Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fate and occurrence of microplastics in wastewater treatment plants
Daniela P.
Mesquita†
 *ab,
Cristina
Quintelas†
*ab,
Cristina
Quintelas†
 ab and
Eugénio C.
Ferreira
ab and
Eugénio C.
Ferreira
 ab
ab
aCEB – Centre of Biological Engineering, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: daniela@deb.uminho.pt
bLABBELS – Associate Laboratory, Braga/Guimarães, Portugal
First published on 16th October 2023
Abstract
Microplastics (MP) are commonly present in our daily life. Reported studies on MP pollution revealed that wastewater treatment plants (WWTPs) serve as pathways for MP to enter terrestrial and aquatic ecosystems, causing adverse effects on the quality of water bodies, aquatic life, and even contamination of soil and groundwater. In WWTPs, variable MP removal efficiencies from liquid streams have been reported. However, many MP particles are still discharged into natural water bodies. Concomitantly, the retention of MP in sewage sludge is reported, and thus, understanding MP fate in WWTPs is of great significance towards MP management. This review discusses the most recent research focused on the abundance and removal of MP in WWTPs, the main methodologies applied to MP sampling, extraction, identification, and quantification in WWTPs, and the current knowledge on MP as transport vectors for other (micro)pollutants. The transfer of MP from wastewater to sludge raises environmental concerns, and efforts to optimize the value of sludge within a circular economy are essential. The potential of bioaugmentation strategies with plastic-degrading microorganisms to enhance MP removal emphasizes the importance of ongoing research, although it is still in its early stages. It is essential to improve and standardize methods for MP sampling, extraction, visual inspection, and chemical quantification in wastewater and sludge samples. The necessity for further investigation into MP interactions with other environmental (micro)pollutants and their potential impact on human health is also highlighted.
Environmental significanceMicroplastics (MP) are gaining wide attention from the scientific community due to their presence in our daily life. Wastewater treatment plants (WWTPs) play an important role in the spread of MP pollution into the environment. This tutorial review gathers recent relevant work regarding the presence of MP in WWTPs. Consistent methods for MP sampling, extraction, identification, and quantification need to be optimized. Cost-effective and efficient strategies that can be readily implemented in WWTPs should be developed aiming to effectively eradicate the release of MP into the environment and protect ecosystems and human health. Bioaugmentation strategies employing plastic-degrading microorganisms could offer significant benefits in the removal of MP. |
1. Introduction
Over the last few decades, the world has witnessed concerns over water quality and a growing demand for wastewater treatment (WWT) processes to overcome water contamination resulting from the increase in world population and intensive industrial activities.1,2 The biological process remains the most appealing approach in WWT, as microorganisms exhibit a remarkable capability to consume organic compounds, thereby mitigating wastewater pollution. This, in turn, makes a valuable contribution to enhancing the overall quality of global aquatic ecosystems. However, it is known that industrial effluents play a significant role in water pollution. Moreover, the discharge of inadequately treated effluents into receiving waters, along with the deteriorating quality and diminishing quantity of accessible groundwater, is having a major impact on the availability of safe drinking and household water resources.3 Also, in the past few years, with the progress and the development of novel products, the pollutant complexity has increased. The presence of a wide range of contaminants of emerging concern, known as (micro)pollutants, has led to a high degree of environmental pollution and to serious health hazards, threatening the quality of life of humans, animals, and plants.4Microplastics (MP) are included in this group of (micro)pollutants due to the increasing demand for products of plastic origin. Over time, all plastic materials gradually degrade into increasingly smaller pieces, with sizes less than 5 mm.5,6 Looking at the numbers, one can verify the extraordinary increase in plastic production over the last few decades, considering that in 1950 it was 2 million metric tons (Mt), and in 2020, it reached 367 Mt, of which 32% was produced in China, followed by the North American Free Trade Agreement (NAFTA) (19%).7,8 Uncontrolled consumption and insufficient waste management practices remain the ongoing influx of plastics into the environment, exerting a substantial influence on the overall degree of environmental pollution across various domains such as the atmosphere, water bodies, soil, and sediment.9 Moreover, human exposure through inhalation, ingestion, and dermal contact is currently recognized as a primary pathway, posing potential health hazards.10–12
MP can be categorized into two groups based on their origin: primary MP and secondary MP.13 Primary MP are commonly found in our daily lives and are used as personal care and cosmetic products, such as toothpaste, facial and body cleansers, and more.14 Primary MP originates from a diverse range of sources, including synthetic fibers from textiles,15,16 polymers after their useful life, and residues from processing industries. On the other hand, secondary MP result from various processes, such as mechanical degradation, photo or ultraviolet (UV) degradation, biodegradation, thermal-oxidative degradation, hydrolysis, and other mechanisms.13
Since late 2019, a significant increase in plastic waste has emerged as a result of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for the viral illness COVID-19. This remarkable increase in plastic waste can be attributed to the widespread use of single-use personal protective equipment, including disposable gloves and face masks.17 The substantial production and consumption of these protective products in the battle against COVID-19 have quickly become the primary drivers behind the growth in plastic production.13,18 The global rise in the production of protective equipment made from polymeric materials such as polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), polyester, polyethylene terephthalate (PET), and polyether sulfone (PES)19,20 has introduced a novel environmental challenge – the emergence of a new source of MP. Recent reports indicate that COVID-19 is expected to cause a twofold increase in plastic debris by 2030,18 raising significant concern.
Concomitantly, MP can be considered vectors or carriers for several toxicants, including pharmaceuticals,21 persistent organic pollutants (POP), and heavy metals.22,23 This is due to MP's large specific surface area,24 a result of their small dimensions and irregular shapes, and lipophilic nature,25 which provide numerous sites for interactions, making them able to absorb or adsorb hydrophobic substances, such as certain chemicals and (micro)pollutants from their surroundings, thus constituting an additional risk for the environment. In this regard, MP may become even more dangerous, transferring harmful chemicals into the food chain, compromising the aquatic life and the ecological system.23,26 Moreover, it was recently found that MP shows potential as a route for antibiotic resistance gene (ARG), promoting its dissemination.27
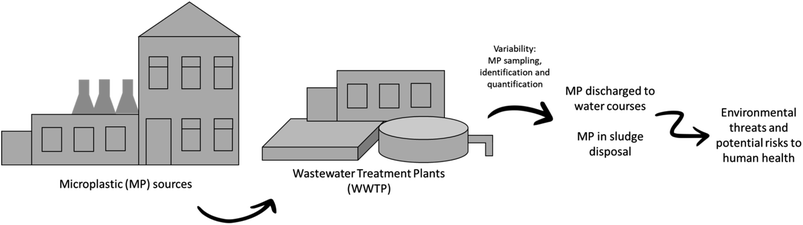
The sources of MP in the oceans are diverse, with some originating from industrial wastewater. It is important to emphasize that the transportation and distribution of MP in ocean ecosystems involve complex processes, and their origins can vary depending on geographical location, coastal currents, and nearby human activities. Moreover, MP can find their way into water bodies through mechanisms such as machine- and hand-laundering, leaching, or flooding. Substantial quantities of MP end up in wastewater treatment plants (WWTPs), which have been identified both as recipients of MP pollution and as a primary pathway for MP to enter the environment.28 WWTP receive MP through multiple pathways: (i) domestic discharge systems; (ii) discharge into municipal wastewater collecting systems; (iii) stormwater runoff; and (iv) landfill leachates.29 To illustrate, synthetic textiles, tires, plastic granules, urban dust, maritime signaling components, marine coatings, WWTP effluents, and personal hygiene products (as depicted in Fig. 1) are among the sources of MP. Fig. 2 provides a schematic representation of the existing challenges posed by MP in WWTP. Consequently, it is imperative to explore solutions for effectively managing these (micro)pollutants.
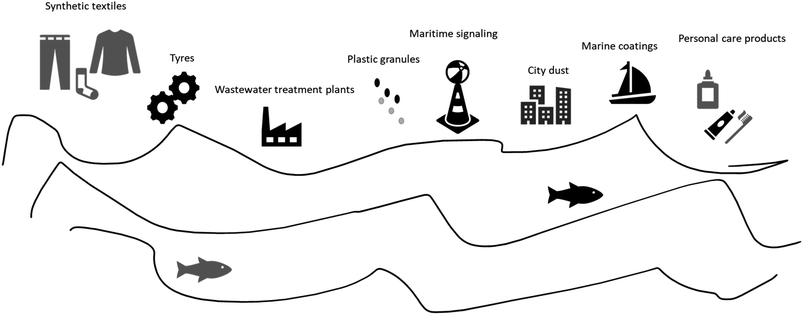 | ||
| Fig. 1 Sources of MP in the ocean. Adapted from Friot and Boucher.53 | ||
The European Union (EU) has been at the forefront of environmental policy and sustainability initiatives, with a central strategy being the promotion of a circular economy.30–32 Within this framework, the management of wastewater and excess sludge plays a fundamental role in achieving sustainability objectives.32 In various regions across the EU, the adoption of water reuse practices is on the rise.32–34 This involves utilizing treated wastewater for purposes such as irrigation, industrial processes, and cooling, thereby aligning with the principles of the circular economy. Such practices effectively increase the lifespan and the usefulness of water resources. Furthermore, it's important to consider sludge, a natural byproduct of WWT, in the context of the circular economy. In several EU regions, innovative approaches have already been implemented for treating and managing sludge in an environmentally responsible manner, including its conversion into biogas or fertilizer.32,35,36
When sustainable practices are implemented, the recycling of treated wastewater and sludge can potentially contribute to the issue of MP pollution, given the presence of MP particles in these materials. This association can result in adverse environmental outcomes, as MP particles have the potential to infiltrate aquatic ecosystems, agricultural soils, and, ultimately, pose risks to marine life while potentially entering the human food chain. These risks highlight the critical need to address the occurrence of MP particles in wastewater and sludge management practices. Such measures are essential to ensure that the principles of the circular economy are effectively integrated with efforts aimed at combating plastic pollution and preserving our environment.
2. Abundance and removal of microplastics in WWTPs
Globally, WWTP exhibit some level of effectiveness in removing MP from wastewater, but the estimated removal efficiencies are often quite variable.37–41 Studies have revealed that several million MP particles are still released daily through WWTP effluents worldwide,23,42,43 contributing to the dispersion of MP in aquatic ecosystems.44 This is primarily because these treatment technologies are not specifically designed for MP removal.45,46 Recent reports highlight that the removal rates are heavily influenced by factors like MP size, density, and shape.47 Consequently, MP are now recognized as a global environmental issue of significant concern, with numerous studies in recent years investigating their distribution48 and impacts,49 particularly within marine environments. The reported concentrations of MP in wastewater vary widely across different WWTP, largely depending on the treatment phases, and the types of polymer materials detected encompass a wide range. Table 1 provides several examples from around the world, illustrating (i) WWTPs sampling sites, (ii) the MP content in liquid streams, (iii) the MP amount, (iv) polymer type and size, (v) extraction and identification methods, and (vi) MP removal efficiency.| Country | Number of facilities | Sampling | MP amount | MP type | MP average size | Polymer type | Extraction method | Identification method | Removal efficiency | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MP m−3 | MP day−1 | ||||||||||
| a PE – polyethylene; PET – polyethylene terephthalate; PP – polypropylene; PA – polyamide; ET – ethylene–propylene; PU – polyurethane; HDPE – high density polyethylene; LDPE – low density polyethylene; WPO – wet peroxide oxidation; OEP – oil extraction protocol; FTIR – Fourier transform infrared spectrometry; μ-FTIR – Fourier transform infrared microscope system; ATR-FTIR – attenuated total reflectance FTIR; Nd–no data. | |||||||||||
| Turkey | 2 | Influent | 1.5 × 104–3.6 × 104 | 1 × 106–6.5 × 106 | Fibres (71.3%); fragments (18.4%); film (10.4%) | 1.57–1.63 mm | Polyester (56.4%); PE (26.3%); PP (12.9%) | WPO | μ-Raman | 73–79% | 54 |
| Secondary effluent | 2.7 × 103–8.6 × 103 | 2.2 × 105–1.5 × 106 | Fibres (65.5%); fragments (18.1%); film (16.5%) | 1.15–1.39 mm | Polyester (56.3%); PE (25.1%); PP (15.7%) | ||||||
| Canada | 1 | Influent | 3.1 × 104 | Nd | Fibres (65.6%); fragments (28.1%); pellets (5.4%) | Nd | Nd | OEP | FTIR | 91.7% | 38 |
| Primary effluent | 2.6 × 103 | Nd | |||||||||
| Secondary effluent | <0.5 × 103 | 3.2 × 106–9.7 × 106 | |||||||||
| Scotland | 1 | Influent | 1.57 × 104 | 4.1 × 109 | Flakes (67.3%); fibres (18.5%); film (9.9%); beads (3.0%); foam (1.3%) | 0.598 mm | Alkyds (28.7%), PS-acrylic (19.1%), polyester (10.8%), PU (8.9%), and acrylic (8.3%) polyester (28%), PA (20%), PP (12%), acrylic (12%), alkyd (8%), PE (4%), PS (4%), and PET (4%) | Nd | FTIR | 98.4% | 40 |
| Grit and grease effluent | 8.7 × 103 | 2.3 × 109 | |||||||||
| Primary effluent | 3.4 × 103 | 8.9 × 108 | |||||||||
| Secondary effluent | 0.25 × 103 | 6.5 × 107 | |||||||||
| China | 2 | Influent | 23.3 × 103–80.5 × 103 | 9.1 × 1010 | Fibres (59.7–73.2%); fragments (21.4–30%) | 80% <1 mm | Nd | Density separation | Raman | 62.7–66.1% | 55 |
| Tertiary effluent | 7.9 × 103–30.3 × 103 | ||||||||||
| China | 1 | Influent | 1.26 × 105 | Nd | Fibres (37.7–60.8%); fragments (30.4–48.3%) | 0.02–5 mm | Polyester (29.5%), PA (20.5%), PET (14.3%), PE (11.6%), PS (9.8%), PP (8.9%) | Density separation | Raman | 75.7% | 56 |
| Primary effluent | 7.26 × 104 | Nd | |||||||||
| Secondary effluent | 3.79 × 104 | Nd | |||||||||
| Tertiary effluent | 3.06 × 104 | Nd | |||||||||
| China | 4 | Influent | 0.07 × 103–8.72 × 103 | Nd | Fibres, fragment and film fibres | 500 μm −5 mm | PP and PE | Density separation | FTIR | 89.2–93.6% | 57 |
| Primary effluent | 0.39 × 103–4.77 × 103 | ||||||||||
| Secondary effluent | 0.07 × 103–0.78 × 103 | 2 × 106–7.8 × 107 | |||||||||
| Spain | 1 | Primary effluent | 2.59 × 103 | Nd | Films (9.1%), fragments (60.5%) and fibers (Nd) | 400–600 μm | LDPE (9.2%), HDPE (2.8%), PP (1.7%), LDPE (2.0%), HDPE (0.6%), PP (0.6%) | Density separation | FTIR | 90.3% | 37 |
| Secondary effluent | 0.31 × 103 | 6.7 × 106 | |||||||||
| Spain | 1 | Primary effluent | 1.71 × 105 | Nd | Fragments and fibres (31%); fragments and fibres (20%) | 25 μm–50 mm | Polyester > PE > PP | Nd | μ-FTIR | 93.7% | 58 |
| Secondary effluent | 1.07 × 104 | 3.0 × 108 | PE > polyester > PP | ||||||||
| Italy | 1 | Influent | 2.5 × 103 | 1.0 × 109 | Films (73%), fragments (21%) and lines (6%) | 0.5–0.1 mm | Co-polymer of acrylonitrile-butadiene (40%), PE (17%) and ET (14%) | Density separation | μ-FTIR | 84% | 59 |
| Secondary effluent | 0.9 × 103 | Films (36%), fragments (36%) and lines (28%) | Polyester (23%), PE (13%), PU (13%), PA (11%), and PP (11%) | ||||||||
| Tertiary effluent | 0.4 × 103 | 1.6 × 108 | Lines (41%), films (38%) and fragments (21%) | Polyesters (35%), PA (17%) and PE (10%) | |||||||
| Iran | 2 | Effluent | 2.04 × 103 | 1.2 × 108 | Fibres > films > fragments > granules | 0.003–5 mm | PE, PP and PA | Density separation | FTIR | Nd | 60 |
| Finland | 4 | Primary/secondary effluent | 0.5 × 103–6.9× 103 | Nd | Fibres, fragments and films | Nd | Polyester (60%), PE (14%), polyacrylates (7%), polyvinylchloride (5%), PS (4%) and PP (3%) | Density separation | FTIR | 40–99.9% | 45 |
| Tertiary effluent | 0.005 × 103–0.3 × 103 | ||||||||||
| Finland | 1 | Tertiary effluent | Nd | 1.7 × 108–1.4 × 108 | Fibres | Nd | Cotton (44%) and PET (33%) | Density separation | FTIR | 97% | 61 |
| Germany | 12 | Secondary/tertiary effluent | Nd | 9 × 107–4 × 109 | Particles and fibers | Nd | PE | Density separation | ATR-FTIR and μ-FTIR | 97% | 43 |
| Australia | 1 | Influent | 1.18 × 104 | 1.07 × 108–1.16 × 108 | Fibres and fragments | Nd | PP (35.91%), polyester (24.64%), PA (89.5%) | Density separation | ATR-FTIR | 76.61% | 62 |
| Primary effluent | 5.23 × 103 | Polyester (26.58%), PA (4.22%), PET (20.68%), PP (19.41%), PE (5.49%) | |||||||||
| Tertiary effluent | 2.7 × 103 | Polyester (45.94%), PP (24.32%), PA (11.48%), PE (9.45%) | |||||||||
| Australia | 3 | Primary effluent | 1.5 × 103 | 4.6 × 108 | Nd | 60–500 μm | Polyester, PE | Nd | FT-IR | 90% | 21 |
| Secondary effluent | 0.48 × 103 | 8.1 × 106 | |||||||||
| Tertiary effluent | 0.28 × 103 | 3.6 × 106 | |||||||||
| South Korea | 3 | Influent | 4.2 × 106–3.14 × 107 | Nd | Microbeads, fragments, fibres, and sheets | Nd | Nd | Nd | Nd | 98.9–99.2% | 63 |
| Primary effluent | 1.57 × 106–1.26 × 107 | ||||||||||
| Secondary effluent | 4.3 × 105–7.86 × 106 | ||||||||||
| Tertiary effluent | 3.3 × 104–2.97 × 105 | ||||||||||
In general, considering Table 1, it becomes evident that studies on the presence of MP in WWTPs worldwide exhibit significant variations. Some studies provide results on the quantity of MP at different treatment steps, while others solely focus on the final effluent. Analyzing the MP concentration across different steps of WWT reveals a substantial decrease leading up to the final effluent. Consequently, the primary effluent exhibits the highest MP concentrations, followed by the secondary effluent. When a WWTP incorporates tertiary treatment, involving for instance filtration, it consistently contributes to improved MP removal in the majority of reported cases. This underscores the importance of the various stages within WWTPs, from primary treatment, through secondary treatment, and, in some cases, tertiary treatment, in mitigating MP pollution in the liquid stream. However, there is a notable variability among different studies regarding the MP concentration detected in each step. This variability may be linked to the location of the WWTPs under investigation (urban/suburban), the proportion of domestic/industrial wastewater entering the plant, and the specific treatment steps designed to meet the plant's requirements in a given region. The diversity in the types of MP and polymers detected primarily correlates with the type of wastewater entering the treatment plant. Fibers tend to dominate in wastewater samples, particularly in WWTP influents sourced from households, indicative of the influence of laundry and textile washing where tiny plastic fibers are released from the fabric due to the mechanical stress of washing, friction, and the use of detergents. Polyesters, PP, and PE are the most commonly identified polymers.
Removal percentages range from 40% to 99.9%, highlighting the substantial impact of different treatment steps on wastewater treatment and MP reduction. Notably, the presence of various types of tertiary treatment significantly enhances MP removal.
Conducting extensive research on MP in wastewater from diverse regions worldwide allows for insights into distribution patterns, sources, and MP behavior. This information can provide targeted interventions, assess the effectiveness of WWT procedures, and guide mitigation plans. However, it is essential to be cautious when evaluating these studies, considering the methodologies used for sampling, extraction, identification, and quantification. Moreover, estimates of the quantities or loads of MP present in water bodies remain uncertain and should be viewed with considerable concern.
Sludge is produced as a result of both primary and secondary treatment processes within wastewater treatment (WWT) systems. The primary treatment phase plays a pivotal role in eliminating grit, grease, and larger debris and sediments from the raw wastewater, which is often referred to as primary sludge. Meanwhile, secondary treatment fosters the generation of secondary sludge due to the active presence of microorganisms. To efficiently manage the diverse types of sludge generated, a systematic approach is employed. Sludge can be collected and then subjected to a series of essential procedures aimed at reducing its volume and stabilizing its composition. These procedures encompass thickening, digestion, and dewatering processes.
In contrast to the numerous studies conducted to assess MP in WWTP liquid streams, there has been relatively little research on sludge (see Table 2). Table 2 reveals significant variability in the measurement of MP in sludge samples from WWTPs. Some studies report results regarding the MP content at various stages of sludge treatment, including both primary and secondary sludge, while others exclusively concentrate on dewatered sludge, making direct comparisons challenging. Studies that have examined the presence of MP in both primary and secondary sludge show that secondary sludge consistently contains a lower quantity of MP. This can be attributed to the fact that primary sludge is typically separated from wastewater at an earlier stage, allowing larger particles and solids to settle, which means that primary sludge may capture more MP during this phase. Secondary sludge, on the other hand, is obtained after biological processes followed by additional settling. Biological treatment involves the action of microorganisms that consume organic materials and can potentially include microorganisms able to promote biodegradation of MP, thereby reducing their presence in secondary sludge. Only one study has provided data on MP levels in sludge after the thickening process (the mixture of primary and secondary sludge) and aerobic digestion. The results seem to indicate a lower presence of MP after sludge thickening. The number of MP at the output of aerobic digestion did not differ significantly from the output of the sludge thickening tank (or input to digestion), suggesting that the reduction of MP is not so evident. Additionally, studies presenting results from dewatering sludge showed variable outcomes. Dewatering techniques are employed to further reduce the moisture content, enhancing the sludge's handling and disposal properties. However, it is clear that different dewatering equipment and techniques may have varying efficiencies in removing MP. Some equipment may be better suited for capturing small particles, while others may perform better with larger particles. The increasing reduction of MP in different steps of sludge processing stages can be also explained by the fact that some lightweight MP particles are separated from the sludge fraction and enter the liquid phase. Therefore, these MP particles are not removed from the process but rather return to treatment with the water collected during sludge processing.
| Country | Number of facilities | Sampling | MP amount | MP type | MP average size | Polymer type | Extraction method | Identification method | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| MP g−1 | MP day−1 | |||||||||
| a Microlitter; PE – polyethylene; PS – polystyrene; PA – polyamide; PP – polypropylene; OEP – oil extraction protocol; FTIR – Fourier transform infrared spectrometry; μ-FTIR – Fourier transform infrared microscope system; ATR-FTIR – attenuated total reflectance FTIR; Nd – no data. | ||||||||||
| Iran | 1 | Primary sludge | 214 | 2.80 × 108 | Fibers (85%) | >37 μm | Polyester and PE | Density separation | μ-Raman | 41 |
| Secondary sludge | 206 | 3.62 × 108 | ||||||||
| Sludge thickener aerobic digestion | 200 | 5.99 × 108 | ||||||||
| Dewatering sludge | 238 | 6.01 × 108 | ||||||||
| 129 | 2.76 × 108 | |||||||||
| Spain | 1 | Primary + secondary sludge | 183 | Nd | Fibers (62%) | 25–104 μm | PE and PP | Density separation | μ-FTIR | 58 |
| Dried sludge | 165 | Fibers (84%) | ||||||||
| Canada | 1 | Primary sludge | 14.9 | Nd | Fibers (>60%) and fragments (>30%) | Nd | Nd | OEP | ATR-FTIR | 38 |
| Secondary sludge | 4.4 | Fibers (>70%) and fragments (>20%) | ||||||||
| Italy | 1 | Secondary sludge | 113 | Nd | Fibers (47%) | 0.5–0.1 mm | Polyester (65%) | Density separation | μ-FTIR | 59 |
| Particles (53%) | ||||||||||
| Germany | 6 | Sewage sludge | 1–24 | Nd | Nd | <500 μm | PE, PP, PA and PS | Density separation | ATR-FTIR and μ-FTIR | 43 |
| Scotland | 1 | Grit fraction | Nd | Nd | Nd | 1.342 mm | Polyester, alkyd, PA, PS | Nd | FTIR | 40 |
| Grease fraction | 7.87 | PE, polyester, alkyd, acrylic, PP, PS | ||||||||
| Primary + secondary sludge | Nd | PE, polyester, alkyd, acrylic, PS | ||||||||
| Finlanda | 1 | Primary + secondary sludge | 76.3 | 1.86 × 1011 | Nd | Nd | Nd | Density separation | FTIR | 61 |
| Dewatering sludge | 186.7 | 1.51 × 1011 | ||||||||
| China | 28 | Dewatering sludge | 1.60–56.4 | Nd | Fibers (63%) | 10 μm−5 mm | Polyolefin, acrylic, PE, PA | Nd | FTIR | 64 |
| China | 4 | Dewatering sludge | 0.2–6.9 | Nd | Fibers (82.7%) | Nd | PP, PE and polyester | Density separation | FTIR | 57 |
Conventionally, following these processes, the treated sludge can normally be disposed of in several environmentally responsible ways. Options include land application as fertilizer, incineration to recover energy, or disposal in a landfill. The choice of disposal method is influenced by various factors, including the composition of the treated sludge and local regulations governing its management.
It is now widely recognized that a substantial portion of MP removed during WWT processes ultimately accumulates in sewage sludge. Each stage of the treatment process can influence the concentration and properties of these MP.39 Given the considerable volume of sewage sludge generated by WWT systems, this issue magnifies into a significant environmental concern, whether in terms of sludge disposal in landfills or its utilization as a fertilizer.50 Consequently, MP can readily find their way into the environment, especially when sewage sludge is commonly used for soil amendment in agriculture, releasing several tons of MP. Furthermore, the presence of MP in excess sewage sludge, along with various absorbed contaminants, elevates the potential for a considerable environmental hazard.51 Effective waste management strategies are imperative, necessitating assessments of potential risks associated with soil application or sludge disposal and the development of specific management approaches. This poses a significant challenge to sustainable agricultural development. To address this challenge, considerable efforts have been invested in the effective valorization of sludge produced in WWTP through various operational strategies. Notably, sludge digestion has emerged as a promising option for sludge treatment from a circular economy perspective, as it converts sludge into biogas and reduces sludge volume. However, it is crucial to evaluate the impact of MP on sludge digestion since MP are present in significant concentrations in the sludge. Recent studies indicate that, in most cases, the toxic substances released from MP, along with the presence of adsorbed contaminants of emerging concern, inhibit methane production during anaerobic digestion.23
The potential of bioaugmentation during the anaerobic digestion step of sludge treatment, to boost biogas and methane production while concurrently degrading MP, has recently been suggested resulting from the microplastics-degrading capabilities of several anaerobes.52 However, further efforts are required to comprehensively examine the mechanisms underlying the effects of MP, such as their forms, particle sizes, contents, and compositions, on anaerobic digestion.
3. Available methods for the identification and quantification of microplastics
The evaluation of MP in WWTPs is a crucial step in understanding their prevalence and mitigating their environmental impact. This section explores various techniques for sampling, extraction, identification, and quantification of MP in WWTPs.65,66 It also highlights the pressing need for standardized protocols to facilitate comparisons across studies and effective policies for combating MP pollution.The choice of sampling method for MP assessment depends on the source, whether it's wastewater or sludge. Consequently, diverse equipment and methods are commonly employed. In the context of wastewater, the separation process can be achieved using mesh/sieve filtration with various openings, enabling the characterization of MPs based on their size.67 Density separation with a salt-saturated solution, followed by MP flotation, filtration, and drying is also commonly used.37 On one hand, filtration facilitates the quantification of MP, providing valuable data for assessment. This approach is relatively accessible and does not demand specialized equipment. However, filtration methods can exhibit size-selectivity, potentially introducing bias by favoring larger MP while overlooking smaller particles. Moreover, filters may become clogged, especially when dealing with wastewater laden with suspended solids, hindering the processing of samples. Density separation demonstrates exceptional efficiency in wastewater treatment, particularly in effectively separating MP from other particles. The solution used creates a high-density environment in which MP are less dense and can float. This facilitates the precise quantification of MP and is generally not reliant on costly equipment. However, it's essential to note that density separation may exhibit selectivity, favoring the capture of larger, less dense MP while potentially overlooking smaller, denser particles. Moreover, the presence of organic compounds and salts is regarded as a bottleneck in these procedures, hindering the efficiency of the separation process.
For assessing MP in sludge, specialized equipment like the van Veen grab sampler68 or even a metal shovel41 is employed for sampling. After collection, several procedures are used for MP extraction. One method involves again the density separation technique as the initial step.57,64 This process entails mixing, settling, and filtering to guarantee the removal of salt residues. In sludge, the limitations of density separation are the same as those mentioned for wastewater.
To eliminate salts and organic compounds57,63 from both wastewater and sludge, various solutions have been tested, but the commonly accepted approach involves chemical digestion with H2O2.69,70 After digestion, the sample is typically subjected to filtration, density separation, or other separation methods to isolate the MP from the remaining wastewater or sludge components.37
Following these procedures, all samples are dried and subsequently characterized using physical and chemical methods.
To prevent overestimation of MP in WWTPs, a staining procedure employing a rose-bengal solution21,41 is sometimes employed. This procedure distinguishes between MP and other substances where researchers can visually confirm the presence and characteristics of MP using fluorescence microscopy. It's important to note that rose-bengal staining specifically aids in distinguishing MP from organic and natural particles but doesn't provide chemical information about the MP. An alternative staining method using a Nile red fluorescence-based protocol,71,72 which has been reported as viable for MP identification; however is not yet commonly utilized in WWTP samples. Nile red selectively adheres to MP minimizing interference from other materials. The intense fluorescence emitted by stained MP makes them easily detectable and distinguishable when examined under a fluorescence microscope. However, it's worth noting that Nile red staining is most effective for hydrophobic MP, and less hydrophobic ones may exhibit weaker fluorescence, potentially leading to underestimations. Like rose-bengal staining, Nile red staining requires access to fluorescence microscopy equipment. As with other visual methods, the interpretation of stained samples can involve subjectivity and may require specialized knowledge for accurate MP identification.
Visual inspection (without staining) is one of the available methods for the analysis of MP in wastewater and sludge samples. It involves the manual observation and identification of MP and can involve optical microscopy (less expensive than fluorescence microscopy), including stereomicroscopy,14,37,38,41 and is commonly complemented by scanning electron microscopy (SEM).73,74 These techniques enable the characterization of MP in terms of size distribution, morphology, and original color.44 However, visual inspection alone has been shown to yield errors of up to 70% in MP classification, underscoring the importance of chemical methods for precise MP analysis.75 For instance, one notable recent advancement is the utilization of polarized-light optical microscopy (PLOM), a technique that enhances the identification of microscopic particles by employing crossed polarizers.74 However, despite its potential benefits, PLOM has yet to gain widespread adoption in research practices.
Several chemical methods are available for investigating the composition of MP. Some are categorized as destructive, such as pyrolysis gas chromatography–mass spectrometry76 and thermo-extraction desorption gas chromatography–mass spectrometry.77 These methodologies can incur higher costs due to equipment and reagent expenses, and they may generate chemical waste or consume significant energy.
Alternatively, less complex and non-destructive methods, like Fourier-transform infrared (FTIR) spectroscopy,43,45,59 Fourier-transform infrared microscopy (μ-FTIR)43,58,59 and Raman spectroscopy-based techniques,55,56,60,69 have gained prominence for WWTP samples, due to their ability to provide precise chemical information, non-destructive nature, and sensitivity to different polymer types. FTIR spectroscopy identifies functional groups within the polymer molecules, allowing for the determination of polymer types. μ-FTIR offers distinct advantages compared to traditional transmission FTIR methods. First, it is designed for analyzing very small samples78 and it can provide high-resolution spectra at the microscale, making it suitable for studying individual MP. Also, it simplifies the sample preparation process especially when analyzing small particles and can offer high sensitivity for the analysis of small quantities of material.78,79 These beneficial features make this technique particularly appealing for studying the microscopic MP heterogeneities within wastewater and sludge samples. On the other hand, Raman spectroscopy (despite being more expensive) can provide information about the distribution of MP within a sample, making it useful for studying their spatial distribution in wastewater and sludge. The combination of visual inspection and chemical analysis is widely accepted for verifying the presence and identifying the suspected particles and polymer types of MP in WWTP samples.66
Considering recent research, it is evident that various techniques have been employed for MP sampling, extraction, identification, and quantification. However, this diversity in methodologies presents a substantial challenge in evaluating the quantities and loads of MP in WWTP and in effectively comparing results. The lack of standardized protocols makes it challenging to draw meaningful comparisons across studies, ultimately hindering our understanding of the extent and distribution of MP pollution.
To address this issue, future research endeavors should prioritize the establishment of standardized protocols for the identification and quantification of MP. By fostering uniformity in methods, advancements in the field of MP research will be possible, enabling more precise assessments and facilitating data interpretation. Such standardization efforts are crucial for the development of policies and strategies aimed at mitigating the impacts of MP pollution on both the environment and human health.
As a practical recommendation, for cost-effective selection, a density separation, followed by digestion and filtration can be consider for sample preparation. Afterwards, stereomicroscopy to efficiently sort and categorize larger MP particles is recommended. This step facilitates the rapid identification of larger MP particles. Subsequently, for the examination of smaller MP particles, optical microscopy is advisable due to its higher magnification capabilities, especially suited for observing MP in the micrometer range. To achieve a comprehensive characterization of MP, incorporating SEM is highly beneficial for providing high-resolution imaging and structural details, which prove especially valuable when dealing with smaller MP. Given the diverse analytical techniques used to explore the chemical composition and properties of MP, it is recommended to employ μ-FTIR due to its capability to provide in-depth chemical insights, allowing for the determination of the polymer composition of MP. This holistic approach ensures effective identification, categorization, and characterization of MP, which are fundamental for tracking the sources of MP pollution and developing effective management strategies.
4. Microplastics as transport vectors for other (micro)pollutants
Beyond their own direct threats to ecosystems and organisms, MP have emerged as transport vectors or carriers for various (micro)pollutants, including heavy metals, pharmaceuticals, and persistent organic pollutants (POP). According to the main literature, one of the primary mechanisms through which MP become vectors for (micro)pollutants is through adsorption. Due to their inherent hydrophobic properties, MP can readily adsorb hydrophobic compounds from their surrounding environments.21–25 This adsorption process is particularly prominent in wastewater, where MP come into contact with a diverse range of (micro)pollutants. The substantial surface area of MP relative to their size makes them highly effective at accumulating these (micro)pollutants. Thus, once (micro)pollutants adhere to the surfaces of MP, they become physically associated with these particles. MP can then be transported over extensive distances within aquatic systems to new locations, facilitating the dispersion of (micro)pollutants to areas distant from their initial sources.Several studies are next reported regarding the interactions between heavy metals, POP and pharmaceuticals with MP, some of the reported potential risks to human health, and some recommendations.
4.1. Heavy metals
Previous research has developed into the adsorption capabilities of MP concerning cadmium (Cd), cobalt (Co), and lead (Pb) when present in sewage sludge. Remarkably, it was found that the adsorption potential of MP increased by a factor of ten after undergoing WWT. This enhancement can be attributed to the physicochemical alterations that take place in sludge-associated MP during the treatment process. Additionally, the research revealed that PE and PP had greater capacities for adsorbing metals, highlighting their efficacy in this context.80 Furthermore, investigations have unveiled substantial adsorption of lead (Pb), chromium (Cr), and zinc (Zn) onto MP, particularly those composed of PE and PVC. Notably, the study findings underscored the critical role played by specific surface area, porosity, and morphological characteristics of MP in influencing their adsorption capacities. It was also deduced that the increased adsorption of metals onto MP is mostly due to the presence of organic matter.81 A comprehensive analysis was also conducted regarding the changes occurring in PA, PE, and PS MP as they pass through the wastewater pipeline, grit chambers, and biological aeration tanks. In general, the research revealed an increased adsorption capacity of MP for cadmium (Cd) following their journey through the wastewater pipeline and biological aeration tanks, attributable to the physicochemical alterations experienced by the MP during this process.82 In eastern India, a substantial presence of MP in both surface water and sediment within treatment ponds and in the associated wastewater canals was found. Notably, these MP were often loaded with toxic metals such as arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), nickel (Ni), lead (Pb), and zinc (Zn). The predominant plastic types identified were PET and PE, which were also detected in fish and macroinvertebrates residing in the treatment ponds. The study also unveiled a close correlation between the content of MP in fish and that in surface water. This observation underscores the potential risks associated with MP for aquatic biota.83 Additionally, it was recently reported that the characteristics and properties of MP and heavy metals in conjunction with environmental factors such as pH, salinity, or natural organic matter have the potential to influence the adsorption capacity of MP for heavy metals.84,854.2. Pharmaceuticals and persistent organic pollutants (POP)
The adsorption of pharmaceuticals to PA was recently investigated. pH higher than 7 and temperature of 20 °C were found to be the suitable conditions for the adsorption of three pharmaceuticals (propranolol, amitriptyline, and fluoxetine) present in wastewater. A low desorption capacity was found after wastewater discharge to the water course, thus indicating that MP is the main driver of pharmaceuticals for long distances after discharge.86 The adsorption capacity of sulfamethoxazole (SMX), an important sulfonamide antibiotic, onto six types of MP highly present in the environment (PA, PE, PET, PS, PVC and PP) was previously studied. It was found that PA had higher affinity for SMX and the adsorption capacity is highly dependent on the pH.24 Despite there not being an investigation carried out in WWTP, it provides significant indicators on MP adsorption capacity. Recently, MP were studied as vectors for exposure to hydrophobic organic chemicals (i.e., 17α-ethinylestradiol, chlorpyrifos and benzo(α)pyrene) in fish (Gasterosteus aculeatus). The study concluded that chemical sorption, desorption, and transfer of chemicals in fish are quite dependent on the physicochemical properties of both MP and chemicals and interactions as well.87Although the study was not conducted in WWTP, MP were analyzed from Taiwan's sandy beaches to assess the presence and composition of several POP. In addition to POP adhering to the surface of the pellets, the authors discovered that POP can penetrate the inner portion of the MP, leading to an increased capacity for POP sorption.88 Another study focused on the impact of plastic aging on the sorption capacity of MP (LDPE, PET, and unplasticized poly(vinyl chloride) (uPVC)) to pharmaceuticals and pesticides. The research revealed that the degree of MP aging plays a crucial role in their sorption capabilities. Aged MP exhibited increased sorption capacities for several pharmaceuticals and pesticides. The authors found that the extent of sorption depended on the specific (micro)pollutant, polymer type, and the effectiveness of the aging treatment.89
4.3. Antibiotic-resistant genes (ARG)
It seems that MP serve as conducive surfaces for microorganisms to attach and form biofilms. These biofilms, characterized by their slimy texture, play a significant role in the subsequent adhesion of various (micro)pollutants and harmful microorganisms. This effectively turns biofilms into central hubs for the transfer of genetic material. The close interactions among microorganisms themselves and between microorganisms and (micro)pollutants have a substantial impact on the increased spread of antibiotic resistance.90 The role of MP as carriers of antibiotic-resistant bacteria (ARB) and pathogens in municipal WWTPs was recently studied, demonstrating that both PE and PS MP enhance the development of biofilms exhibiting sulfonamide resistance. The presence of SMX further amplifies the absolute abundances of antibiotic-resistant genes (ARG) and MP selectively promote antibiotic-resistant and pathogenic taxa, facilitating the proliferation of ARB and pathogens.91 Although studies have identified variations in the composition of ARG between MP and their adjacent environments, a unanimous agreement regarding the quantity and diversity of ARG on MP has not been achieved.92 WWTPs often present both ARB and MP, creating an environment characterized by selection pressure resulting from the presence of antibiotics and the coexistence of resistant bacteria. Notable potential for the enrichment and distribution of pathogenic bacteria and ARG into marine ecosystems via MP is noticed. Although MP have been observed to facilitate horizontal gene transfer (HGT), their precise impact on the evolution and dissemination of antibiotic resistance among pathogens and environmental bacteria remains unexplored.934.4. Potential risk to human health and recommendations
When released into the aquatic ecosystem, MP can be ingested by a variety of organisms, and can generally induce adverse effects on biota,4 and consequently, bioaccumulate in the food chain.94 Thus, the spread of MP still represents a neglected hazard for human health.95 Studies suggest that humans could potentially ingest a significant amount of MP weekly.10,11,96–99 Adverse effects on human health may include inflammation, genotoxicity, tissue abrasion, intestinal obstruction, chronic inflammation, and more.100–102 Research has found the presence of synthetic polymer particles and fibers in human lung tissues, primarily derived from commonly consumed plastics like PP and PE. The COVID-19 pandemic introduced new challenges, with different mask types posing varying risks of MP inhalation.103,104 Dermal exposure to MP can induce oxidative stress in epithelial cells and may be linked to various health issues, including cancer. While dermal exposure is considered less harmful than ingestion or inhalation, further research is needed to fully understand the risks and establish safe exposure limits.11,99,105–107To comprehensively assess the threats posed by MP pollution to both the environment and human health, it is crucial to understand the mechanisms governing the interactions between MP and (micro)pollutants. There are some reports suggesting that in some cases, the presence of MP reduced the (micro)pollutants' bioavailability.108 Despite a significant number of studies pointing to the adsorption of (micro)pollutants, the majority of experiments have been conducted in the laboratory and for short durations. Therefore, to gain a deeper understanding of the actual and long-term effects of coexisting MP and (micro)pollutants, it is imperative to conduct extended investigations. The impact of MP contamination on the bioavailability of (micro)pollutants may be influenced by undisclosed factors, highlighting the need for more comprehensive and extensive research. Furthermore, research efforts should encompass the examination of real-world scenarios involving aged MP in the presence of (micro)pollutants, thus simulating environmentally realistic conditions. This should also include an exploration of their potential ecotoxicological effects on organisms, as well as an assessment of the associated risks to the food chain.
5. Conclusions and perspectives
Based on the studies presented in this tutorial review, it is evident that WWTPs serve as both sinks and sources of MP. This underscores the significant role that WWTPs play in the dispersion of MP pollution in the environment. Notably, there is considerable variability in the efficiency of MP removal within these facilities. The heterogeneous outcomes can be attributed to factors such as the treatment process stages, MP characteristics, and the diverse methodologies employed for the identification and quantification of MP in WWTPs. The primary treatment phase has been found to make a substantial contribution to the elimination of MP from wastewater. However, it is important to note that MP are subsequently transferred from wastewater to sludge, posing an additional environmental concern. To embrace a circular economy perspective, there is a pressing need to explore ways to maximize the value of sludge, considering aspects such as nutrient and energy recovery. Nevertheless, the presence of MP introduces challenges and needs the exploration of methodologies to ensure the sustainable and safe use of sludge resources. The incorporation of bioaugmentation strategies involving plastic-degrading microorganisms holds promise for enhancing MP removal from wastewater and sludge. It is crucial to acknowledge that investigations into the effectiveness of bioaugmentation for MP removal are still in their early stages. Furthermore, interactions between MP and other environmental (micro)pollutants may amplify the impact of MP on WWTPs, thereby exerting additional adverse effects on the environment. Investigations into the impacts of both MP and (micro)pollutants on human health are still in their early stages. Consequently, in-depth research is imperative to better comprehend the specific short-term and long-term effects of MP and environmental factors, as well as the impact on bioavailability of (micro)pollutants.There is a crucial necessity to optimize consistent methods for MP sampling, extraction, identification, and quantification in wastewater and sludge samples. To analyze MP efficiently and cost-effectively, sample preparation could involve density separation, followed by digestion and filtration. Larger MP particles could be effectively assessed using stereomicroscopy, while optical microscopy could be employed for smaller MP particles, SEM for a comprehensive characterization of MP, and μ-FTIR to investigate the chemical composition and properties of MP. These procedures hold the potential to enhance our understanding of MP in wastewater and sludge, and standardizing them will facilitate global comparisons of results, thereby improving our comprehension of the fate of MP in WWTPs, its dispersion into the environment, and its subsequent impacts throughout the food chain.
In addition to previous recommendations, mitigating the release of MP into the environment is a global concern demanding action. To accomplish this objective, it is crucial to promote interdisciplinary collaborations among scientists, policymakers, and industries for advancing our knowledge in this field and effectively translating research findings into practical applications. Promoting this collaboration, will enable the development of effective strategies for eliminating MP pollution and preserving the health of our ecosystems.
Author contributions
Daniela P. Mesquita: conceptualization, investigation, writing – original draft; Cristina Quintelas: investigation, writing – original draft; Eugénio C. Ferreira: supervision, resources, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of the UIDB/04469/2020 unit, and by LABBELS – Associate Laboratory in Biotechnology, Bioengineering and Microelectromechanical Systems, LA/P/0029/2020. Daniela P. Mesquita and Cristina Quintelas also acknowledge FCT for funding under the Scientific Employment Stimulus – Institutional Call (CEECINST/00018/2021) and under the DL57/2016 Transitory Norm Programme, respectively.References
- E. Abascal, L. Gómez-Coma, I. Ortiz and A. Ortiz, Sci. Total Environ., 2022, 810, 152233 CrossRef CAS PubMed.
- M. Varol and C. Tokatlı, Chemosphere, 2023, 311, 137096 CrossRef CAS PubMed.
- K. Saravanakumar, S. De Silva, S. S. Santosh, A. Sathiyaseelan, A. Ganeshalingam, M. Jamla, A. Sankaranarayanan, V. P. Veeraraghavan, D. MubarakAli, J. Lee, G. Thiripuranathar and M. H. Wang, Chemosphere, 2022, 307, 135593 CrossRef CAS PubMed.
- R. Álvarez-Ruiz and Y. Picó, Trends Environ. Anal. Chem., 2020, 25, e00082 CrossRef.
- L. M. Hernandez, N. Yousefi and N. Tufenkji, Environ. Sci. Technol. Lett., 2017, 4, 280–285 CrossRef CAS.
- Y. Zhou, M. Kumar, S. Sarsaiya, R. Sirohi, S. K. Awasthi, R. Sindhu, P. Binod, A. Pandey, N. S. Bolan, Z. Zhang, L. Singh, S. Kumar and M. K. Awasthi, Sci. Total Environ., 2022, 802, 149823 CrossRef CAS PubMed.
- P. Europe, Plastics—The Facts 2021. An Analysis of European Plastics Production, Demand and Waste Data, 2021 Search PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, 3–8 Search PubMed.
- D. Hu, M. Shen, Y. Zhang and G. Zeng, Sci. Total Environ., 2019, 657, 108–110 CrossRef CAS PubMed.
- K. D. Cox, G. A. Covernton, H. L. Davies, J. F. Dower, F. Juanes and S. E. Dudas, Environ. Sci. Technol., 2019, 53, 7068–7074 CrossRef CAS PubMed.
- R. Kumar, C. Manna, S. Padha, A. Verma, P. Sharma, A. Dhar, A. Ghosh and P. Bhattacharya, Chemosphere, 2022, 298, 134267 CrossRef CAS PubMed.
- J. C. Prata, Environ. Pollut., 2018, 234, 115–126 CrossRef CAS PubMed.
- N. K. Shahi, M. Maeng, D. Kim and S. Dockko, Process Saf. Environ. Prot., 2020, 141, 9–17 CrossRef CAS.
- S. A. Carr, J. Liu and A. G. Tesoro, Water Res., 2016, 91, 174–182 CrossRef CAS PubMed.
- M. A. Browne, P. Crump, S. J. Niven, E. Teuten, A. Tonkin, T. Galloway and R. Thompson, Environ. Sci. Technol., 2011, 45, 9175–9179 CrossRef CAS PubMed.
- S. Raju, M. Carbery, A. Kuttykattil, K. Senathirajah, S. R. Subashchandrabose, G. Evans and P. Thavamani, Rev. Environ. Sci. Biotechnol., 2018, 17, 637–653 CrossRef CAS.
- O. O. Fadare and E. D. Okoffo, Sci. Total Environ., 2020, 737, 140279 CrossRef CAS PubMed.
- A. L. Patrício, J. C. Prata, T. R. Walker, A. C. Duarte, W. Ouyang, D. Barcelò and T. Rocha-santos, Chem. Eng. J., 2021, 405, 126683 CrossRef PubMed.
- S. Jung, S. Lee, X. Dou and E. E. Kwon, Chem. Eng. J., 2021, 405, 126658 CrossRef CAS PubMed.
- N. Parashar and S. Hait, Sci. Total Environ., 2021, 759, 144274 CrossRef CAS PubMed.
- S. Ziajahromi, P. A. Neale, L. Rintoul and F. D. L. Leusch, Water Res., 2017, 112, 93–99 CrossRef CAS PubMed.
- B. Ma, W. Xue, Y. Ding, C. Hu, H. Liu and J. Qu, J. Environ. Sci., 2019, 78, 267–275 CrossRef CAS PubMed.
- X. Zhang, J. Chen and J. Li, Chemosphere, 2020, 251, 126360 CrossRef CAS PubMed.
- X. Guo, C. Chen and J. Wang, Chemosphere, 2019, 228, 300–308 CrossRef CAS PubMed.
- L. M. Rios, C. Moore and P. R. Jones, Mar. Pollut. Bull., 2007, 54, 1230–1237 CrossRef CAS PubMed.
- F. Wang, C. S. Wong, D. Chen, X. Lu, F. Wang and E. Y. Zeng, Water Res., 2018, 139, 208–219 CrossRef CAS PubMed.
- Y. Su, Z. Zhang, J. Zhu, J. Shi, H. Wei, B. Xie and H. Shi, Environ. Pollut., 2021, 270, 116278 CrossRef CAS PubMed.
- E. C. Quintelas, C. Mesquita and D. P. Ferreira, in Biological Treatment of Industrial Wastewater, ed. E. Maulin P. Shah, Royal Society of Chemistry, 2022, pp. 15–39 Search PubMed.
- P. L. Ngo, B. K. Pramanik, K. Shah and R. Roychand, Environ. Pollut., 2019, 255, 113326 CrossRef CAS PubMed.
- European Commission, The European Green Deal, Brussels, 2019, vol. 53 Search PubMed.
- European Commission, A New Circular Economy Action Plan for a Cleaner and More Competitive Europe, Brussels, 2020 Search PubMed.
- European Economic Area (EEA), Beyond Water Quality-Sewage Treatment in a Circular Economy, 2022 Search PubMed.
- V. Shrivastava, I. Ali, M. M. Marjub, E. R. Rene and A. M. F. Soto, Chemosphere, 2022, 293, 133553 CrossRef CAS PubMed.
- K. Ramm and M. Smol, Sustainability, 2023, 15, 12781 CrossRef CAS.
- P. Kaszycki, M. Głodniok and P. Petryszak, Adv. Biotechnol., 2021, 61, 80–89 CAS.
- K. Ignatowicz, G. Filipczak, B. Dybek and G. Wałowski, Energies, 2023, 16, 798 CrossRef CAS.
- J. Bayo, S. Olmos and J. López-Castellanos, Chemosphere, 2020, 238, 124593 CrossRef CAS PubMed.
- E. A. Gies, J. L. LeNoble, M. Noël, A. Etemadifar, F. Bishay, E. R. Hall and P. S. Ross, Mar. Pollut. Bull., 2018, 133, 553–561 CrossRef CAS PubMed.
- P. U. Iyare, S. K. Ouki and T. Bond, Environ. Sci.: Water Res. Technol., 2020, 6, 2664–2675 RSC.
- F. Murphy, C. Ewins, F. Carbonnier and B. Quinn, Environ. Sci. Technol., 2016, 50, 5800–5808 CrossRef CAS PubMed.
- S. S. Petroody, S. H. Hashemi and C. A. M. van Gestel, Chemosphere, 2021, 278, 130471 CrossRef PubMed.
- S. A. Mason, D. Garneau, R. Sutton, Y. Chu, K. Ehmann, J. Barnes, P. Fink, D. Papazissimos and D. L. Rogers, Environ. Pollut., 2016, 218, 1045–1054 CrossRef CAS PubMed.
- S. M. Mintenig, I. Int-Veen, M. G. J. Löder, S. Primpke and G. Gerdts, Water Res., 2017, 108, 365–372 CrossRef CAS PubMed.
- J. Sun, X. Dai, Q. Wang, M. C. M. van Loosdrecht and B. J. Ni, Water Res., 2019, 152, 21–37 CrossRef CAS PubMed.
- J. Talvitie, A. Mikola, A. Koistinen and O. Setälä, Water Res., 2017, 123, 401–407 CrossRef CAS PubMed.
- Q. Xu, Q. S. Huang, T. Y. Luo, R. L. Wu, W. Wei and B. J. Ni, Chem. Eng. J., 2021, 416, 129123 CrossRef CAS.
- A. H. Hamidian, E. J. Ozumchelouei, F. Feizi, C. Wu, Y. Zhang and M. Yang, J. Cleaner Prod., 2021, 295, 126480 CrossRef CAS.
- M. Cole, P. Lindeque, C. Halsband and T. S. Galloway, Mar. Pollut. Bull., 2011, 62, 2588–2597 CrossRef CAS PubMed.
- A. J. R. Watts, M. A. Urbina, R. Goodhead, J. Moger, C. Lewis and T. S. Galloway, Environ. Sci. Technol., 2016, 50, 5364–5369 CrossRef CAS PubMed.
- L. Hou, D. Kumar, C. G. Yoo, I. Gitsov and E. L. W. Majumder, Chem. Eng. J., 2021, 406, 126715 CrossRef CAS.
- G. Gatidou, O. S. Arvaniti and A. S. Stasinakis, J. Hazard. Mater., 2019, 367, 504–512 CrossRef CAS PubMed.
- K. H. D. Tang, J. Cleaner Prod., 2023, 387, 135864 CrossRef CAS.
- J. Boucher and D. Friot, Primary Microplastics in the Oceans: A Global Evaluation of Sources, IUCN, Gland, Switzerland, 2017 Search PubMed.
- S. Gündoğdu, C. Çevik, E. Güzel and S. Kilercioğlu, Environ. Monit. Assess., 2018, 190, 626 CrossRef PubMed.
- N. Tang, X. Liu and W. Xing, Sci. Total Environ., 2020, 745, 141026 CrossRef CAS PubMed.
- J. Jiang, X. Wang, H. Ren, G. Cao, G. Xie, D. Xing and B. Liu, Sci. Total Environ., 2020, 746, 141378 CrossRef CAS PubMed.
- L. Zhang, J. Liu, Y. Xie, S. Zhong and P. Gao, J. Cleaner Prod., 2021, 291, 125968 CrossRef CAS.
- C. Edo, M. González-Pleiter, F. Leganés, F. Fernández-Piñas and R. Rosal, Environ. Pollut., 2020, 259, 113837 CrossRef CAS PubMed.
- S. Magni, A. Binelli, L. Pittura, C. G. Avio, C. Della Torre, C. C. Parenti, S. Gorbi and F. Regoli, Sci. Total Environ., 2019, 652, 602–610 CrossRef PubMed.
- A. Naji, S. Azadkhah, H. Farahani, S. Uddin and F. R. Khan, Chemosphere, 2021, 262, 128039 CrossRef CAS PubMed.
- J. Talvitie, A. Mikola, O. Setälä, M. Heinonen and A. Koistinen, Water Res., 2017, 109, 164–172 CrossRef CAS PubMed.
- S. Raju, M. Carbery, A. Kuttykattil, K. Senthirajah, A. Lundmark, Z. Rogers, S. SCB, G. Evans and T. Palanisami, Water Res., 2020, 173, 115549 CrossRef CAS PubMed.
- H. Hidayaturrahman and T. G. Lee, Mar. Pollut. Bull., 2019, 146, 696–702 CrossRef CAS PubMed.
- X. Li, L. Chen, Q. Mei, B. Dong, X. Dai, G. Ding and E. Y. Zeng, Water Res., 2018, 142, 75–85 CrossRef CAS PubMed.
- C. Alvim, J. A. Mendoza-Roca and A. Bes-Piá, J. Environ. Manage., 2020, 255, 109739 CrossRef PubMed.
- A. B. Silva, A. S. Bastos, C. I. L. Justino, J. P. da Costa, A. C. Duarte and T. A. P. Rocha-Santos, Anal. Chim. Acta, 2018, 1017, 1–19 CrossRef CAS PubMed.
- N. Turan, H. Sari Erkan and G. Onkal Engin, Process Saf. Environ. Prot., 2021, 146, 77–84 CrossRef.
- H. Ou and E. Y. Zeng, in Microplastic Contamination in Aquatic Environments, Elsevier, 2018, pp. 317–338 Search PubMed.
- S. Fortin, B. Song and C. Burbage, Mar. Pollut. Bull., 2019, 149, 110579 CrossRef CAS PubMed.
- A. S. Tagg, M. Sapp, J. P. Harrison and J. J. Ojeda, Anal. Chem., 2015, 87, 6032–6040 CrossRef CAS PubMed.
- G. Erni-Cassola, M. I. Gibson, R. C. Thompson and J. A. Christie-Oleza, Environ. Sci. Technol., 2017, 51, 13641–13648 CrossRef CAS PubMed.
- T. Maes, R. Jessop, N. Wellner, K. Haupt and A. G. Mayes, Sci. Rep., 2017, 7, 1–10 CrossRef PubMed.
- M. Shen, T. Hu, W. Huang, B. Song, G. Zeng and Y. Zhang, Chem. Eng. J., 2021, 421, 129918 CrossRef CAS.
- I. Sierra, M. R. Chialanza, R. Faccio, D. Carrizo, L. Fornaro and A. Pérez-Parada, Environ. Sci. Pollut. Res., 2020, 27, 7409–7419 CrossRef CAS PubMed.
- V. Hidalgo-Ruz, L. Gutow, R. C. Thompson and M. Thiel, Environ. Sci. Technol., 2012, 46, 3060–3075 CrossRef CAS PubMed.
- M. Funck, A. Yildirim, C. Nickel, J. Schram, T. C. Schmidt and J. Tuerk, MethodsX, 2020, 7, 100778 CrossRef CAS PubMed.
- E. Dümichen, P. Eisentraut, C. G. Bannick, A. K. Barthel, R. Senz and U. Braun, Chemosphere, 2017, 174, 572–584 CrossRef PubMed.
- C. Rathore, M. Saha, P. Gupta, M. Kumar, A. Naik and J. de Boer, Sci. Total Environ., 2023, 888, 164157 CrossRef CAS PubMed.
- Y. Chen, D. Wen, J. Pei, Y. Fei, D. Ouyang, H. Zhang and Y. Luo, Curr. Opin. Environ. Sci. Health, 2020, 18, 14–19 CrossRef.
- X. Li, Q. Mei, L. Chen, H. Zhang, B. Dong, X. Dai, C. He and J. Zhou, Water Res., 2019, 157, 228–237 CrossRef CAS PubMed.
- V. Godoy, G. Blázquez, M. Calero, L. Quesada and M. A. Martín-Lara, Environ. Pollut., 2019, 255, 113363 CrossRef CAS PubMed.
- X. Li, M. Li, Q. Mei, S. Niu, X. Wang, H. Xu, B. Dong, X. Dai and J. L. Zhou, Sci. Total Environ., 2021, 797, 148940 CrossRef CAS PubMed.
- D. J. Sarkar, S. Das Sarkar, B. K. Das, B. K. Sahoo, A. Das, S. K. Nag, R. K. Manna, B. K. Behera and S. Samanta, Water Res., 2021, 192, 116853 CrossRef CAS PubMed.
- S. Liu, J. H. Huang, W. Zhang, L. X. Shi, K. X. Yi, H. B. Yu, C. Y. Zhang, S. Z. Li and J. N. Li, J. Environ. Manage., 2022, 302, 113995 CrossRef CAS PubMed.
- Y. Cao, M. Zhao, X. Ma, Y. Song, S. Zuo, H. Li and W. Deng, Sci. Total Environ., 2021, 788, 147620 CrossRef CAS PubMed.
- A. Wagstaff, L. A. Lawton and B. Petrie, Chemosphere, 2022, 288, 132578 CrossRef CAS PubMed.
- G. Ašmonaitė, M. Tivefälth, E. Westberg, J. Magnér, T. Backhaus and B. Carney Almroth, Front. Environ. Sci., 2020, 8, 1–12 CrossRef.
- L. C. Wang, J. C. Te Lin, C. Di Dong, C. W. Chen and T. K. Liu, J. Hazard. Mater., 2021, 420, 126658 CrossRef CAS PubMed.
- M. N. Miranda, A. R. Lado Ribeiro, A. M. T. Silva and M. F. R. Pereira, Sci. Total Environ., 2022, 850, 158073 CrossRef CAS PubMed.
- J. Nath, J. De, S. Sur and P. Banerjee, Pathogens, 2023, 12, 888 CrossRef CAS PubMed.
- D. N. Pham, L. Clark and M. Li, J. Hazard. Mater. Lett., 2021, 2, 100014 CrossRef CAS.
- Y. Liu, W. Liu, X. Yang, J. Wang, H. Lin and Y. Yang, Sci. Total Environ., 2021, 773, 145643 CrossRef CAS PubMed.
- N. P. M. Marathe and M. S. Bank, in Microplastic in the Environment: Pattern and Process, ed. M. S. Bank, Springer Cham, Cham, Switzerland, 2022, pp. 311–322 Search PubMed.
- W. C. Li, H. F. Tse and L. Fok, Sci. Total Environ., 2016, 566–567, 333–349 CrossRef CAS PubMed.
- G. Caruso, Mar. Pollut. Bull., 2019, 146, 921–924 CrossRef CAS PubMed.
- B. Toussaint, B. Raffael, A. Angers-Loustau, D. Gilliland, V. Kestens, M. Petrillo, I. M. Rio-Echevarria and G. Van den Eede, Food Addit. Contam., Part A: Chem., Anal., Control, Exposure Risk Assess., 2019, 36, 639–673 CrossRef CAS PubMed.
- S. A. Mason, V. G. Welch and J. Neratko, Front. Chem., 2018, 6, 407 CrossRef PubMed.
- Q. Li, C. Ma, Q. Zhang and H. Shi, Curr. Opin. Food Sci., 2021, 40, 192–197 CrossRef CAS.
- M. S. L. Yee, L. W. Hii, C. K. Looi, W. M. Lim, S. F. Wong, Y. Y. Kok, B. K. Tan, C. Y. Wong and C. O. Leong, Nanomaterials, 2021, 11, 1–23 CrossRef PubMed.
- K. Senathirajah, S. Attwood, G. Bhagwat, M. Carbery, S. Wilson and T. Palanisami, J. Hazard. Mater., 2021, 404, 124004 CrossRef CAS PubMed.
- Q. Liu, Z. Chen, Y. Chen, F. Yang, W. Yao and Y. Xie, J. Agric. Food Chem., 2021, 69, 10450–10468 CrossRef CAS PubMed.
- C. Pironti, M. Ricciardi, O. Motta, Y. Miele, A. Proto and L. Montano, Toxics, 2021, 9, 1–29 CrossRef PubMed.
- L. F. Amato-Lourenço, R. Carvalho-Oliveira, G. R. Júnior, L. dos Santos Galvão, R. A. Ando and T. Mauad, J. Hazard. Mater., 2021, 416, 126124 CrossRef PubMed.
- L. Li, X. Zhao, Z. Li and K. Song, J. Hazard. Mater., 2021, 411, 1–9 Search PubMed.
- S. Abbasi, Curr. Opin. Toxicol., 2021, 27, 41–46 CrossRef CAS.
- M. Llorca and M. Farré, Front. Toxicol., 2021, 3, 1–24 Search PubMed.
- Q. Shi, J. Tang, R. Liu and L. Wang, Crit. Rev. Environ. Sci. Technol., 2021, 52, 3863–3895 CrossRef.
- C. Li, H. Sun, Y. Shi, Z. Zhao, Z. Zhang, P. Zhao, Q. Gao, X. Zhang, B. Chen, Y. Li and S. He, J. Hazard. Mater., 2023, 445, 130638 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |