Open Access Article
Open Access ArticleMetal halides RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+ with yellow and cyan emissions obtained via a facile hydrothermal process
Dayu
Huang
ab,
Pan
Zheng
ab,
Ziyong
Cheng
ab,
Qiuyun
Ouyang
*a,
Hongzhou
Lian
*ab and
Jun
Lin
 *ab
*ab
aKey Laboratory of In-Fiber Integrated Optics of Ministry of Education, College of Physics and Opotoelectronic Engineering, Harbin Engineering University, Harbin 150001, China. E-mail: qyouyang@hrbeu.edu.cn
bState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: hzlian@ciac.ac.cn; jlin@ciac.ac.cn; Tel: +86-431-85698041
First published on 13th November 2023
Abstract
It is of great interest to obtain multiple luminescent products from a single reaction, such as existing core–shell nanomaterials and heterojunction nanomaterials, all with the aim of enriching the photoelectric properties of the materials. Here, we report a hydrothermal approach for creating crystals of needle-shaped RbCdCl3:Sb3+ and bulk-shaped Rb4CdCl6:Sb3+, two antimony doped-cadmium based metal halides. Two crystal materials with different shapes and phases can be synthesized simultaneously at the same stoichiometric ratio. RbCdCl3:Sb3+ is needle-shaped, whereas Rb4CdCl6:Sb3+ is bulk-shaped. Under 320 nm excitation wavelength irradiation, blue emission (490 nm) of Rb4CdCl6:Sb3+ and yellow emission (560 nm) of RbCdCl3:Sb3+ can be attained. Interestingly at low temperatures RbCdCl3:Sb3+ shows double peaked emissions (460 and 560 nm). This study investigates Sb3+-doped RbCdCl3 crystals that exhibit double luminescence as a result of intrinsic host self-trapped excitons and extrinsic dopant-induced self-trapped excitons (STEs), which are referred to as host STEs and Sb3+ dopant STEs, respectively. The construction of multicolor light-emitting devices is made possible by their color kinetics characteristics.
1. Introduction
With the gradual deepening of research based on metal halides and their applications, the requirements for the properties of metal halides will become more precise and demanding. Therefore, it can be expected that how to further develop the synthetic chemistry of metal halides will remain the core of their future research.1–3 However, the synthetic chemistry of metal halides has already passed the early stage of exploration and has gradually formed a fixed synthesis mode and system. In order to break through the existing synthetic bottlenecks to achieve further development, it is necessary to systematically study the influence of various factors on the growth behaviour of metal halides during the synthesis process, so as to make relevant improvements. Phosphors with multiexcitonic emissions are necessary for building illumination devices like white light-emitting diodes (LEDs), as they improve their color rendering index performance.4,5 Now, fluorescence/phosphorescence and exciton phase-space filling are the main methods used to produce multiexcitonic emissions in organic fluorophores and heterostructured semiconductor quantum dots. However, the main drawbacks of these types of multiexcitonic emissions are low controllability driven by the monodirectional intersystem crossover and low efficiency caused by quenching due to Auger recombination.2,6 Because of their high tolerance for structural defects, which effectively prevents nonradiative energy losses, metal halides have recently demonstrated tremendous potential in lighting applications.7 Given their great designability and tenability, metal halide variants with a vacancy-ordered structural arrangement have evolved in particular in recent years.8 These so-called metal halides, such as Cs2SnCl6, Rb4CdCl6, and Cs2ZrCl6, have closely packed photogenerated charge carriers which foster charge recombination and light emission.9–12 The addition of different dopant ions, which produce additional energy levels for sustaining excitons of adjustable energies in the host lattice, also makes it possible to easily adjust the emission lines of these crystals. For instance, Sb3+-doped metal halides with coordination-dependent emission have been found to exhibit highly effective and low-toxic multicolor photoluminescence properties.12–23 Although controlling the luminescence of metal halide crystals has been very effective, rational regulation of many excitonic emissions in a single reaction still has to be achieved. The dopant self-trapped excitons (STEs) often predominate the emission spectra regardless of the excitation conditions, because of the strong interaction between the host and dopant STEs in a great deal of doped metal halide materials. In this study, two different types of metal halides (RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+) are produced in a reactor under the same reaction conditions. RbCdCl3:Sb3+ exhibits yellow emission (at about 560 nm) under 320 nm UV light irradiation, while Rb4CdCl6:Sb3+ exhibits cyanotic emission (at about 490 nm). RbCdCl3:Sb3+ has another characteristic at a low temperature of −180 °C. A pure blue emission peak of the host appears, mixing with the yellow emission peak to form a single-phase white light emission. Therefore, the categorisation of multi-resonance emission in Sb3+ doped RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+ metal halides was investigated by spectroscopic measurements. We demonstrate that the different luminescence peaks of the RbCdCl3:Sb3+ material resulting from host and dopant STEs are independent of each other. By precisely varying the temperature, dynamic color transmission can be achieved using multi-resonance emission.2. Experimental
2.1 Chemicals
All chemical reagents were used without further purification: CdO (cadmium oxide, Aladdin), RbCl (rubidium chloride, Aladdin), HCl aqueous solution, SbCl3 (antimony chloride, Aladdin). All reactions were performed under ambient conditions.2.2 Synthesis of RbCdCl3:Sb3+ and bulk-shaped Rb4CdCl6:Sb3+
RbCdCl3:Sb3+ and bulk-shaped Rb4CdCl6:Sb3+ were synthesized by using a hydrothermal method. RbCl (3 mmol), SbCl3 (0.1–0.3 mmol) and CdO (1 mmol) were added into a 25 mL Teflon-lined autoclave. Then, 10 mL of HCl aqueous solution was added and heated at 150 °C for 2 h and then slowly cooled at 2 °C h−1 to room temperature. Finally, the obtained single crystal particles were washed two times with ethanol and dried at 80 °C for 1 h in a furnace. It is vital to note that only one reaction can produce two crystals.2.3 Characterization
Phase identification was confirmed by X-ray diffraction using a D8 Focus diffractometer (Bruker) with Cu-Kα radiation (λ = 1.5405 Å). Analysis of the elements was performed using an energy-dispersive spectrometer equipped with a scanning electron microscope (S-4800; Hitachi, Japan). X-ray photoelectron spectroscopy (XPS) spectra were recorded employing a VG ESCALAB MK II electron spectrometer using the Mg Kα (1200 eV) line as an excitation source. Photoluminescence (PL) spectra and temperature-dependent (at −180 to 150 °C) PL spectra were recorded on a photoluminescence spectrophotometer with a 450 W xenon lamp as the excitation source equipped with a temperature controller (an Edinburgh Instrument FLS-920). Photoluminescence quantum yield (PLQY) values were collected on an absolute PL quantum yield measurement system (Hamamatsu Photonics K.K., C9920-02 Japan). The electroluminescence (EL) performance of the fabricated WLED devices was evaluated using a HAAS 2000 photoelectric measuring system (380–1100 nm, EVERFINE, China).3. Results and discussion
Given their exceptional photoluminescence performance, metal halides are selected as host materials. To introduce dopant STEs by creating Sb3+-based polyhedrons, the Sb3+ ion with an ns2 electronic configuration was chosen. SbCl3, CdO, RbCl, and HCl aqueous solutions were used as the starting materials in a co-precipitation process to create RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+ metal halides. The reaction process cools down very slowly. In the Experimental section, the synthesis process is described in extensive detail. After four days of controlled cooling, centimeter-sized crystals appeared. It is interesting to note that the synthetic crude product includes samples that resemble needle-shaped RbCdCl3:Sb3+ and bulk-shaped Rb4CdCl6:Sb3+ (Fig. 1a). The needle-shaped RbCdCl3:Sb3+ material has a length of approximately 1 cm. The bulk Rb4CdCl6:Sb3+ material's maximum width is roughly 0.5 cm. XRD was used to determine the material's phase (Fig. 1b and c). The needle-shaped RbCdCl3:Sb3+ and bulk-shaped Rb4CdCl6:Sb3+ show distinct Pnma and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c space groups (Fig. 1d and e). The structural components of RbCdCl3 are depicted in Fig. 1d as [CdCl6]4− and [RbCl9]8− polyhedrons. It is known that Sb3+ ions preferentially occupy the 6-coordinated Cd2+ sites. The structural unit of Rb4CdCl6 is the polyhedron composed of [CdCl6]4−, [RbCl6]5− and [RbCl8]7− polyhedrons. With the development of a Cl vacancy ([VCl]+) to maintain the charge balance, we determined that the Sb3+ dopant most likely existed as [SbCd]+.9
c space groups (Fig. 1d and e). The structural components of RbCdCl3 are depicted in Fig. 1d as [CdCl6]4− and [RbCl9]8− polyhedrons. It is known that Sb3+ ions preferentially occupy the 6-coordinated Cd2+ sites. The structural unit of Rb4CdCl6 is the polyhedron composed of [CdCl6]4−, [RbCl6]5− and [RbCl8]7− polyhedrons. With the development of a Cl vacancy ([VCl]+) to maintain the charge balance, we determined that the Sb3+ dopant most likely existed as [SbCd]+.9
X-ray photoelectron spectroscopy (XPS), which verified the existence of Rb, Cd, Cl, and Sb elements in the RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+ crystals, also provided confirmation for the successful doping of Sb3+ ions (Fig. 2a and b). Fine peaks of Rb, Cd, Cl, and Sb are presented. The Rb 3d5/2 orbital binding energies of the two materials are between 108 and 112 eV. The binding energy of Cd 3d5/2 orbitals of both materials is located at 405 eV, and that of the other 3d3/2 orbitals is located at 412 eV. The binding energy of Cl 2p1/2 orbitals of the two materials is located at 200 eV, and that of the other 2p3/2 orbitals is located at 198 eV. The Sb 3d5/2 orbital binding energy of the two materials is located at 531 eV. Fig. 3a and b displays the SEM images of RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+. RbCdCl3:Sb3+ is primarily needle-like, with a minimum length of around 30 μm and a maximum length of about 1 cm. The elemental distribution obtained from SEM mapping images is displayed in order. Rb4CdCl6:Sb3+ has a significantly different shape. It appears as a bulk with a minimum width of more than 30 μm and a maximum width in centimeters, as in daylight photographs. The SEM mapping images of the Rb, Cd, Cl and Sb elements in Rb4CdCl6:Sb3+ are shown in Fig. 3, respectively.
 | ||
| Fig. 3 (a) and (b) SEM images and elemental mapping of Rb4CdCl6:Sb3+ and RbCdCl3:Sb3+ showing the uniform distribution of Cd, Cl, Rb and Sb elements. | ||
The optical characteristics of the Sb3+-doped RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+ crystals were then investigated. Photoluminescence excitation (PLE) demonstrated that Sb3+ doping adds extra excitation peaks in the 250–360 nm range to the sample (Fig. 4a and b). According to the results of the simulation, the large absorption peaks at 250–300 nm and 300–360 nm, respectively, were attributed to the 1S0–1P1 and 1S0–3P1 transitions of Sb3+ ions.9 As a result, the Sb3+ dopant also increased the emission intensity of the RbCdCl3 and Rb4CdCl6 host crystals. An optimal doping concentration of 20% was found by connecting the emission intensity and luminous quantum yield with the amount of Sb3+ present, at which point the greatest quantum yield of doping-induced emission was measured at 78%. Higher dopant concentrations may cause polyhedral cluster formation and a drop in the particle size and crystallinity, leading to a fall in emission intensity.
The photoluminescence (PL) spectra of the RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+ crystals were recorded in order to investigate the recombination processes in the Sb3+-doped RbCdCl3 and Rb4CdCl6 phosphors. The undoped Rb4CdCl6 phosphor had a weak emission peak centered at 490 nm under excitation at 297 nm, which was in line with a prior result.11 RbCdCl3 was much weaker and did not show the phenomenon of luminescence. Due to strong electron–phonon interactions in the metal halide materials with a soft lattice, the Rb4CdCl6 host STEs were thought to be responsible for the broad emission band with a significant Stokes shift of 193 nm. The [CdCl6]4−, [RbCl6]5− and [RbCl8]7− charge transition was attributed to the solitary peak at 490 nm in the emission spectra. Numerous instances of this charge transition were also seen in metal halides. In addition to the host excitation band at 290 nm, a novel excitation band centered at 320 nm was found for the RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+ crystals. The direct excitation of Sb3+ ions through the 1S0 → 1P1 transition, which was also frequently observed in Sb3+-doped metal halides such RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+, was assigned to the excitation band at 320 nm. Rb4CdCl6:Sb3+ has cyan emission (490 nm) under 320 nm excitation (Fig. 4d). However, RbCdCl3:Sb3+ has broadband yellow emission (560 nm) under 320 nm excitation (Fig. 4c). It was most likely dopant-induced STEs in the [SbCl6]3− polyhedrons that were responsible for the related broad emissions in the 400–700 nm region under 320 nm excitation. Photographs of the luminescence of the RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+ materials under 365 nm excitation are shown in Fig. 4e.
The dynamic process of electronic changes was then investigated. When the RbCdCl3:Sb3+ crystals were excited at 320 nm, their broad emission showed a gradual decline with a long lifetime of 7.9 μs, which is compatible with the STE emission (Fig. 5a). The STE emission of the Rb4CdCl6:Sb3+ crystals had a lifetime of 4.2 μs, which was a little bit shorter than that of RbCdCl3:Sb3+(Fig. 5b). This energy transfer from the host to Sb3+ could be accelerated by increasing the concentration of Sb3+ dopant.9
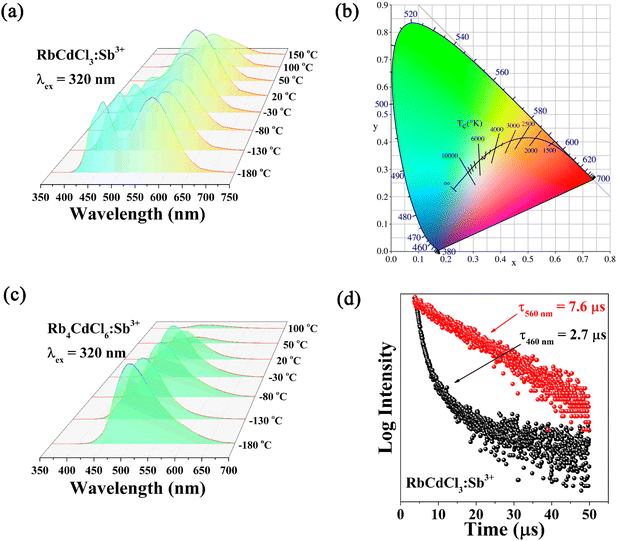
In addition, we used temperature-dependent PL spectra to investigate the thermal stability of RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+ crystals. Fig. 6a depicts the change in the PL spectra of RbCdCl3:Sb3+ with temperature in the −180 to 150 °C range. Interestingly, there was a double emission peak (460 and 560 nm) at low temperatures. The fact that isolated units exist in the vacancy-ordered metal halides is largely responsible for this result, which shows that the emission processes of host STEs and dopant STEs are mutually independent. The RbCdCl3:Sb3+ crystals' double luminescence is also made possible by the little energy transfer from host to dopant STEs, which would otherwise extinguish the host emission. At this time, white light emission can be obtained by mixing blue light and yellow light (Fig. 6b). The intensity of the emission peak at 460 nm decreases rapidly with increasing temperature, however, the intensity of the emission peak at 560 nm decreases slowly and the intensity of the emission increases at a certain temperature (−30 to 20 °C). It is noteworthy that the intensity of the emission peaks of Sb3+ ions is well maintained relative to the charge transition band of the host. Fig. 6c demonstrates that the PL spectra of Rb4CdCl6:Sb3+ change as a function of temperature in the −180 to 100 °C range. The intensity of the emission peak at 490 nm decreases with increasing temperature and the peak position is blue-shifted. This phenomenon is common; as the temperature increases, the lattice of the Rb4CdCl6:Sb3+ material expands, resulting in a weakening of the crystal field strength, and the 5S2 → 5S1/5P1 transition energy of Sb3+ increases as the crystal field strength decreases, resulting in a shift of the emission peaks towards higher energies.
Due to defects caused by the Sb3+ dopant, which disrupted the electron cloud and encouraged carrier entrapment in the nearby [CdCl6]4− octahedra, as previously reported,24 the charge transition band recombination was slowed down.9 The RbCdCl3:Sb3+ crystals displayed a double-exponential decay with a short component of 2.7 μs and a long component of 7.6 μs (Fig. 6d), which corresponded to the two emission components (460 and 560 nm). It is important to note that the Sb3+ dopant in metal halide structures was responsible for the lifetimes of the dopant STEs being essentially constant when the temperature varied. Finally, the light-emitting diode (LED) device is obtained by using both fabricated RbCdCl3:Sb3+ (560 nm) and Rb4CdCl6:Sb3+ (490 nm) materials. LED device results show that a good color rendering index (91) and correlated color temperature (4303 K) can be obtained using synthetic RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+ materials (Fig. 7a and b).
4. Conclusions
In summary, the same reactor produces two distinct crystal types with various crystal phases and shapes. This process of simultaneous synthesis of materials is also distinctive. RbCdCl3:Sb3+ needle-shaped crystals with yellow emission (560 nm) and Rb4CdCl6:Sb3+ bulk-shaped crystals with blue emission (460 nm) are synthesized. A new multi-resonance luminescence is found in Sb3+ doped RbCdCl3 crystals at low temperatures. The bimodal emission of the RbCdCl3:Sb3+ crystals is attributed to the host and dopant STEs, respectively. The crystal structure of the RbCdCl3:Sb3+ metal halide is vacancy-ordered, which is principally responsible for the isolation of the host and dopant STEs. The temperature-dependent spectra demonstrate that energy transfers from the host to Sb3+ as temperature rises. White light emission is obtained at −180 °C, slowly changing to yellow light emission as the temperature increases. The simultaneous acquisition of RbCdCl3:Sb3+ and Rb4CdCl6:Sb3+ crystals largely simplifies the synthesis process and adds members to the luminescent material family.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the National Key Research and Development Program of China (2022YFB3503800), the National Natural Science Foundation of China (NSFC no. 51932009, 51902226, 52172166, U2005212), the Natural Science Fund of Heilongjiang Province (LH2019F013), the 111 Project (B13015) of Ministry Education of China to Harbin Engineering University, and the Project of High-level Scientific Research Guidance of Harbin Engineering University (no. 3072022TS2508).Notes and references
- K. Han, J. Jin, B. Su and Z. Xia, Trends Chem., 2022, 4, 1034–1044 CrossRef CAS.
- K. Han, J. Qiao, S. Zhang, B. Su, B. Lou, C. G. Ma and Z. Xia, Laser Photonics Rev., 2023, 17, 2200458 CrossRef CAS.
- L.-y Huang and W. R. Lambrecht, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 165203 CrossRef.
- J. Mao, H. Lin, F. Ye, M. Qin, J. M. Burkhartsmeyer, H. Zhang, X. Lu, K. S. Wong and W. C. Choy, ACS Nano, 2018, 12, 10486–10492 CrossRef CAS PubMed.
- S. Pimputkar, J. S. Speck, S. P. DenBaars and S. Nakamura, Nat. Photonics, 2009, 3, 180–182 CrossRef CAS.
- C. Bi, Z. Yao, J. Hu, X. Wang, M. Zhang, S. Tian, A. Liu, Y. Lu, N. H. de Leeuw, M. Sui and J. Tian, ACS Energy Lett., 2023, 8, 731–739 CrossRef CAS.
- B. Wang, Y. H. Zhou, S. Yuan, Y. H. Lou, K. L. Wang, Y. Xia, C. H. Chen, J. Chen, Y. R. Shi, Z. K. Wang and L. S. Liao, Angew. Chem., 2023, e202219255 CAS.
- G. Zhou, B. Su, J. Huang, Q. Zhang and Z. Xia, Mater. Sci. Eng., R, 2020, 141, 100548 CrossRef.
- B. Chen, Y. Guo, Y. Wang, Z. Liu, Q. Wei, S. Wang, A. L. Rogach, G. Xing, P. Shi and F. Wang, J. Am. Chem. Soc., 2021, 143, 17599–17606 CrossRef CAS PubMed.
- Z. Tan, J. Li, C. Zhang, Z. Li, Q. Hu, Z. Xiao, T. Kamiya, H. Hosono, G. Niu, E. Lifshitz, Y. Cheng and J. Tang, Adv. Funct. Mater., 2018, 28, 1801131 CrossRef.
- D. Huang, Q. Ouyang, Y. Kong, B. Wang, Z. Cheng, A. A. Al Kheraif, H. Lian and J. Lin, Dalton Trans., 2023, 52, 5715–5723 RSC.
- G. Zhang, P. Dang, H. Xiao, H. Lian, S. Liang, L. Yang, Z. Cheng, G. Li and J. Lin, Adv. Opt. Mater., 2021, 9, 2101637 CrossRef CAS.
- J. Jin, Y. Peng, Y. Xu, K. Han, A. Zhang, X.-B. Yang and Z. Xia, Chem. Mater., 2022, 34, 5717–5725 CrossRef CAS.
- Y. Jing, Y. Liu, X. Jiang, M. S. Molokeev, Z. Lin and Z. Xia, Chem. Mater., 2020, 32, 5327–5334 CrossRef CAS.
- Y. Jing, Y. Liu, M. Li and Z. Xia, Adv. Opt. Mater., 2021, 9, 2002213 CrossRef CAS.
- Y. Jing, Y. Liu, J. Zhao and Z. Xia, J. Phys. Chem. Lett., 2019, 10, 7439–7444 CrossRef CAS PubMed.
- B. Su, M. Li, E. Song and Z. Xia, Adv. Funct. Mater., 2021, 31, 2105316 CrossRef CAS.
- Q. Wei, T. Chang, R. Zeng, S. Cao, J. Zhao, X. Han, L. Wang and B. Zou, J. Phys. Chem. Lett., 2021, 12, 7091–7099 CrossRef CAS PubMed.
- J.-B. Luo, J.-H. Wei, Z.-Z. Zhang and D.-B. Kuang, Inorg. Chem., 2022, 61, 338–345 CrossRef CAS PubMed.
- J. Nie, B. Zhou, S. Fang, H. Zhong, H. Li and Y. Shi, Chem. Mater., 2022, 34, 6288–6295 CrossRef CAS.
- D.-Y. Li, J.-H. Song, Y. Cheng, X.-M. Wu, Y.-Y. Wang, C.-J. Sun, C.-Y. Yue and X.-W. Lei, Angew. Chem., Int. Ed., 2022, 134, e202206437 CrossRef.
- A. Zhang, J. Jin and Z. Xia, Adv. Opt. Mater., 2022, 10, 2200720 CrossRef CAS.
- D.-Y. Li, J.-H. Song, Z.-Y. Xu, Y.-J. Gao, X. Yin, Y.-H. Hou, L.-J. Feng, C.-Y. Yue, H. Fei and X.-W. Lei, Chem. Mater., 2022, 34, 6985–6995 CrossRef CAS.
- Q.-L. Wei, X.-F. Meng, W.-C. Lin, S.-G. Ge, X.-X. Han, L. Chen, R.-S. Zeng and B. S. Zou, J. Phys. Chem. Mater., 2022, 13, 8436–8446 CAS.
| This journal is © The Royal Society of Chemistry 2023 |