dead biomass, 27 Mt in total. This equates to 8.9 MtC of Blue Carbon.
Modelling the emissions from this shows that if the ecosystem was allowed to grow for 100 years, but then removed, GHG emissions would be 33MtCO2 (100% CO2) or up to 488MtCO2e (50% CO2 and 50% CH4).
3.5 Global forward model
The global model predicts that by 100 years since installation, there will be 360 Mt of live biomass and 43 Mt of dead biomass, 403 Mt in total. This equates to 264 MtC of Blue Carbon.Modelling the emissions from this shows that if the ecosystem was allowed to grow for 100 years, but then removed, GHG emissions would be 970 MtCO2 (100% CO2) or up to 14,241MtCO2e (50% CO2 and 50% CH4).
4 Discussion
The model shows that ecosystems around OGIMMS sequester and store carbon over the long term in both the live biomass of species living and breeding in these ecosystems, and importantly also the dead biomass which accumulates over time. This is the first model to quantify blue carbon associated with OGIMMS and shows that these ecosystems provide a highly valuable and large carbon store, which has until now not been recognised. Furthermore, this study shows that the potential future growth of these ecosystems will lead to increasing carbon sequestration due to ecosystem population growth and consequential build-up of dead biomass.The potential GHG emissions produced as a consequence of the degradation of the biomass depends on the end point of the biomass, known as marine waste, it usually ends its life in landfill18 where 50% of the carbon content will be available to produce methane due to the availability of other gases and environmental conditions of landfill sites and could produce up to 96.2 MtCO2e in the UK North Sea alone, modelled at up to 2,730 MtCO2e globally. These are significant volumes of GHG emissions that have not been recognised until now.
Interestingly, the solutions to reducing GHG emissions is also the solution to avoiding biodiversity loss. By leaving these valuable and thriving ecosystems in place, the ecosystem is conserved and therefore the carbon sequestered up to that point will not be released.
The GHG emissions produced from the complete removal of all OGIMMS in the UK north sea (mainly due to fossil fuel use by vessels and waste management practises) has been shown by Davies19 to be 176MtCO2e and this study has shown that the removal of biomass will release up to 96 MtCO2e, this equates to 272 MtCO2e. Considering that UK annual emissions were 505 MtCO2e in 202148 this is a substantial volume of GHG emissions, that could be avoided by leaving the OGIMMS in place. This shows that decommissioning in situ is the best method for dealing with OGIMMS in the marine environment and that OSPAR 98/3 needs to be re-evaluated.
Conservation and enhancement of the ecosystem may now also be possible with this new information. For example, MMS could in future be designed to specifically enhance and encourage biodiversity growth.
This model assumed the carrying capacity of the environment would be reached at 100 years since installation, however reefs are known to be exceedingly long-lived ecosystems. Wolfe24 estimated the age of the L. pertusa reef to be 1000 s of years old and thick sequences of coral reefs forming large sedimentary layers show their importance over geological time.49 The main factor that may play a part in determining the carrying capacity in these ecosystems could be the structural integrity of the MMS/BC system. Many reefs today and in the geological record become self-supporting structures, could the ecosystems here develop in the same way? What would happen to the ecosystem if there was a catastrophic collapse of the MMS? There is no knowledge currently in terms of the longevity of these MMS in the marine environment, and no published studies looking at the interaction of the MMS and ecosystem in this way. Future model development should include this vital interplay between engineered MMS structures and the ecosystem, which could help to inform many fields of study, as well as design and engineering decisions.
The results from this study show that instead of protecting the natural environment, OSPAR 98/3 is in fact responsible for the destruction of these thriving ecosystems, including the destruction of the protected and endangered species L. pertusa. Furthermore, it shows that OSPAR 98/3 and other clear seabed policies are incompatible with climate change and biodiversity loss mitigations, and the PARIS Agreement.50 Clear seabed policies will produce at least 38 MtCO2 by 203019 from the decommissioning activity, and the loss of the associated ecosystem will continue to produce GHG emissions from the degradation of the biomass, as well as the potential long term storage loss of the carbon sequestered by the biomass. Plus, a currently unquantified loss to other potential pools of carbon such as phytoplankton, the larger tropic species that consume carbon from smaller species such as fish, mammals and birds and the wider food web. Furthermore, according to Henry et al. (2018) connectivity will be reduced if MMS are removed, further reducing the potential carbon sequestration pools.
Over time the ecosystem will continue to evolve becoming more complex and supporting bigger populations of both sessile and free-swimming communities. Birds colonise the topsides, and their population has also been modelled to increase, along with the amount of guano that is deposited. This potentially increases the nutrients available to the organisms living in the water column. Also, to note is that the steel is itself deteriorating through rusting and possible structural integrity loss, possibly adding minerals and nutrients to the ecosystem.
Fig. 10 illustrates the ecosystem and OIMMS at 100 years since installation. This shows dead matter has increased significantly and will now be bigger than the mass of the living organisms. The ecosystem has continued to grow, and the complexity has also increased. Many free-swimming organisms are supported by this ecosystem and many more birds are present on the topsides. The build up of guano continues which may lead to the development of plant material on the topsides.
Considering most other global ecosystems are deteriorating, degrading or nearing extinction51 the fact that the ecosystems around OGIMMS are thriving, is intriguing. A theory developed by this study, is that OGIMMS increase PP on a very local scale. This increases the available nutrients to opportunistic organisms that attach to the structures enabling them to thrive and multiply. The majority of OGIMMS are made from steel31 and as steel degrades over time it produces iron oxide (rust), especially in the high moisture, high salt marine environment. Frost's52 study showed that the introduction of iron into the marine environment led to the production of a phytoplankton bloom. Consideration has not been given to the potential that this process could also be happening around OGIMMS, and if so, that the PP would provide nutrients, not otherwise available in that location for the prolific marine growth observed at OGIMMS.
There is some evidence for this theory in satellite imagery of PP in the north sea. Fig. 11 shows a satellite image from the Moderate Resolution Imaging Spectroradiometer (MODIS) instruments on NASA's Terra and Aqua satellites,53 with a visible phytoplankton bloom, the white box indicates the location of shows a satellite image of an offshore wind farm with individual wind turbines seem as an equidistance grid of white spots. The wind turbines each have what appears to be an associated plume, for some distance along the sea surface and perpendicular to the wind turbine tower. Platis54 notice a similar feature in the southern north sea and attribute it to sediment plumes. However, the colour of the plume in may indicate high phytoplankton content rather than high sediment rates (notice the plume is the same colour as the larger PP bloom).
 | ||
| Fig. 11 Satellite image of the southern north sea (see Fig. 11 for location), showing the green and blue whirls of a phytoplankton bloom and the equidistance grid like layout of a wind farm, with individual wind turbines visible as a white spot. The red outline indicates the feature of interest; is this a sediment or phytoplankton plume?.53 | ||
Although Insite's55 study didn't find any evidence for an influence on PP from MMS, their study was conducted on a wide scale (local to regional) and it may be that the scale was too large for the localised PP signatures that may be produced from MMS to be identified. Further study would be needed to answer this question, including possibly sampling at sea. This requires further study not only to investigate these questions further, but also because it has big implications for BC directly associated with MMS once the scale of global infrastructure is taken into consideration.
According to Price et al.26 reefs with greater vertical relief display a wider rugosity range, meaning that these structures are complex and attractive for species to colonize. OGI MMS such as steel jackets that stretch throughout the water column are structurally complex that provide a large surface area of hard substrate for many organisms to adhered to. This may also be a reason for the prolific nature of OGI MMS. Further study would be required to attempt to answer this question.
Like all marine environments, the north sea is being and will continue to be impacted by climate change including sea level rise, warming temperatures, increasing storms and storm intensity, changing circulation patterns and stratification of ocean waters (anoxic conditions), ocean acidification with the consequential reduction in the ocean's ability to absorb atmospheric CO2. However, the Mayk et al.56 study found that the shells of Nucella lapillus in the UK north sea have grown thicker over the last 130 years, despite ocean acidification, indicating that organisms are taking up more excess carbon in the water column than expected. This is surprising as it bucks the global trend of general ecosystem and biodiversity degradation. Further studies would be required to investigate further, and it would also be worthwhile to compare the growth and number of individual species adhered to MMS to the same species but located on a natural hard substrate.
Many assumptions were made during model development mainly because of the lack of available data. Biomass was estimated based on marine waste; the biomass removed at the same time as the infrastructure it is attached to during decommissioning. However, it is unclear what proportion of the biomass is removed and how much is left behind. A more detailed study to establish these masses would enhance and improve the model. This is also necessary to more accurately quantify the dead biomass as this is currently very uncertain and has been estimated based on warm coral reefs, which may not be a good analogue for the ecosystems around OGIMMS.
The study used a sigmoidal population growth pattern which required that a carrying capacity be assumed. 100 years was arbitrarily chosen for this model as this allowed the earlier growth stages to be assessed. If a larger carrying capacity is used, the total biomass will also be larger and the potential carbon sequestration would also be larger. There is also evidence in the pattern of evolution of the biomass around OGI MMS, in the developing complexity and number of species present and the fact that the number of larger, long lived, reef building organisms seem to be increasing that it is unlikely that the carrying capacity has been reached.
This study has assumed that the biomass would store the carbon over the long term (i.e. on geological timescale), based on analogues drawn from the geological record. However, deposition and burial depend on a large number of factors and would require much more research before the uncertainty of this assumption could be reduced.
The carbon content of the biomass was determined to be between 18% of the total biomass, but this was based on very limited number of sources as more detailed data is absent. There is high level of uncertainty with this value because not only will different species contain different amounts of carbon, but different components of the same specimen can themselves have differing carbon contents; Baker & Baker37 for example, found that some proteins in mussels contained 35–40% carbon compared to 12% carbon in the shell. It is likely that the actual range of carbon content will be much more variable, for example MMS with large volumes of L. pertusa biomass are likely to have a higher carbon content than those without because it is a calcium carbonate skeletal reef building organism and Wolfe24 found that up to 73% of a single structure will be dead.
It is essential for model development that further study is undertaken to assess each species in terms of carbon content and potential for long term blue carbon storage. Furthermore, specific types and numbers of species, as well as size distribution, with total mass values for each species would greatly enhance this model. A more complex model would be more accurate, and ideally the biomass would be mapped in 3D and a detailed inventory would be taken to enable a detailed understanding of the extent of biomass present, for each individual MMS. This should now be possible with the advent of remotely operated vehicles and operational centres, meaning less personnel are required offshore for survey and mapping work, reducing costs significantly. One such example is by Gormley57 where they showed the potential for machine learning with the aim of developing remote sensing techniques for ecosystem surveying and monitoring.
OGI operators routinely survey and map their MMS. It would be useful to have a mechanism whereby this data was routinely available for research. It would also be useful to investigate the types of data collection that is now possible, as there have been many advancements including LIDAR and AI.
In the UK, the OGI operators have to give their exploration data to the governing body as part of their license agreements, this includes data such as petrophysics data from exploration drilling campaigns, a similar arrangement should be developed for other data useful for research purposes. For example the UK governing body Department for Energy Security and Net Zero (DESNZ) have the power to request extra survey data be taken, and that all this data becomes available in a database such as the UK National Data Repository (NDR).
In order to usefully model a very complex environment this study developed a very parsimonious representation of the system it is representing. It would be greatly enhanced by the acquisition of more detailed data on the amount of live and dead biomass present over particular timescales. Furthermore, because the biomass can develop on any surface of the MMS a more detailed 3D model should be developed. This should include data such as bio-volume and bio-thickness over time as well as biomass. Furthermore, the question of the degradation process in landfill also needs further study. We have assumed 100% of the dead biomass will be available to degrade to GHG emissions, but there is no data currently.
Data is also required to understand the main differences and similarities between biomass type, growth rate and evolutionary development on different structures in different locations and over different timescales.
Model assumptions also include that evolution is homogenous and does not consider the differing ages of the MMS. A complex interplay between nutrient levels, light levels, wind and storm processes could limit the growth of the biological system and different location, connectivity, weather or current patterns, may determine which organisms arrive at the structure and which do not.
This study only looked at sessile communities of species adhered to MMS, and did not take into account the wider potential for carbon sequestration in higher trophic levels such as mammals, fish and invertebrates, nor did it investigate the potential impacts of MMS on PP. Furthermore, this study also did not look at the impact of MMS on production and deposition of inorganic carbon, bacteria and viruses, nor the carbon dioxide/oxygen respiration flux.
The study did not look at potential risks of leaving the structures in terms of pollution or biodiversity from invasive species or climate change directly as it was out with the scope of this work.
Future plans to erect large numbers of wind turbines58 and associated infrastructure as well as the continued growth of HC exploration and production in the marine environment59 mean that the large and significant gaps in the knowledge in regard to the carbon content and potential carbon sequestration afforded by the ecosystems directly associated with MMS should be urgently closed.
The study looked at the potential growth rates of current BC stocks and did not take into consideration expected growth of either the OGI or offshore renewables such as wind. Davies & Hastings24 showed that emissions from the decommissioning of marine energy infrastructure will increase 200-fold over the coming decades, if this is the case then the levels of BC will also increase 200-fold.
5 Conclusions
• The potential for carbon sequestration (blue carbon) around MMS has until now not been recognised. This study shows the value that these ecosystems have in terms of both current BC and future potential BC growth.• The model presented used growth rates and a biological understanding of ecosystem evolution to make predictions about the amount of biomass and blue carbon that is currently adhered or attached to OGIMMS in the marine environment. It found that these thriving ecosystems provide carbon sequestration and have the potential to store vast amounts of carbon if allowed to continue to grow.
• Importantly, this ‘forgotten’ solution would also contribute to significant GHG emissions savings by not requiring the complete removal of all MMS at the time of decommissioning, but instead, decommission in situ.
• The study revealed a possible relationship between localised PP and MMS that requires further study.
• The study showed that the lack of data and detailed understanding of the benefits of these ecosystems is limiting decision making, increasing GHG emissions and destroying highly productive and thriving ecosystems that are bucking the global trend of biodiversity degradation and loss.
• The dual climate and biodiversity crisis requires radical and fast action. Decommissioning in situ whilst contributing to significant cost savings, significantly provides a highly productive mechanism for long term carbon sequestration.
Conflicts of interest
There are no conflicts to decalre.Acknowledgements
This research is funded by OGTC and the University of Aberdeen, through their partnership in the UK National Decommissioning Centre. This work contributed to the NERC funded FAB-GGR project and UKERC-4 projects.References
- S. M. Watson, D. L. McLean, B. J. Balcom, S. N. R. Birchenough, A. M. Brand, E. C. M. Camprasse, J. T. Claisse, J. W. P. Coolen, T. Cresswell, B. Fokkema, S. Gourvenec, L.-A. Henry, C. L. Hewitt, M. S. Love, A. E. MacIntosh, M. Marnane, E. McKinley, S. Micallef, D. Morgan, J. Nicolette, K. Ounanian, J. Patterson, K. Seath, A. G. L. Selman, I. M. Suthers, V. L. G. Todd, A. Tung and P. I. Macreadie, Offshore decommissioning horizon scan: Research priorities to support decision-making activities for oil and gas infrastructure, Sci. Total Environ., 2023, 878, 163015, DOI:10.1016/j.scitotenv.2023.163015.
- D. L. McLean, L. C. Ferreira, J. A. Benthuysen, K. J. Miller, M. Schläppy, M. J. Ajemian, O. Berry, S. N. R. Birchenough, T. Bond, F. Boschetti, A. S. Bull, J. T. Claisse, S. A. Condie, P. Consoli, J. W. P. Coolen, M. Elliott, I. S. Fortune, A. M. Fowler, B. M. Gillanders, H. B. Harrison, K. M. Hart, L. Henry, C. L. Hewitt, N. Hicks, K. Hock, K. Hyder, M. Love, P. I. Macreadie, R. J. Miller, W. A. Montevecchi, M. M. Nishimoto, H. M. Page, D. M. Paterson, C. B. Pattiaratchi, G. T. Pecl, J. S. Porter, D. B. Reeves, C. Riginos, S. Rouse, D. J. F. Russell, C. D. H. Sherman, J. Teilmann, V. L. G. Todd, E. A. Treml, D. H. Williamson and M. Thums, Influence of offshore oil and gas structures on seascape ecological connectivity, Global Change Biol., 2022, 28, 3515–3536, DOI:10.1111/gcb.16134.
- C. A. Layman and J. E. Allgeier, An ecosystem ecology perspective on artificial reef production, J. Appl. Ecol., 2020, 57, 2139–2148, DOI:10.1111/1365-2664.13748.
- A. M. Fowler, A.-M. Jørgensen, J. W. P. Coolen, D. O. B. Jones, J. C. Svendsen, R. Brabant, B. Rumes and S. Degraer, the ecology of infrastructure decommissioning in the North Sea: what we need to know and how to achieve it, ICES J. Mar. Sci., 2020, 77, 1109–1126, DOI:10.1093/icesjms/fsz143.
- A. J. Lemasson, A. M. Knights, M. Thompson, G. Lessin, N. Beaumont, C. Pascoe, A. M. Queirós, L. McNeill, M. Schratzberger and P. J. Somerfield, evidence for the effects of decommissioning man-made structures on marine ecosystems globally: a systematic map protocol, Environ. Evid., 2021, 10, 4, DOI:10.1186/s13750-021-00218-y.
- S. van Leeuwen, P. Tett, D. Mills and J. van der Molen, Stratified and nonstratified areas in the North Sea: Long-term variability and biological and policy implications, J. Geophys. Res.: Oceans, 2015, 120, 4670–4686, DOI:10.1002/2014JC010485.
- R. Callaway, G. Engelhard, J. Dann, J. Cotter and H. Rumohr, a century of North Sea epibenthos and trawling: comparison between 1902-1912, 1982-1985 and 2000, Mar. Ecol.: Prog. Ser., 2007, 346, 27–43, DOI:10.3354/meps07038.
- IMSA, Ecosystems associated with the North Sea oil and gas facilities and the impact of decommissioning options, Background Report Phase 1, Living North Sea Initiative, IMSA Amsterdam, 2011 Search PubMed.
- B. Sommer, A. M. Fowler, P. I. Macreadie, D. A. Palandro, A. C. Aziz and D. J. Booth, decommissioning of offshore oil and gas structures – Environmental opportunities and challenges, Sci. Total Environ., 2019, 658, 973–981, DOI:10.1016/j.scitotenv.2018.12.193.
- A. J. Guerin, Marine Communities of North Sea Offshore Platforms, and the Use of Stable Isotopes to Explore Artificial Reef Food Webs, PhD thesis, University of Southampton faculty of engineering, science & mathematics, School o Ocean & Earth Sciences, 2009.
- A. Burden, C. Smeaton, S. Angus, A. Garbutt, L. Jones, H. D. Lewis and S. M. Rees, Impacts of climate change on coastal habitats, relevant to the coastal and marine environment around the UK, MCCIP Science Review, 2020, 228–255, DOI:10.14465/2020.ARC11.CHB.
- J. Järnegren, S. Brooke and H. Jensen, effects and recovery of larvae of the cold-water coral Lophelia pertusa (Desmophyllum pertusum) exposed to suspended bentonite, barite and drill cuttings, Mar. Environ. Res., 2020, 158, 104996, DOI:10.1016/j.marenvres.2020.104996.
- J. S. Gray and M. Elliott, Ecology of Marine Sediments: from Science to Management, Oxford University Press, 2nd edn, 2009, DOI:10.1093/oso/9780198569015.001.0001.
- L.-A. Henry, C. G. Mayorga-Adame, A. D. Fox, J. A. Polton, J. S. Ferris, F. McLellan, C. McCabe, T. Kutti and J. M. Roberts, Ocean sprawl facilitates dispersal and connectivity of protected species, Sci. Rep., 2018, 8, 11346, DOI:10.1038/s41598-018-29575-4.
- A. MacIntosh, K. Dafforn, B. Penrose, A. Chariton and T. Cresswell, ecotoxicological effects of decommissioning offshore petroleum infrastructure: A systematic review, Crit. Rev. Environ. Sci. Technol., 2022, 52, 3283–3321, DOI:10.1080/10643389.2021.1917949.
- D. L. McLean, L. C. Ferreira, J. A. Benthuysen, K. J. Miller, M. Schläppy, M. J. Ajemian, O. Berry, S. N. R. Birchenough, T. Bond, F. Boschetti, A. S. Bull, J. T. Claisse, S. A. Condie, P. Consoli, J. W. P. Coolen, M. Elliott, I. S. Fortune, A. M. Fowler, B. M. Gillanders, H. B. Harrison, K. M. Hart, L. Henry, C. L. Hewitt, N. Hicks, K. Hock, K. Hyder, M. Love, P. I. Macreadie, R. J. Miller, W. A. Montevecchi, M. M. Nishimoto, H. M. Page, D. M. Paterson, C. B. Pattiaratchi, G. T. Pecl, J. S. Porter, D. B. Reeves, C. Riginos, S. Rouse, D. J. F. Russell, C. D. H. Sherman, J. Teilmann, V. L. G. Todd, E. A. Treml, D. H. Williamson and M. Thums, Influence of offshore oil and gas structures on seascape ecological connectivity, Global Change Biol., 2022, 28, 3515–3536, DOI:10.1111/gcb.16134.
- OSPAR, OSPAR Convention, 1992 Search PubMed.
- Oil & Gas UK, The Management of Marine Growth during Decommissioning, Oil & Gas UK, 2013 Search PubMed.
- A. J. Davies and A. Hastings, Greenhouse Gas Emissions from Decommissioning Manmade Structures in the Marine Environment; Current Trends and Implications for the Future, JMSE, 2023, 11, 1133, DOI:10.3390/jmse11061133.
- B. W. Griscom, J. Adams, P. W. Ellis, R. A. Houghton, G. Lomax, D. A. Miteva and W. H. Schlesinger, Nature Climate Solutions, Proc. Natl. Acad. Sci. U.S.A., 2017, 114(44), 11645–11650 CrossRef CAS PubMed . https://www.pnas.org/content/114/44/11645.
- IPCC, Climate Change 2021 – the Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, 1st edn, 2021, DOI:10.1017/9781009157896.
- D. Moore, M. Heilweck, W. Fears, P. Petros, S. Squires, E. Tamburini and R. Waldron, Potential of ocean calcifiers to sequester atmospheric carbon in quantity and even reverse climate change, Earth, Atmospheric, And Planetary Sciences, 2023, 7, 132, DOI:10.35841/aajfr-7.1.132.
- E. Atar, A. C. Aplin, V. Lamoureux-Var, C. März and T. Wagner, Sedimentation of the Kimmeridge Clay Formation in the Cleveland Basin (Yorkshire, UK), Minerals, 2020, 10, 977, DOI:10.3390/min10110977.
- K. Wolfe, T. M. Kenyon and P. J. Mumby, The biology and ecology of coral rubble and implications for the future of coral reefs, Coral Reefs, 2021, 40, 1769–1806, DOI:10.1007/s00338-021-02185-9.
- UNESCO, Great Barrier Reef, 2023. https://whc.unesco.org/en/list/154 (accessed 6.20.23) Search PubMed.
- D. M. Price, K. Robert, A. Callaway, C. Lo lacono, R. A. Hall and V. A. I. Huvenne, Using 3D photogrammetry from ROV video to quantify cold-water coral reef structural complexity and investigate its influence on biodiversity and community assemblage, Coral Reefs, 2019, 38, 1007–1021, DOI:10.1007/s00338-019-01827-3.
- L.-A. Henry and J. M. Roberts, Global Biodiversity in Cold-Water Coral Reef Ecosystems, in Marine Animal Forests, ed. S. Rossi, L. Bramanti, A. Gori and C. Orejas Saco del Valle, Springer International Publishing, Cham, 2016, pp. 1–21, DOI:10.1007/978-3-319-17001-5_6-1.
- C. M. Roberts, B. C. O'Leary, D. J. McCauley, P. M. Cury, C. M. Duarte, J. Lubchenco, D. Pauly, A. Sáenz-Arroyo, U. R. Sumaila, R. W. Wilson, B. Worm and J. C. Castilla, Marine reserves can mitigate and promote adaptation to climate change, Proc. Natl. Acad. Sci. U.S.A., 2017, 114, 6167–6175, DOI:10.1073/pnas.1701262114.
- FAO, What Are Vulnerable Marine Ecosystems?, Food and Agriculture Organisation of the United Nations, 2017 Search PubMed.
- J. Cresswell, Offshore Wind Starts Squaring up to Massive Future Decom Challenge, Energy Voice, 2020, https://www.energyvoice.com/renewables-energy-transition/wind/uk-wind/274744/offshore-wind-starts-squaring-up-to-massive-future-decom-challenge/, (accessed 1.10.23) Search PubMed.
- A. J. Davies and A. Hastings, Quantifying greenhouse gas emissions from decommissioned oil and gas steel structures: Can current policy meet NetZero goals?, Energy Policy, 2022, 160, 112717, DOI:10.1016/j.enpol.2021.112717.
- OEUK, Decommissioning Insight, OEUK, 2022 Search PubMed.
- P. Palmerini and C. N. Bianchi, biomass measurements and weight-to-weight conversion factors: a comparison of methods applied to the mussel Mytilus galloprovincialis, Mar. Biol., 1994, 120, 273–277, DOI:10.1007/BF00349688.
- Healthy Reefs, Coral Mortality, Healthy Reefs for Healthy People, 2023, https://www.healthyreefs.org/cms/healthy-reef-indicators/coral-mortality/#:%7E:text=Foracolonywithlivingandnonlivingsections%2C,75%25living%2C5%25recentlydead%2Cand20%25olddead, (accessed 7.13.23) Search PubMed.
- I. Galan, F. P. Glasser and C. Andrade, Calcium carbonate decomposition, J. Therm. Anal. Calorim., 2013, 111, 1197–1202, DOI:10.1007/s10973-012-2290-x.
- J. M. Fry, Carbon Footprint of Scottish Suspended Mussels and Intertidal Oysters, Scottish Aquaculture Research Forum, Pitlochry, 2012 Search PubMed.
- P. Baker and S. Baker, Carbon Fixation by Hard Clam Aquaculture in Florida, 2010 Search PubMed.
- IFAS, Carbon Fixation by Florida Cultured Clam, Florida Shellfish Aquaculture, 2011, https://shellfish.ifas.ufl.edu/projects/shellfish-farm-environment/carbon-fixation/, (accessed 7.13.23) Search PubMed.
- ATSDR, Chapter 2: Landfill Gas Basicshttps://www.atsdr.cdc.gov/HAC/landfill/html/ch2.html#:%7E:text=PhaseIIIdecompositionstartswhencertainkindsof,inwhichmethane-producingbacteriabegintoestablishthemselves, Agency for Toxic Substances & Disease Registry, 2001, (accessed 7.13.23).
- W. L. Geary, M. Bode, T. S. Doherty, E. A. Fulton, D. G. Nimmo, A. I. T. Tulloch, V. J. D. Tulloch and E. G. Ritchie, a guide to ecosystem models and their environmental applications, Nat. Ecol. Evol, 2020, 4, 1459–1471, DOI:10.1038/s41559-020-01298-8.
- R. Kratz, Biology: How to Track Changes in Populations for Dummies, 2006, https://www.dummies.com/article/academics-the-arts/science/biology/biology-how-to-track-changes-in-populations-169037/, (accessed 7.13.23) Search PubMed.
- J. R. Fanchi, Modern Reservoir Characterization Techniques, Shared Earth Modeling. Elsevier, 2002, pp. 182–198, DOI:10.1016/B978-075067522-2/50011-2.
- D. R. Cox, A. M. W. Newton and M. Huuse, an introduction to seismic reflection data: acquisition, processing and interpretation, Regional Geology and Tectonics, Principles of Geologic Analysis, Elsevier, 2020, pp. 571–603, DOI:10.1016/B978-0-444-64134-2.00020-1.
- P. M. Burgess, A brief review of developments in stratigraphic forward modelling, 2000–2009, Phanerozoic Regional Geology of the World, Elsevier, 2012, pp. 378–404, DOI:10.1016/B978-0-444-53042-4.00014-5.
- K. A. Barnhill, J. M. Roberts, I. Myers-Smith, M. Williams, K. G. Dexter, C. Ryan, U. Wolfram and S. J. Hennige, Incorporating dead material in ecosystem assessments and projections, Nat. Clim. Change, 2023, 13, 113–115, DOI:10.1038/s41558-022-01565-5.
- A. Freiwald, J. H. Fosså, A. Grehan, T. Koslow and J. M. Roberts, Cold-water Coral Reefs: Out of Sight – No Longer Out of Mind, UNEP-WCM, Cambridge, UK, 2004 Search PubMed.
- R. A. Ronconi, K. A. Allard and P. D. Taylor, Bird interactions with offshore oil and gas platforms: Review of impacts and monitoring techniques, J. Environ. Manage., 2015, 147, 34–45, DOI:10.1016/j.jenvman.2014.07.031.
- Office for National Statistics (ONS), Greenhouse Gas Emissions, UK, Provisional Estimates: 2021, ONS, 2022, https://www.ons.gov.uk/economy/environmentalaccounts/bulletins/greenhousegasintensityprovisionalestimatesuk/2021, (Accessed 7.14.23) Search PubMed.
- J. L. Drake, T. Mass, J. Stolarski, S. Von Euw, B. Schootbrugge and P. G. Falkowski, how corals made rocks through the ages, Global Change Biol., 2020, 26, 31–53, DOI:10.1111/gcb.14912.
- UNFCCC, Paris Agreement United Nations Framework Convention on Climate Change, UNFCCC, United Nations, 2015 Search PubMed.
- UN, Nature's Dangerous Decline ‘Unprecedented’; Species Extinction Rates ‘Accelerating’, UN Report, Sustainable Development Goals, 2019, https://www.un.org/sustainabledevelopment/blog/2019/05/nature-decline-unprecedented-report/, (Accessed 7.14.23) Search PubMed.
- B. W. Frost, Phytoplankton bloom on iron rations, Nature, 1996, 383, 475–476, DOI:10.1038/383475a0.
- NASA Earth Observatory, A Sea of Color and Wind, 2020, https://earthobservatory.nasa.gov/images/146556/a-sea-of-color-and-wind, (Accessed 7.14.23) Search PubMed.
- A. Platis, S. K. Siedersleben, J. Bange, A. Lampert, K. Bärfuss, R. Hankers, B. Cañadillas, R. Foreman, J. Schulz-Stellenfleth, B. Djath, T. Neumann and S. Emeis, First in situ evidence of wakes in the far field behind offshore wind farms, Sci. Rep., 2018, 8, 2163, DOI:10.1038/s41598-018-20389-y.
- Insite, Signal, Influence of Man-Made Structures in the Ecosystem: Is There a Planktonic Signal?, 2017 Search PubMed.
- D. Mayk, L. S. Peck, T. Backeljau and E. M. Harper, Shell thickness of Nucella lapillus in the North Sea increased over the last 130 years despite ocean acidification, Commun. Earth Environ., 2022, 3, 158, DOI:10.1038/s43247-022-00486-7.
- K. Gormley, F. McLellan, C. McCabe, C. Hinton, J. Ferris, D. Kline and B. Scott, Automated Image Analysis of Offshore Infrastructure Marine Biofouling, JMSE, 2018, 6, 2, DOI:10.3390/jmse6010002.
- S. Gourvenec, F. Sturt, E. Reid and F. Trigos, Global assessment of historical, current and forecast ocean energy infrastructure: Implications for marine space planning, sustainable design and end-of-engineered-life management, Renewable Sustainable Energy Rev., 2022, 154, 111794, DOI:10.1016/j.rser.2021.111794.
- M. Cavcic, 2022 Seen as a Bumper Year for Global Oil & Gas Exploration, Says WoodMac, Offshore Energy, 2023, (accessed 14/09/2023) Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |