Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Band-bowing effects in lead-free double Cs2AgBixSb1−xCl6 perovskites and their anion-exchanged derivatives†
Oleksandr
Stroyuk
 *a,
Oleksandra
Raievska
a,
Anastasia
Barabash
b,
Riley W.
Hooper
*a,
Oleksandra
Raievska
a,
Anastasia
Barabash
b,
Riley W.
Hooper
 c,
Vladimir K.
Michaelis
c,
Jens
Hauch
ab and
Christoph J.
Brabec
ab
c,
Vladimir K.
Michaelis
c,
Jens
Hauch
ab and
Christoph J.
Brabec
ab
aForschungszentrum Jülich GmbH, Helmholtz-Institut Erlangen Nürnberg für Erneuerbare Energien (HI ERN), 91058 Erlangen, Germany. E-mail: o.stroyuk@fz-juelich.de
bFriedrich-Alexander-Universität Erlangen-Nürnberg, Materials for Electronics and Energy Technology (i-MEET), Martensstrasse 7, 91058 Erlangen, Germany
cDepartment of Chemistry, University of Alberta, 11227 Saskatchewan Drive, Edmonton, Alberta T6G 2G2, Canada
First published on 4th December 2023
Abstract
Green synthesis of lead-free Cs2AgBixSb1−xCl6 solid-solution double perovskites and their conversion into bromide and iodide derivatives is reported. Interaction of the single-phase Cs2AgBixSb1−xCl6 double perovskites with sodium iodide in 2-propanol/glycerol yields Cs3Bi2xSb2(1−x)I9 derivatives with varied Bi/Sb ratio, while the similar anion exchange with sodium bromide results in mixtures of Cs3Bi2xSb2(1−x)Cl3Br6 and Cs2AgBixSb1−xCl2Br4. A band-bowing effect is revealed for the starting chloride perovskites and the anion-exchange products, with the indirect band gap of intermediate Bi/Sb mixed compounds being significantly lower than the band gaps of corresponding Bi- and Sb-pure products. Solid-state nuclear magnetic resonance spectroscopy uncovered that the band-bowing originates from the lattice disorder of mixed Bi/Sb halide compounds, thus highlighting a new approach for the bandgap design of halide perovskites.
Introduction
Lead-free halide materials attract considerable attention as a potential alternative for lead-based hybrid perovskites, the latter excelling as a new class of highly efficient and variable absorbers for thin-film photovoltaics (PV).1–5 The substitution of Pb2+ with various bi-valent cations as well as the introduction of M+/M3+ combinations instead of Pb2+/Pb2+ pairs, yields lead-free perovskites with a broad compositional variability, unrivalled by their Pb-based ancestors. The efficiency of PV devices incorporating lead-free perovskites is, though, still comparatively low,1–5 mostly due to larger band gaps and inferior charge carrier mobility, showing room for further progress in the functional design of such PV materials.The field of double lead-free AI2MIMIIIX6 perovskites, where AI is an alkali cation and X is a halide, is dominated by bismuth(III) compounds where Bi3+ is typically combined with Ag+ or Na+, and AI = Cs+, for example, Cs2NaBiCl6 or Cs2AgBiBr6.1–8 The double perovskite structure is very labile allowing multiple substitutions to be realized simultaneously on MI, MIII, and X sites. In this way, Bi3+ is typically alloyed with In3+ or Sb3+ on the MIII sites, while the MI sites are filled with mixtures of Ag+ and Na+.1–8 Recently, many new Bi-halide compounds have been synthesized and tested as PV materials, such as CsBi3I10 or Cu2AgBiI6, combined into the class of “perovskite-inspired materials” and revealing a broad compositional and structural versatility and promises for indoor photovoltaics.1,4,9,10
Similar to the Pb-based halide perovskites, the lead-free Bi-based compounds show a strong dependence of the light sensitivity range on the nature of halide X, with band gaps of bromide compounds approaching 2 eV and those of iodide compounds – coming below this imaginary threshold and approaching values typical for Pb perovskites.2,6,7 At the same time, the synthesis of Bi-based double bromide and iodide perovskites is typically challenging, as it requires a large thermal input, an inert atmosphere, and reactive, toxic, or volatile precursors (such as HBr or HI) as well as due to the inherent thermal and/or redox instability of many final perovskites.6,7
The broadly explored chemistry of lead perovskites indicates that this problem can be addressed by an indirect synthesis of bromide or iodide compounds from corresponding chlorides via anion exchange reactions.6,7 The feasibility of this approach for a controlled transformation of lead-free double perovskites was shown by Gamelin et al. who reported the anion-exchange-driven conversion of nanocrystalline Cs2AgBiCl6 into Cs2AgBiBr6 and further – into Cs2AgBiI6 by using trimethylsilyl halides as exchange agents.11 This pioneering report showed the potential and flexibility of anion-exchange reactions in the chemistry of lead-free halide perovskites.6,7 Simultaneously, it outlined the challenges, particularly the need for more stable and sustainable anion exchange agents that can be used at lower temperatures and in polar solvents required by green chemistry.
In the present paper, we report the structural and spectral properties of bromide- and iodide-containing materials produced via anion exchange from Cs2AgBixSb1−xCl6 (CABSC) perovskites with a varied Bi/Sb ratio. The key point of our report is that the anion exchange is performed in very mild conditions, including room temperature, an open-air atmosphere, and the application of NaBr and NaI as readily available, stable, and non-volatile bromide and iodide precursors. At that, the original microcrystalline CABSC double perovskites are synthesized by using a “green” protocol based on the recently introduced mild synthesis of Cs2AgxNa1−xBiyIn1−yCl6 perovskites.12,13
The paper focuses on the spectral characteristics of Cs2AgBixSb1−xCl6 perovskites and their anion-exchanged derivatives probed by absorption, Raman, and solid-state nuclear magnetic resonance (NMR) spectroscopies. We discuss optical band-bowing observed both for the original perovskites and for the products of chloride-to-bromide and chloride-to-iodide exchange as well as spectral evidence of the local lattice disorder as one of the reasons for the band-bowing behavior.
Materials and methods
Synthesis of Cs2AgBixSb1−xCl6 microcrystals
Two precursors were prepared, separately containing MIII and MI compounds. The first precursor was prepared by adding 200 μL of 12.0 M aqueous HCl solution and 500 μL of 2-propanol to 100 μL of 1.0 M aqueous SbCl3 (BiCl3, SbCl3 + BiCl3) solution, the latter containing 4.0 M HCl to avoid hydrolysis of MIII salts. The second precursor solution was prepared by adding 100 μL of 1.0 M aqueous AgNO3 solution to 100 μL of deionized (DI) water followed by 25 μL of aqueous 25% ammonia solution, 500 μL of 2-propanol, and 60 μL of aqueous 4.0 M solution of Cs acetate (CsAc). All additions were performed under vigorous stirring. An excess of CsAc (20%) was introduced to shift the equilibrium toward the deposition of the perovskite phase from the water/2-propanol mixture. The synthesis of microcrystalline CABSC is performed at ambient temperature in an open-air environment.The presence of excessive CsAc enhances the precipitation and suppresses the formation of Cs3(Sb,Bi)2Cl9 compounds with a lower CsI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MIII ratio (1.5
MIII ratio (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0), routing the reaction to the target Cs2Ag(Sb,Bi)Cl6 double perovskites (2.0
1.0), routing the reaction to the target Cs2Ag(Sb,Bi)Cl6 double perovskites (2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0). We note that the stock solutions with concentrations above 1.0 M should be prepared by dissolving the required amount of the salt in a minimal volume of water and adding then DI water to reach the total calculated volume of solution.
1.0). We note that the stock solutions with concentrations above 1.0 M should be prepared by dissolving the required amount of the salt in a minimal volume of water and adding then DI water to reach the total calculated volume of solution.
The second precursor solution is then rapidly added to the first precursor solution at intense stirring for a minimum of 5 min. Afterward, the resulting suspension is kept in a closed vial without stirring overnight (for at least 12–14 h) to reach complete crystallization. After ripening for 12–14 h the supernatant solution is eliminated from the precipitate, 1 mL of 2-propanol is added to the precipitate, and the resulting suspension is centrifuged at 2000 rpm for 2 min. The supernatant is discarded again, 1.0 mL of 2-propanol is added, and the centrifugation is repeated to purify the product from residual salts. The final samples are dried and stored in the dark at room temperature. The above-described synthetic procedure yields ca. 50 mg of each product.
The samples for structural characterization and optical measurements are produced from freshly prepared and purified precipitates by adding 200 μL 2-propanol and drop-casting 30 μL of the final suspension into a 1 cm2 glass substrate.
Anion exchange
CABSC perovskites with varied Bi/Sb ratios were reacted with NaBr and NaI dissolved in 2-propanol/glycerol mixtures at room temperature and in an open-air environment. The 2-propanol/glycerol mixtures were prepared by mixing 50 g of glycerol with 100 mL of 2-propanol. In a typical procedure, 1.4 mL of 2-propanol/glycerol mixture was mixed with ca. 0.05 g of freshly prepared and purified chloride double perovskite, and then 0.2 mL of aqueous 4.0 M NaBr (NaI) solution was added under vigorous stirring. The stirring was continued for 30 min, and the precipitate was separated from the supernatant containing dissolved NaCl by centrifugation at 2000 rpm for 2 min. Then, 1 mL of 2-propanol was added, and centrifugation was repeated. In a series with varied NaBr concentrations, the total volume of water in the mixture was kept constant. The presence of glycerol is necessary to dissolve NaCl released during the anion exchange, which has a low solubility in 2-propanol.X-Ray diffraction (XRD) patterns were registered using a Panalytical X'pert powder diffractometer with filtered Cu Kα radiation (λ = 1.54178![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Å) and an X'Celerator solid-state stripe detector in the Bragg–Brentano geometry in an angle range of 2θ = 5–100° with a step rate of 0.05° per min. The XRD patterns were subjected to a Rietveld refinement procedure using MAUD software. Scanning electron microscopy (SEM) imaging and energy-dispersive X-ray spectroscopic (EDX) analysis were performed using a JEOL JSM-7610F Schottky field emission scanning electron microscope operating under 15–20 kV acceleration voltage equipped with an X-Max 80 mm2 silicon drift detector (Oxford Instruments).
Å) and an X'Celerator solid-state stripe detector in the Bragg–Brentano geometry in an angle range of 2θ = 5–100° with a step rate of 0.05° per min. The XRD patterns were subjected to a Rietveld refinement procedure using MAUD software. Scanning electron microscopy (SEM) imaging and energy-dispersive X-ray spectroscopic (EDX) analysis were performed using a JEOL JSM-7610F Schottky field emission scanning electron microscope operating under 15–20 kV acceleration voltage equipped with an X-Max 80 mm2 silicon drift detector (Oxford Instruments).
Reflectance spectra were recorded using a BlackComet spectrometer (StellarNet Inc.) and a 75 W Xenon lamp (Thorlabs) as an excitation source. The spectra were registered with an optical Y-fiber probe in identical geometry for samples and a scattering reference (ultra-pure BaSO4, Alfa-Aesar). The reflectance spectra were transformed into absorption spectra using the Kubelka–Munk formula and the reference.
Raman spectra were registered on a WITec alpha700 confocal Raman microscope equipped with a UHTS 300 spectrometer and a 532 nm laser.
Solid-state nuclear magnetic resonance (NMR) spectroscopy was performed on either a Bruker AVANCE III HD 400 (B0 = 9.39 T) or Bruker Avance NEO 500 (B0 = 11.75 T) NMR spectrometer equipped with a 4 mm double-resonance (H/X) Bruker magic-angle spinning (MAS) probe. The 133Cs MAS NMR spectra were acquired using a Bloch-decay experiment with a 4.0 μs π/2 pulse (νrf = 62.5 kHz), 4 co-added transients, a recycle delay of 300 s, and a spinning frequency of 14 kHz. 133Cs NMR spectra were referenced to a secondary standard (solid CsCl to 223.2 ppm) with respect to 0.5 M CsCl (δ(133Cs) = 0.00 ppm). 209Bi NMR (MAS; νrot = 14 kHz, and non-spinning) spectra were acquired using a Hahn echo experiment with a 1.0 μs π/2 pulse (νrf = 250 kHz), 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 co-added transients, and recycle delay of 0.1 s. NMR spectra were referenced with respect to saturated Bi(NO3)3 in HNO3 (δ(209Bi) = 0.00 ppm). All NMR spectra were processed with Bruker TopSpin 3.6.2.
000 co-added transients, and recycle delay of 0.1 s. NMR spectra were referenced with respect to saturated Bi(NO3)3 in HNO3 (δ(209Bi) = 0.00 ppm). All NMR spectra were processed with Bruker TopSpin 3.6.2.
Results and discussion
Chloride-based compounds
X-ray diffraction patterns of Cs2AgBixSb1−xCl6 (CABSC) double perovskites show a set of reflections characteristic for a cubic Fm3m space group14–19 in the entire range of Bi-to-Sb ratios (Fig. 1a). Rietveld refinement of the XRD patterns (see examples in ESI,† Fig. S1) shows that CABSC double perovskites are present as a single phase with no detectable phase admixtures.EDX analysis of CABSC compounds revealed that the actual Bi fraction in final products (xa) follows very closely the nominal Bi fraction (x) set during the synthesis in the precursor solutions (ESI,† Table S1). The relationship between xa and x is linear with the slope very close to unity (ESI,† Fig. S2a). The Cs/(Bi + Sb) and Ag/(Bi + Sb) ratios are close respectively to 2 and 1, following the expected stoichiometry, while the Cl/(Bi + Sb) ratio is typically around 5–5.5, indicating a certain deficiency of chloride anions in the perovskite structure (ESI,† Fig. S3 and Table S1).
The lattice parameter of CABSCs increases linearly with the actual Bi fraction xa (Fig. 1b) showing the double perovskites to be ideal solid solutions obeying Vegard's law.18,20 The lattice parameters of individual Cs2AgSbCl6 (L = 10.713 Å) and Cs2AgBiCl6 (L = 10.788 Å) are close to typically reported values.14–19 The lattice expansion observed for Bi-containing compounds is caused by a larger cation size of Bi3+ (117 pm) as compared to Sb3+ (90 pm).18
Scanning electron microscopy of CABSC samples revealed randomly aggregated microcrystals with a broad distribution of grain sizes from ca. 0.5 to 5 μm (ESI,† Fig. S4, left column of images). No significant changes in the morphology were detected as x was elevated from 0 to 1.
Raman spectra of CABSC compounds are typical for double perovskites with prominent A1g, Eg, and T2g vibrational bands16,17,19 observed for Cs2AgSbCl6 at 288 cm−1, 215 cm−1, and 121 cm−1, respectively (Fig. 1c). As antimony is gradually substituted with bismuth the positions of phonon peaks shift to lower frequencies, reaching 282 cm−1, 212 cm−1, and 115 cm−1, respectively, for Cs2AgBiCl6. Both sets of frequencies are in accordance with previous reports.16,17,19 The Raman data are consistent with the above conclusions about the single-phase and solid-solution character of CABSC double perovskites made from the powder XRD data.
The peak positions and relative intensities of the A1g phonon band can be used for the spectral identification of the perovskite composition. Both the position (Fig. 1d, scatter 1) and relative intensity (Fig. 1d, scatter 2) show polynomial dependences on xa, allowing Raman spectroscopy to be used as an express analytical tool for evaluating the bismuth content in CABSC double perovskites. The continuous changes of phonon frequencies of mixed double perovskites typically indicate homogeneous alloying, while the segregation of components results in discontinuous frequency variations.19 The shift of the A1g band peak from 288 cm−1 for CASC to 282 cm−1 for CABC is related to the increase in the metal(III)–chloride bond length, from 2.35 Å for Sb–Cl16 to 2.70 Å for Bi–Cl.20
The absorption spectrum of antimony-pure (CASC) double perovskite shows a continuous band with an extended low-energy edge at ca. 2.8–3.0 eV (Fig. 2a, curve 1). The absorption edge of bismuth-pure (CABC) double perovskite is shifted to lower energy as compared to CASC and reveals an additional high-energy peak at ca. 3.5 eV (Fig. 2a, curve 4). Surprisingly, the intermediate mixed CABSC compounds showed a shift of the absorption edge to lower energies (see exemplary curves 2 and 3 in Fig. 2a), as compared to both pure compounds. This shift is evidenced by a much deeper yellow coloration of mixed compounds as compared to Sb- and Bi-pure phases (photographs in the insert in Fig. 2a).
By theory and experimental observations, CASC and CABC double perovskites were reported as indirect-band gap semiconductors.15–17,19,21–23 The absorption spectra of these compounds show extended linear sections of the absorption edge when plotted in the coordinates of the Tauc equation both for indirect and direct allowed interband transitions (see examples in ESI,† Fig. S5). At that, the presentation of the spectra in the Tauc coordinates for indirect transitions gives a higher correspondence with the original spectrum, as compared with the direct-transition Tauc presentation. For this reason, as well as in agreement with previous reports, the indirect bandgaps were calculated and discussed in the present work for the CABSC series.
The indirect bandgap decreases as Sb is substituted with Bi from Eg = 2.82 eV for Sb-pure compound to 2.59 eV for CABSC with xa = 0.26 (Fig. 2b). At higher Bi contents, the bandgap starts to increase reaching 2.77 eV for Bi-pure CABC perovskite.
Considering the single-phase character of CABSC double perovskites, this behavior can be attributed to the band-bowing effect, often observed for solid-solution semiconductors24,25 including mixed-metal halide perovskite and perovskite-like compounds.26–30 The band-bowing in perovskites was attributed to local inhomogeneities in the composition and lattice of the alloyed semiconductor compounds.26,27 Alternatively, the non-linear bandgap behavior was related to an energy mismatch between atomic orbitals of two different metal cations contributing to the band edges.28,31 For example, the band-bowing effects observed for alloyed Cs2AgBixSb1−xBr6 (CABSB) perovskites were interpreted as a result of a mixing of Bi- and Sb-related electronic states.31 Calculations based on the relativistic density functional theory (DFT) showed the conduction band minimum of CABSB to be dominated by Bi–Br interactions, and the valence band maximum – by Sb–Ag–Br interactions, resulting in the alloys having lower bandgaps as compared to CABB and CASB. At that, the bandgap reduction upon alloying was predicted even for CABSB alloys with uniform atomic distribution.31 The DFT calculations of the compositional bandgap dependence of Cs2AgBixSb1−xCl6 perovskites showed indications of the band-bowing when spin–orbit coupling effects were taken into consideration.32
Similar band-bowing effects originating from the non-linear orbital mixing at the band edges on the bandgap of Bi/Sb-based halide alloys were reported for solution-precipitated microcrystalline Cs3(BixSb1−x)2Br9 double salts.29 For these compounds, the minimal bandgap was found at Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb ratio close to 1
Sb ratio close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 both experimentally and by DFT calculations.29 For the same phase-pure Cs3(BixSb1−x)2Br9 alloys, but produced via a mechano-chemical treatment of mixtures of Cs3Bi2Br9 and Cs3Sb2Br9, the lowest bandgap was observed at x = 0.2–0.3.30 The ambiguity in the reported position of the lowest bandgap29,30 can indicate that a combination of different reasons is responsible for the observed band-bowing effects. These reasons can be both of a chemical nature as assumed in ref. 28 and 31 but also can stem from local lattice disorder and local strain effects, related to the presence and inhomogeneous mixing of two different MIII cations. For double halide perovskites, such disorder-induced bandgap narrowing was reported for Cs2AgBiBr6, originating from Ag–Bi disorder.33 Theoretical modeling of this compound showed the bandgap decreasing from 1.93 eV for a totally ordered lattice to 0.44 eV for randomly distributed cations.34 The strain was reported to alter dramatically the lattice and electronic structure of Sb-pure CASC perovskite. For example, highly strained CASC single crystals17 showed a lattice parameter and bandgap of 10.388 Å and 1.82 eV, respectively, which increased to 10.713 Å and 2.55 eV after the strain release by grinding with no detectable changes in the chemical composition between the original single crystal and ground polycrystalline CASC powder.17
1 both experimentally and by DFT calculations.29 For the same phase-pure Cs3(BixSb1−x)2Br9 alloys, but produced via a mechano-chemical treatment of mixtures of Cs3Bi2Br9 and Cs3Sb2Br9, the lowest bandgap was observed at x = 0.2–0.3.30 The ambiguity in the reported position of the lowest bandgap29,30 can indicate that a combination of different reasons is responsible for the observed band-bowing effects. These reasons can be both of a chemical nature as assumed in ref. 28 and 31 but also can stem from local lattice disorder and local strain effects, related to the presence and inhomogeneous mixing of two different MIII cations. For double halide perovskites, such disorder-induced bandgap narrowing was reported for Cs2AgBiBr6, originating from Ag–Bi disorder.33 Theoretical modeling of this compound showed the bandgap decreasing from 1.93 eV for a totally ordered lattice to 0.44 eV for randomly distributed cations.34 The strain was reported to alter dramatically the lattice and electronic structure of Sb-pure CASC perovskite. For example, highly strained CASC single crystals17 showed a lattice parameter and bandgap of 10.388 Å and 1.82 eV, respectively, which increased to 10.713 Å and 2.55 eV after the strain release by grinding with no detectable changes in the chemical composition between the original single crystal and ground polycrystalline CASC powder.17
The assumption of random Sb/Bi substitution made from powder XRD and Raman data is also supported by the 133Cs MAS NMR spectroscopy of selected CABSC double perovskites. Each Cs cation is surrounded by 12 halide anions making it an ideal indicator that responds to any changes in the symmetry or composition of the perovskite lattice.35–37
The CABSC double perovskites reveal a single Cs-related peak (Fig. 2c) following the expected lattice symmetry. This observation supports the conclusion on the solid-solution character of pure crystalline materials, with trace quantities (<1%) of residual CsCl salt (δiso = 223 ppm).35–37 The isotropic chemical shift (δiso) is observed at 80 ppm for CASC (xa = 0), lowering to 76 ppm for the mixed CABSC compound with xa = 0.50 and further, to 72 ppm, for CABC double perovskite (xa = 1.00).
The monotonous shift of the 133Cs peak indicates the formation of solid-solution Cs2AgBi0.5Sb0.5Cl6 compound, complimenting the XRD and Raman data, above. At the same time, the spectral width of the peaks increases, from 2.6 ppm for CASC to 3.3 ppm for CABSC, then decreasing again to 2.7 ppm for CABC perovskite. Analysis of the first-order quadrupolar interaction confirms a negligible second-order quadrupolar contribution to the central transition. Hence, the broadened 133Cs resonance of the mixed CABSC can be related to a greater medium-range lattice disorder of this compound due to Sb/Bi mixing in the second coordination sphere as compared to antimony- and bismuth-pure lattices.36,37
Further analysis reveals a single spinning sideband pair which is due to residual Cl vacancies, as previously discussed.37 Surveying the 209Bi MAS NMR (ESI,† Fig. S6) reveals a sizable chemical shift change of ∼120 ppm between CABC (δiso = 4050 ppm, FWHM = 3.2 kHz) and CABSC (δiso = 3967 ppm, FWHM = 5.8 kHz). Furthermore, an increase in the spinning sideband manifold by ca. 50% (∼320 vs. 460 kHz) is detected for the CABSC double perovskite phase. This experimental observation is consistent with an increasing perturbation of the bismuth electric field gradient (increase in quadrupole coupling) which would occur with an increase in atomic-level Sb/Bi disorder.38
Anion exchange with bromide: (i) the simpler case of substitutions in Cs2AgBiCl6
The Cl-to-Br exchange in CABSC double perovskites results in several events, including the anion exchange itself and the partial decomposition of the double perovskite into two different phases. To provide a more detailed description of these events, the Cl-to-Br substitutions in the Bi-only CABC double perovskite are first discussed in this section as the simpler case yielding single-phase products.Interaction of CABC double perovskite with NaBr results in a change of coloration from pale yellow to deep orange, visually indicating the transformation of the original phase. A series of Cs2AgBi(Cl1−yBry)6 compounds was obtained by adding different amounts of NaBr. The nominal Br fraction y was elevated up to 1.6 with respect to the stoichiometric amount needed to completely substitute Cl anions (that is y = 1.0).
The substitution of Cl is incomplete even at the over-stoichiometric amount of NaBr, at y = 1.4–1.6. The actual Br fraction in final products, ya, determined by EDX, increases gradually with y reaching a plateau of ya = 0.65–0.66 at y = 1.4–1.6 (Fig. 3a).
Powder XRD analysis of the anion-exchange products showed them to be single-phase cubic double perovskites similar to the original CABC with no new phases detected (ESI,† Fig. S7, left panel). From these data, the composition of exchanged products with the maximal substitution depth can be inferred as Cs2AgBiCl2Br4 (1/3 of original chloride remaining in the perovskite).
At lower amounts of Br the exchange results in the gradual substitution of Cl with the actual Br fraction ya following the nominal Br fraction y in an almost linear manner (Fig. 3a). A closer look at the XRD reflections (ESI,† Fig. S7, right panel) shows several substituted forms to be present in equilibrium in each case. Nevertheless, the average lattice parameter shows a linear increase with ya (Fig. 3b) indicating the formation of Cs2AgBi(Cl1−yBry)6 solid solutions obeying Vegard's law.6,7,19 The lattice expansion, in this case, originates from the larger size of the Br− (192 pm) as compared to Cl− (181 pm).19
SEM examination of the substitution products with different y (ESI,† Fig. S8) shows the same polycrystalline morphology observed earlier for the original Cl double perovskite.
Raman spectra of Cs2AgBi(Cl1−yBry)6 compounds (ESI,† Fig. S9a) showed a gradual shift of the A1g phonon frequency with a linear dependence on the actual Br fraction ya (Fig. 3c), confirming the formation of solid-solution compounds.6,7,19 This dependence encompasses a rather broad variation range, from 282 cm−1 for CABC to 223 cm−1 for the substituted compound with ya = 0.66, allowing the composition of the Br–Cl mixed perovskites to be reliably identified from their Raman spectra.
The increase in the nominal Br fraction results in a gradual shift of the absorption band edge to lower energies, visualized by the change of coloration of the final products (see photograph in the insert in Fig. 3d). Similar to the original chloride perovskite, the absorption spectra of substitution products show extended linear edge sections when presented in the Tauc coordinates for both direct and indirect allowed electron transitions (ESI,† Fig. S9b and c), however, indirect bandgaps reflect more adequately the positions of absorption edge in original spectra. Moreover, the Br-pure and mixed Br/Cl Bi-based double perovskites revealed indirect bandgaps in theoretic modeling and experimental measurements.6,7,19,22,23 In the present case, the indirect bandgap shows a linear dependence on the actual Br fraction ya (Fig. 3d), indicating the formation of homogeneous solid solutions,19 in agreement with the above-discussed XRD and Raman data as well as theoretical predictions.23
Anion exchange with bromide: (ii) the more complex case of Cs2AgBixSb1−xCl6
The interaction between Bi/Sb-mixed CABSC perovskites and NaBr results in a more complicated case with phase transformations of Sb-rich compounds, most probably due to the instability of double Ag–Sb bromide compounds.In this series, freshly synthesized Cs2AgBixSb1−xCl6 perovskites with a varied Bi/Sb ratio x interacted with a fixed excess of NaBr (y = 1.33). EDX analysis of the final products showed the actual fraction of Bi, xa, in all compounds to be close to the nominal one, x, set during the synthesis (Table 1). The xa changes linearly with x (ESI,† Fig. S2b) and has a slope of approximate unity indicating a reliable control over the Bi/Sb ratio in the final anion-exchange products.
| x | x a | (Br + Cl)/(Bi + Sb) | y a | Cs/(Bi + Sb) |
|---|---|---|---|---|
| 0 | 0 | 4.70 | 0.64 | 1.51 |
| 0.05 | 0.07 ± 0.04 | 4.45 | 0.65 | 1.53 |
| 0.10 | 0.12 ± 0.03 | 4.60 | 0.65 | 1.47 |
| 0.25 | 0.25 ± 0.02 | 5.55 | 0.66 | 1.68 |
| 0.50 | 0.47 ± 0.02 | 5.90 | 0.68 | 1.89 |
| 0.75 | 0.82 ± 0.02 | 6.00 | 0.67 | 1.96 |
| 0.90 | 0.94 ± 0.01 | 6.05 | 0.68 | 1.97 |
| 1.00 | 1.00 | 5.80 | 0.68 | 2.01 |
Similar to the above-discussed case of the Cl-to-Br exchange in CABC double perovskite, the anion exchange is incomplete even at the given excess of NaBr. The actual fraction of Br, ya, varies around 0.66 (Table 1), indicating chloride is a third of the halide component of the final double perovskite.
The products synthesized in the range of x = 0–0.10 showed different ratios of total halide to total MIII, (Br + Cl)/(Bi + Sb) and Cs/(Bi + Sb) ratio, as compared to Sb-richer samples. For these compounds the ratios are around 4.5 and 1.5, respectively, suggesting a Cs3M2X9 composition (Table 1) and confirmed by NMR, vide infra. The rest of the products with higher Bi contents shows (Br + Cl)/(Bi + Sb) of ca. 6 and Cs/(Bi + Sb) of ca. 2, indicating the formation of Cs2AgMIIIX6 double perovskites. Taking into account the incomplete Br-to-Cl exchange with 1/3 Cl remaining in the structure, the chemical formulas for these cases can be presented as Cs3MIII2Cl3Br6 and Cs2AgMIIICl2Br4, respectively. The powder XRD results in general support these assessments and provide additional indications on the composition of the products of Cl-to-Br exchange. SEM examination of the exchange products showed them to be polycrystalline powders (ESI,† Fig. S4, middle column) with a relatively broad distribution of grain sizes in the range of a few μm. The morphology is roughly the same for the entire range of x. A fraction of thin-flake-like products was also observed for x = 0.10–0.25.
Rietveld analysis of the XRD patterns of Br-exchanged CABSC double perovskites revealed them to be a mixture of phases for all x's, except for the CABC compound (Fig. 4a and ESI,† Fig. S9). For the range of x = 0–0.10, the XRD patterns were satisfactorily fitted as mixtures of Cs3Bi2xSb2(1−x)Cl3Br6 (CBSCB) and AgBr (Fig. 4b and ESI,† Fig. S10). At higher contents of Bi (x > 0.10) the phase of double Cs2AgBixSb1−xCl2Br4 (CABSCB) was detected. The relative content of this phase increased with x until the single phase was observed at x = 1.00.
The EDX analysis of Br-substituted samples revealed sodium admixtures in the entire range of xa (ESI,† Table S2 and Fig. S11). At the same time, the ratio of Ag/(Bi + Sb) was found to be very close to 1 for all samples where the presence of double perovskite Cs2AgBixSb1−xCl2Br4 was detected by XRD (xa > 0.12, Fig. 4b). This observation indicates that no appreciable substitution of Ag+ with Na+ took place in the double perovskite during the anion exchange, the sodium admixture observed by EDX stemming most probably from residual NaBr/NaCl.
The elementary cell volume of Cs3Bi2xSb2(1−x)Cl3Br6 compounds changes linearly with xa (Fig. 4c, scatter 1) indicating the formation of mixed Bi-Sb solid-solution series. Similarly, the lattice parameter of the double perovskite phase shows a linear increase with xa, indicating the formation of the Cs2AgBixSb1−xCl2Br4 solid solution.
The above XRD results (Fig. 4) indicate that mixed Bi/Sb double Br/Cl perovskites are unstable in the present conditions decomposing partially or completely with the formation of a Cs3Bi2xSb2(1−x)Cl3Br6 phase and silver being excluded in the form of AgBr. The instability of Cs2AgBixSb1−xCl2Br4 double perovskites observed in the present conditions is in agreement with previous reports, indicating that antimony-substituted Cs2AgBiBr6 double perovskites can only accommodate ca. 40% of SbIII, decomposing at higher Sb contents.39,40
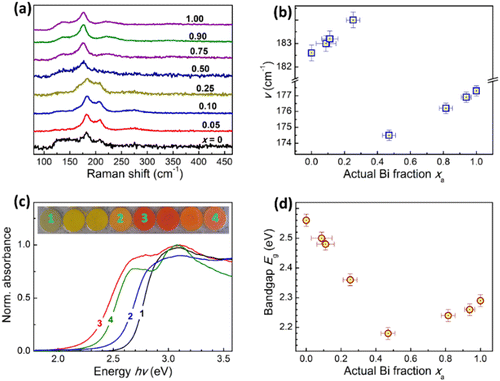
Raman spectroscopy of the products of Cl-to-Br exchange supports the above assessments. The spectra registered for compounds with x = 0–0.25 have a distinctly different structure as compared with the Raman spectra of the products with higher Bi contents (Fig. 5a). The most prominent peaks in the Raman spectra of Cs3Sb2Cl3Br6 (the range of xa < 0.5) can be observed at 208–209 cm−1 and 182–183 cm−1, following A1g and Eg frequencies reported for Cs3Sb2Br9, 214 cm−1 and 184 cm−1.41 As the Bi content increases this peak shows a minor shift to a higher frequency (Fig. 5b), indicating that it comes from a phase with varied composition.
Raman spectra of the anion-exchanged products with xa > 0.5 show the main peak at lower frequencies, gradually increasing from ca. 175 cm−1 for xa = 0.57 to ca. 177 cm−1 for xa = 1.00 (Fig. 5b), the latter value close to the reported A1g frequencies of 177–180 cm−1 for Cs2AgBiBr6 double perovskite.42,43 The gradual shift of the peak frequency with x is in line with the formation of solid-solution Cs2AgBixSb1−xCl2Br4 compounds. The rather sharp change of the shape of Raman spectra observed at xa of ca. 0.5 is also in line with the previous reports on the instability of Cs2AgBixSb1−xBr6 double perovskites at xa < 0.6.39,40
Similar to the above-discussed chloride double perovskites, the products of Cl-to-Br exchange show a deeper coloration for intermediate composition, as compared with the Sb-pure and Bi-pure products (photograph in the insert in Fig. 5c). The absorption edge of mixed compounds is shifted to lower energies reaching ca. 2.3 eV for the compound with xa = 0.47 (Fig. 5c). The similarity with chloride compounds is continued by the fact that the absorption spectra of brominated products can be plotted in the Tauc coordinates of both direct and indirect allows transitions with linear sections of the absorption edge long enough for reliable determination of corresponding bandgaps (ESI,† Fig. S12), but the indirect Eg describing more realistically the position of the absorption band edge.
The bandgap of Bi-pure Cs2AgBiCl2Br4 is higher than the values reported for Br-pure Cs2AgBiBr6, 1.9–2.19 eV,14,22 additionally confirming the presence of chloride in the double chloro-bromide perovskite lattice. Similarly, the Sb-pure Cs3Sb2Cl3Br6 compound shows a somewhat higher indirect bandgap than reported for Br-pure Cs3Sb2Br9, 2.43 eV.41
For mixed Bi/Sb products of anion exchange, the bandgap was found to be dependent on the Bi fraction, decreasing from Eg = 2.56 eV for Sb-pure product to 2.18 eV for xa = 0.47 and increasing at higher actual fractions of Bi, reaching 2.29 eV for Bi-pure double perovskite (Fig. 5d). As with chloride perovskites, this trend indicates the band-bowing effect in Cs3Bi2xSb2(1−x)Cl3Br6 and Cs2AgBixSb1−xCl2Br4. At that, the band-bowing in CBSCB perovskites is a major contributor to the descending section of Eg(xa) dependence (Fig. 5d) at xa < 0.5, while the ascending “wing” of this dependence forms due to the band-bowing effect in CABSCB dominating at xa > 0.5.
To ascertain atomic-level homogeneous mixing in the phases identified by XRD, complimentary 133Cs MAS NMR spectroscopy measurements were performed for the identical series. The 133Cs MAS NMR spectrum (Fig. 6a) of the Cl-to-Br exchange products of the Cs2AgSbCl6 phase with 0% Bi loading (x = 0) shows two resonances. The lower frequency resonance (δiso = 63 ppm) is consistent with the dominant form of Cs3Sb2(Br,Cl)9 which has two distinct crystallographic Cs+ sites as previously reported.35,44–46 The higher frequency chemical shift at 83 ppm, is unresolved but should be in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with the lower frequency resonance. The small additional intensity is attributed to residual Cs2SbAg(Cl,Br)6 double perovskite phase (Cs2SbAgCl6, δiso = 82 ppm) reported by Karmakar et al.36 These results are consistent with previous reports for Cs3Sb2Cl9 (δiso of 70 and 35 ppm),36 Cs3Bi2Br9 (δiso of 73 and 64 ppm),46 and Cs3Bi2I9 (δiso of ∼60 and ∼40 ppm).34 A similar structure is observed in the 133Cs MAS NMR spectra for the products with xa = 0.07–0.12, where Cs3(Sb,Bi)2(Br,Cl)9 phase dominates at low Bi concentrations. This finding supports the XRD observations and is consistent with atomic-level mixing of the MIII and X sites.
1 ratio with the lower frequency resonance. The small additional intensity is attributed to residual Cs2SbAg(Cl,Br)6 double perovskite phase (Cs2SbAgCl6, δiso = 82 ppm) reported by Karmakar et al.36 These results are consistent with previous reports for Cs3Sb2Cl9 (δiso of 70 and 35 ppm),36 Cs3Bi2Br9 (δiso of 73 and 64 ppm),46 and Cs3Bi2I9 (δiso of ∼60 and ∼40 ppm).34 A similar structure is observed in the 133Cs MAS NMR spectra for the products with xa = 0.07–0.12, where Cs3(Sb,Bi)2(Br,Cl)9 phase dominates at low Bi concentrations. This finding supports the XRD observations and is consistent with atomic-level mixing of the MIII and X sites.
 | ||
| Fig. 6 133Cs MAS NMR spectra of the anion-exchange products of CABSC double perovskites with NaBr (a) and NaI (b). | ||
The trend changes at xa = 0.25 where only a small residual shoulder can be observed at 63 ppm with a dominant CABSCB double perovskite phase (ESI,† Fig. S13a). At higher xa values only a single resonance is observed at ca. 84 ppm (Fig. 6a, xa = 0.47–1.00) assigned to the CABSCB phase with a single Cs site that appears to have a constant Cl/Br ratio. At the same time, the linewidth of this resonance shows a more regular dependence on the actual Bi fraction, increasing at small xa, coming to saturation at xa = 0.3–0.5, and then decreasing at higher Bi content (ESI,† Fig. S13b). This dependence can also indicate increased disorder in the mixed Cs3(Sb,Bi)2(Br,Cl)9 and CABSCB double perovskite phases,44–46 consistent with the optical band-bowing phenomena. The small shoulder (xa > 0.25) is attributed to a minor secondary phase of Cs3(Sb,Bi)2(Br,Cl)9.
Anion exchange with iodide
Interaction of CABSC double perovskites with NaI results in an instant transformation of chloride compounds into brightly colored reddish anion-exchange products. Inspection of the products with EDX analysis showed a close correspondence between the nominal Bi fraction x and the actual Bi fraction xa (ESI,† Table S3 and Fig. S14). The xa(x) dependence is linear with a slope close to unity (ESI,† Fig. S2c), showing a perfect control over the Bi/Sb ratio in the final products. At the same time, the ratios of I/(Bi + Sb) and Cs(Bi + Sb) are close to 4.5 and 1.5 for the whole series range of x (ESI,† Table S3), respectively, both expected for the Cs3MIII2I9 compounds.In agreement with these expectations, the Rietveld analysis of XRD patterns of the NaI-treatment products revealed the presence of a single crystalline phase isostructural to Cs3Bi2I9 (CBI)47–49 with a varied Bi/Sb ratio (Fig. 7a).
The elementary cell volume increases linearly with an increase of the Bi fraction (Fig. 7b), indicating the formation of homogeneously alloyed solid-solution Cs3Bi2xSb2(1−x)I9 compounds. No other phases were detected, with silver cations most probably excluded in the form of AgI and removed during the purification.
Similar to chloride and bromo-chloride compounds, SEM inspection revealed the I-exchanged products to be polycrystalline powders with no particular dependence of the morphology on the composition (ESI,† Fig. S4, right column). At high Bi contents (x = 0.75–1.00) formation of a minor fraction of hexagonal platelets was observed along with randomly aggregated perovskite microcrystals.
The 133Cs MAS NMR spectra show two chemical shifts for the Cs3(Sb,Bi)I9 phase assigned to the two inequivalent Cs sites (Fig. 6b),44,45 further supporting the conclusions made from the XRD data. One of the NMR peaks (Fig. 6b) shows a constant isotropic chemical shift of 42 ppm at different Bi fractions, while the second peak reveals a distinct compositional dependence, shifting from 67 ppm for xa = 0 (i.e., Cs3Sb2I9, δiso(133Cs) of 42 and 67 ppm) to 66 ppm for xa = 0.52 to 62 ppm for xa = 1.00 (i.e., Cs3Bi2I9, δiso(133Cs) of 42 and 62 ppm), the latter is consistent with ref. 35 and their reported chemical shift values for the Cs3Bi2I9 phase.
The linewidth of this composition-dependent resonance increases from 6 ppm for xa = 0 to 8 ppm for the mixed CBSI compound with xa = 0.52 and then decreases again to 5 ppm for the bismuth-pure CBI compound. These observations are due to an increase in Sb/Bi random mixing,44,45 and mirror the behavior observed in the CABSC double perovskites discussed above. The nature of a minor peak at ca. 52 ppm observed for the sample with xa = 0 is an unidentified cesium-containing impurity. Further, this site mixing-induced disorder is also reflected in the 209Bi NMR spectra for Cs3Bi2I9 and Cs3(Sb,Bi)2I9, with the broadening of the main Bi resonance for the mixed Sb/Bi compound (Fig. S15, ESI†).
By analogy with the original CABSC double perovskites the I-exchange products show deeper coloration for the intermediate composition, as compared to Sb- and Bi-only compounds (see photograph in the insert in Fig. 7c). The differences can also be observed in the position of the absorption edge, the mixed Cs3Bi2xSb2(1−x)I9 compounds showing a shift of the edge position to lower energies (curve 2 in Fig. 7c) as compared to individual components (curves 1 and 3).
As in the cases of chloride and bromo-chlorides, both direct and indirect bandgaps can be estimated, the indirect Eg's showing a better match with the original absorption spectra (ESI,† Fig. S16). The indirect bandgap of Cs3Bi2xSb2(1−x)I9 compounds decreases from Eg = 2.03 eV for Sb-pure compound down to 1.95 eV for xa = 0.52, growing again at higher Bi content up to 2.00 eV for Cs3Bi2I9 (Fig. 7d). Given the fact of the presence of a single phase, this bandgap variation can be attributed to the disorder-activated band-bowing effect, following the observations discussed above for 133Cs MAS NMR spectra. This effect has not been reported before for Cs–Bi–I compounds, to the best of our knowledge, neither in experimental reports nor in theoretical predictions. It seems to be general for all studied mixed Bi/Sb materials, opening a new approach to bandgap design of metal halide perovskite and perovskite-inspired semiconductors by disorder-activated electronic effects. The family of Cs3Bi2X9 (X = Cl, Br, I) compounds were reported to show a high degree of inherent disorder, even in the single-crystalline form,47 allowing the even higher disorder to exist in mixed Bi/Sb analogs. A large room for bandgap variation can be expected from various disorder-activated effects in CBI perovskites, most probably reflected in a large scatter of reported bandgaps for this semiconductor, varying from 1.87 eV48 to 2.24 eV.49
Conclusions
We report a mild synthesis of lead-free microcrystalline Cs2AgBixSb1−xCl6 (CABSC) double perovskites in the form of single-phase solid solutions with a varied Bi/Sb ratio. The CABSC perovskites revealed a compositional dependence of the bandgap, which decreases due to the BiIII introduction into the Cs2AgSbCl6 matrix, reaches a minimum at x = 0.26, and then increases again for Bi-richer compositions. This band-bowing effect is attributed to a lattice disorder in CABSC perovskites based on disorder-related effects in 133Cs MAS NMR spectra.Interaction of CABSC perovskites with NaBr results in partial exchange of chloride with bromide as well as in the decomposition of the double Bi-Sb perovskite into a mixture of Bi/Sb phases, enriched with Cs3Bi2xSb2(1−x)Cl3Br6 at lower Bi contents and with Cs2AgBixSb1−xCl2Br4 – at higher Bi contents. The Cl-to-Br anion-exchange products show a band-bowing behavior, with the minimal Eg reached at x = 0.47.
The Cl-to-I exchange in CABSC perovskites yields single-phase solid-solution Cs3Bi2xSb2(1−x)I9 (CBSI) double salts with a varied Bi-to-Sb ratio. The CBSI compounds also reveal a band-bowing behavior with the minimal Eg observed at x = 0.52. Based on NMR results, this effect was also attributed to the lattice disorder caused by the Bi/Sb intermixing.
The present report highlights the possibility of a strong influence of lattice disorder and strain on the electronic properties of halide perovskite and perovskite-like compounds with two different MIII cations, in particular, by contributing to the optical band-bowing behavior. A deeper understanding and mastering of such band-bowing effects can lead to new PV materials with reduced band gaps.
Author contributions
O. Stroyuk: conceptualization (lead), investigation (equal), writing – original draft preparation (lead); O. Raievska: investigation (lead), methodology (lead); A. Barabash: investigation (equal), writing – review & editing (equal); R. W. Hooper: investigation (equal), writing – review & editing (equal); V. K. Michaelis: conceptualization (equal), writing – review & editing (equal), project administration (lead), funding acquisition (lead); J. Hauch: conceptualization (equal), project administration (lead), writing – review & editing (equal); C. J. Brabec: conceptualization (equal), funding acquisition (lead), writing – review & editing (equal).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support of The German Federal Ministry for Economic Affairs and Climate Action (project Pero4PV, FKZ: 03EE1092A) and The Bavarian State Government (project “ELF-PV-Design and development of solution-processed functional materials for the next generations of PV technologies”, No. 44-6521a/20/4). VKM acknowledges research support from the Natural Sciences and Engineering Research Council of Canada Discovery Grant (RGPIN-2021-02540) and Create (ATUMS), Alberta Innovates Strategic Project, and Canada Research Chairs (CRC-2020-00352) programs. The Canada Foundation for Innovation (CFI), the Government of Alberta, and the University of Alberta are acknowledged for supporting the Chemistry Centre for Magnetic Resonance (C2MR), a core characterization facility within the Faculty of Science, CNAS.References
- Y. Cui, L. Yang, X. Wu, J. Deng, X. Zhang and J. Zhang, J. Mater. Chem. C, 2022, 10, 16629 RSC.
- H. Yin, Y. Xian, Y. Zhang, W. Li and J. Fan, Sol. RRL, 2019, 1900148 CrossRef.
- W. Ning and F. Gao, Adv. Mater., 2019, 1900326 CrossRef PubMed.
- Y. Li, Z. Shi, W. Liang, J. Ma, X. Chen, D. Wu, Y. Tian, X. Li, C. Shan and X. Fang, Mater. Horiz., 2021, 8, 1367 RSC.
- X. Li, X. Gao, X. Zhang, X. Shen, M. Lu, J. Wu, Z. Shi, V. L. Colvin, J. Hu, X. Bai, W. W. Yu and Y. Zhang, Adv. Sci., 2021, 8, 2003334 CrossRef CAS.
- H. Lei, D. Hardy and F. Gao, Adv. Funct. Mater., 2021, 31, 2105898 CrossRef CAS.
- X. Yang, W. Wang, R. Ran, W. Zhou and Z. Shao, Energy Fuels, 2020, 34, 10513 CrossRef CAS.
- S. Attique, N. Ali, S. Ali, R. Khatoon, N. Li, A. Khesro, S. Rauf, S. Yang and H. Wu, Adv. Sci., 2020, 7, 1903143 CrossRef CAS.
- Y. Peng, T. N. Huq, J. Mei, L. Portilla, R. A. Jagt, L. G. Occhipinti, J. L. MacManus-Driscoll, R. L. Z. Hoye and V. Pecunia, Adv. Energy Mater., 2021, 11, 2002761 CrossRef CAS.
- N. Glück and T. Bein, Energy Environ. Sci., 2020, 13, 4691 RSC.
- S. E. Creutz, E. N. Crites, M. C. De Siena and D. R. Gamelin, Nano Lett., 2018, 18, 1118 CrossRef CAS PubMed.
- O. Stroyuk, O. Raievska, A. Barabash, M. Batentschuk, A. Osvet, S. Fiedler, U. Resch-Genger, J. Hauch and C. J. Brabec, J. Mater. Chem. C, 2022, 10, 9938 RSC.
- O. Stroyuk, O. Raievska, A. Barabash, C. Kupfer, A. Osvet, V. Dzhagan, D. R. T. Zahn, J. Hauch and C. J. Brabec, Mater. Adv., 2022, 3, 7894 RSC.
- E. T. McClure, M. R. Ball, W. Windl and P. M. Woodward, Chem. Mater., 2016, 28, 1348 CrossRef CAS.
- J. Zhou, X. Rong, M. S. Molokeev, X. Zhang and Z. Xia, J. Mater. Chem. A, 2018, 6, 2346 RSC.
- J. E. F. S. Rodrigues, C. A. Escanhoela, Jr., B. Fragoso, G. Sombrio, M. M. Ferrer, C. Álvarez-Galván, M. T. Fernández-Díaz, J. A. Souza, F. F. Ferreira, C. Pecharromán and J. A. Alonso, Ind. Eng. Chem. Res., 2021, 60, 18918 CrossRef CAS.
- A. Singh, R. Chaurasiya, A. Bheemaraju, J. S. Chen and S. Satapathi, ACS Appl. Energy Mater., 2022, 5, 3926 CrossRef CAS.
- B. Yang, F. Hong, J. Chen, Y. Tang, L. Yang, Y. Sang, X. Xia, J. Guo, H. He, S. Yang, W. Deng and K. Han, Angew. Chem., Int. Ed., 2019, 58, 1 CrossRef.
- A. C. Dakshinamurthy, M. Gupta, B. R. Kumar Nanda and C. Sudakar, J. Phys. Chem. C, 2023, 127, 1588 CrossRef CAS.
- D. Manna, J. Kangsabanik, T. K. Das, D. Das, A. Alam and A. Yella, J. Phys. Chem. Lett., 2020, 11, 2113 CrossRef CAS PubMed.
- A. S. Kshirsagar and A. Nag, J. Chem. Phys., 2019, 151, 161101 CrossRef PubMed.
- M. R. Filip, S. Hillman, A. A. Haghighirad, H. J. Snaith and F. Giustino, J. Phys. Chem. Lett., 2016, 7, 2579 CrossRef CAS.
- J. Su, T. Mou, J. Wen and B. Wang, J. Phys. Chem. C, 2020, 124, 5371 CrossRef CAS.
- D. Mourad and G. Czycholl, Eur. Phys. J. B, 2012, 85, 153 CrossRef.
- A. Zunger and J. E. Jaffe, Phys. Rev. Lett., 1983, 51, 662 CrossRef CAS.
- J. Im, C. C. Stoumpos, H. Jin, A. J. Freeman and M. G. Kanatzidis, J. Phys. Chem. Lett., 2015, 6, 3503 CrossRef CAS.
- G. E. Eperon, T. Leijtens, K. A. Bush, R. Prasanna, T. Green, J. T. W. Wang, D. P. McMeekin, G. Volonakis, R. L. Milot, R. May, A. Palmstrom, D. J. Slotcavage, R. A. Belisle, J. B. Patel, E. S. Parrott, R. J. Sutton, W. Ma, F. Moghadam, B. Conings, A. Babayigit, H. G. Boyen, S. Bent, F. Giustino, L. M. Herz, M. B. Johnston, M. D. McGehee and H. J. Snaith, Science, 2016, 354, 861 CrossRef CAS.
- A. Goyal, S. McKechnie, D. Pashov, W. Tumas, M. Van Schilfgaarde and V. Stevanovic, Chem. Mater., 2018, 30, 3920 CrossRef CAS.
- S. Dai, X. Gan, K. Li, Q. Huang, L. Guo and H. Liu, Phys. Chem. Chem. Phys., 2023, 25, 30993 RSC.
- G. Giovilli, B. Albini, V. Grisci, S. Bonomi, M. Moroni, E. Mosconi, W. Kaiser, F. De Angelis, P. Galinetto and L. Malavasi, J. Mater. Chem. C, 2023, 11, 10282 RSC.
- Z. Li, S. R. Kavanagh, M. Napari, R. G. Palgrave, M. Abdi-Jalebi, Z. Andaji-Garmaroudi, D. W. Davies, M. Laitinen, J. Julin, M. A. Isaacs, R. H. Friend, D. O. Scanlon, A. Walsh and R. L. Z. Hoye, J. Mater. Chem. A, 2020, 8, 21780 RSC.
- H. Chen, S. Ming, M. Li, B. Wang and J. Su, J. Phys. Chem. C, 2021, 125, 11271 CrossRef CAS.
- F. Ji, J. Klarbring, F. Wang, W. Ning, L. Wang, C. Yin, J. S. Mendoza Figueroa, C. K. Christensen, M. Etter, T. Ederth, L. Sun, S. I. Simak, I. A. Abrikosov and F. Gao, Angew. Chem., 2020, 132, 15303 CrossRef.
- J. Yang, P. Zhang and S. H. Wei, J. Phys. Chem. Lett., 2018, 9, 31 CrossRef CAS PubMed.
- D. J. Kubicki, M. Saski, S. MacPherson, K. Galkowski, J. Lewinski, D. Prochowicz, J. J. Titman and S. D. Stranks, Chem. Mater., 2020, 32, 8129 CrossRef CAS.
- A. Karmakar, M. S. Dodd, S. Agnihotri, E. Ravera and V. K. Michaelis, Chem. Mater., 2018, 30, 8280 CrossRef CAS.
- A. Karmakar, G. M. Bernard, A. Meldrum, A. O. Oliynyk and V. K. Michaelis, J. Am. Chem. Soc., 2020, 142, 10780 CrossRef CAS PubMed.
- A. Karmakar, G. M. Bernard, A. Pominov, T. Tabassum, R. Chaklashiya, S. Han, S. K. Jain and V. K. Michaelis, J. Am. Chem. Soc., 2023, 145, 4485 CrossRef CAS.
- E. M. Hutter, M. C. Gelvez-Rueda, D. Bartesaghi, F. C. Grozema and T. J. Savenje, ACS Omega, 2018, 3, 11655 CrossRef CAS PubMed.
- K. Du, W. Meng, X. Wang, Y. Yan and D. B. Mitzi, Angew. Chem., Int. Ed., 2017, 56, 8158 CrossRef CAS.
- A. Pradhan, M. K. Jena and S. L. Samal, ACS Appl. Energy Mater., 2022, 5, 6952 CrossRef CAS.
- R. Kentsch, M. Scholz, J. Horn, D. Schlettwein, K. Oum and T. Lenzer, J. Phys. Chem. C, 2018, 122, 25940 CrossRef CAS.
- P. Pistor, M. Meyns, M. Guc, H. C. Wang, M. A. L. Marques, X. Alcobe, A. Cabot and V. Izquierdo-Roca, Scr. Mater., 2020, 184, 24 CrossRef CAS.
- Yu. N. Ivanov, A. A. Sukhovskii, V. V. Lisin and I. P. Aleskandrova, Inorg. Mater., 2001, 37, 623 CrossRef CAS.
- C. J. Krajewska, S. R. Kavanagh, L. Zhang, D. J. Kubicki, K. Dey, K. Gałkowski, C. P. Grey, S. D. Stranks, A. Walsh, D. O. Scanlon and R. G. Palgrave, Chem. Sci., 2021, 12, 14686 RSC.
- A. Elattar, L. Kobera, J. Kangsabanik, H. Suzuki, S. Abbrent, T. Nishikawa, K. S. Thygesen, J. Brus and Y. Hayashi, J. Mater. Chem. C, 2022, 10, 12863 RSC.
- N. K. Tailor and S. Satapathi, ACS Appl. Energy Mater., 2020, 3, 11732 CrossRef CAS.
- A. Nila, M. Baibarac, A. Matea, R. Mitran and I. Baltog, Phys. Status Solidi B, 2017, 254, 1552805 CrossRef.
- S. K. Shil, F. Wang, Z. Lai, Y. Meng, Y. Wang, D. Zhao, M. K. Hossain, K. O. Egbo, Y. Wang, K. M. Yu and J. C. Ho, Nano Res., 2021, 14, 4116 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Elemental composition of chloride, bromo-chloride, and iodide compounds; examples of XRD patterns of CABSC perovskites and products of partial chlorine-to-bromine substitution; relationships between nominal and actual element fractions; a collection of SEM images and EDX spectra of chloride, bromide, and iodide compounds with different Bi/Sb ratio; examples of absorption spectra of chloride, bromide, and iodide compounds plotted in Tauc coordinates for direct and indirect transitions; 209Bi MAS NMR spectra for chloride and iodide compounds; relationships between NMR spectral features and composition of Br-substituted CABSC compounds; examples of Raman spectra of bromide compounds. See DOI: https://doi.org/10.1039/d3tc04004f |
| This journal is © The Royal Society of Chemistry 2024 |