Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tuning the emission and exciton utilization mechanisms of pyrazine-based multi-carbazole emitters and their use in organic light-emitting diodes†‡
Dongyang
Chen
ac,
Le
Zhang
 ab,
Tomas
Matulaitis
ab,
Tomas
Matulaitis
 a,
David B.
Cordes
a,
David B.
Cordes
 a,
Alexandra M. Z.
Slawin
a,
Alexandra M. Z.
Slawin
 a,
Xiao-Hong
Zhang
a,
Xiao-Hong
Zhang
 c,
Ifor D. W.
Samuel
c,
Ifor D. W.
Samuel
 *b and
Eli
Zysman-Colman
*b and
Eli
Zysman-Colman
 *a
*a
aOrganic Semiconductor Centre, EaStCHEM School of Chemistry, University of St Andrews, St Andrews, Fife KY16 9ST, UK. E-mail: eli.zysman-colman@st-andrews.ac.uk
bOrganic Semiconductor Centre, SUPA, School of Physics and Astronomy, University of St Andrews, North Haugh, St Andrews, Fife KY16 9SS, UK. E-mail: idws@st-andrews.ac.uk
cInstitute of Functional Nano & Soft Materials (FUNSOM), Joint International Research Laboratory of Carbon-Based Functional Materials and Devices, Soochow University, Suzhou, 215123, Jiangsu, P. R. China
First published on 6th September 2023
Abstract
Thermally activated delayed fluorescence (TADF) and hot excitons are two distinct exciton harvesting mechanisms that can lead to 100% internal quantum efficiency in organic light-emitting diodes (OLEDs). Herein, we show that with judicious molecular engineering, the resulting structurally similar compounds emit via distinct photophysical mechanisms, which has a direct consequence on the OLED efficiency. When the pyrazine core is substituted with four carbazoles, the molecule 4CzPyz shows TADF in doped PPT film, with ΦPL of 75%, ΔEST of 0.23 eV and τd of 150 μs. The device based on 4CzPyz emits in the sky-blue (λEL = 486 nm) with an EQEmax of 24.1%. When one carbazole is replaced by an ortho-biphenyl, the ΔEST of 3CzBPz increases to 0.29 eV, the ΦPL decreases to 56%, and the TADF character is largely suppressed in the PPT film. However, a RISC process between higher-lying triplet excited states and the S1 state is hypothesized to be operational, supported by a combined photophysical and DFT study, to rationalize how the device with 3CzBPz shows an EQEmax of 9.6% (λEL = 464 nm), reflecting that greater than 86% of the excitons are converted into light in the OLED. When two ortho-biphenyl groups are connected to the pyrazine core, the ΔEST of 2CzBPz is further increased to 0.34 eV while the ΦPL is reduced to 45% in the PPT film. The DFT and photophysical studies indicate that 2CzBPz should act as a traditional blue fluorescence emitter. The OLED devices with 2CzBPz bear this out and exhibit an EQEmax 3.2% at a λEL of 446 nm. These results show how subtle structural changes modulate the efficiency of the triplet exciton harvesting mechanisms and provide new design directions for highly efficient blue emitters for OLEDs.
Introduction
Reverse intersystem crossing (RISC), is a photophysical process that converts triplet excitons to singlet excitons.1–3 RISC is central to the thermally activated delayed fluorescence (TADF) mechanism where the process is endothermic and converts non-emissive triplet excitons to emissive singlet excitons.4–6 Organic light-emitting diodes (OLEDs) with compounds that emit TADF can achieve 100% internal quantum efficiency (IQE).7,8 For TADF to be operational, the energy gap between the lowest-lying singlet (S1) and triplet (T1) excited states, ΔEST, must be sufficiently small, normally taken to be less than 200 meV.9,10 The most common design rule for TADF emitters includes poorly electronically coupled electron donor (D) and acceptor (A) moieties in order to confine the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) on the donor and acceptor units, respectively. The resulting small exchange integral induces a small ΔEST. Using this molecular design requires very electronically weak donor and acceptor groups in order to achieve high-energy emissive S1 states for blue TADF emitters. Frequently, the limited choice of these groups leads to compounds that show too delocalized frontier molecular orbitals (FMO), leading to larger ΔEST and inefficient RISC.11–13 Meanwhile, the presence of higher-energy triplet states has been suggested to assist in the harvesting of triplet exciton in the device.14,15 One possible mechanism requires that the higher-energy triplet states Tn (n > 1) lie essentially degenerate with S1 yet be much higher in energy than T1, which renders RISC competitive with IC.16–18 The nature of the excited states involved in this mechanism usually show mixed locally-excited (LE) and charge transfer (CT) character [so called hybridized local and charge-transfer (HLCT)] that consequently leads to enhanced spin–orbit coupling between S1 and these higher-lying triplet states.14,15 The nature of the HLCT state provides a balance between fast radiative transition rates manifested in high ΦPL and sufficiently small energy gaps between S1 and Tn (ΔES1Tn), facilitating RISC.16,17Compounds emitting via either a traditional TADF mechanism, involving RISC between T1 and S1 mediated by a small ΔEST, or emitting via a mechanism involving RISC from a Tn state to S1 (n > 1) have been widely explored as emitters for OLEDs (Fig. 1). For TADF emitters based on a twisted D–A structure, the S1 and T1 states are usually of CT character, and according to El Sayed's rules, direct RISC from T1 to S1 of states with the same orbital symmetry is not allowed, which is reflected in the very low spin–orbit coupling (SOC) between these two states.19–21 Previous research results have shown that intermediate triplet states between S1 and T1 can assist the RISC process, and as the orbital type of Tn usually differs from that of the S1 state, strong SOC exists.21–23 Thus, the RISC process is facilitated by spin-vibronic coupling between T1 and Tn and enhanced SOC between Tn and S1 when the excited states involved in RISC are energetically closely aligned.22 Our previous work on the pyrazine-based TADF emitter DTCz-Pz also showed that the presence of an intermediate T2 state of HLCT character that is of different orbital type to S1 (CT character) can provide an indirect route for RISC to occur despite the relatively large ΔEST of 0.27 eV in 2,8-bis(diphenyl-phosphoryl)-dibenzo[b,d]thiophene (PPT).12 Makhseed et al. recently reported a TADF emitter 4CzPyz where four carbazoles were connected to a pyrazine core, and the multiple carbazoles can present slightly different electronic coupling with the central acceptor, thus providing a dense population of excited states.244CzPyz exhibited narrowed ΔEST of 0.23 eV and shorter delayed lifetime (τd) of 144 μs in 10 wt% bis[2-(diphenylphosphino)phenyl]ether oxide (DPEPO) film compared to corresponding values of 0.27 eV and 5.5 ms for DTCz-Pz in 7% PPT film.12,24 However, the electroluminescence behavior of 4CzPyz was not explored and the increased molecular conjugation caused significantly red-shifted emission as 4CzPyz showed sky-blue emission with λPL of 502 nm, while for DTCz-Pz which only contains two tert-butylcarbazoles were connected to pyrazine, the emission is deep blue with λPL of 460 nm.12,24
ortho-Substituted biphenyl (BP) has been used to improve the RISC process by introducing a LE state in the two-step RISC mechanism without substantially increasing the conjugation in the molecule.25 The compound 4mCzBN-BP emits from a CT state [E(S1) = 2.80 eV] and has a T1 state of HLCT character [E(T1) = 2.69 eV] in toluene, both of which are energetically close to the triplet LE state of BP (2.95 eV). As a result, 4mCzBN-BP exhibited faster kRISC of 2.28 × 106 s−1 in toluene, compared to 1.26 × 106 s−1 for the reference emitter 4mCzBN.25 Inspired by this work, here we modified the reference emitter 4CzPyz by replacing one or two of the carbazole donors with ortho-substituted biphenyl groups and obtained two blue/deep blue emitters, 3CzBPz and 2CzBPz. The three compounds exhibit distinct photophysics and exciton harvesting mechanisms in OLEDs. The S1 energies of 3CzBPz and 2CzBPz are destabilized to 2.94 eV and 3.00 eV, respectively, compared to 2.88 eV for 4CzPyz in 10 wt% PPT films, while the ΔEST values progressively increase from 0.23 eV (4CzPyz) to 0.29 eV (3CzBPz) and 0.34 eV (2CzBPz), respectively. The smaller ΔEST for 4CzPyz makes it possible to utilize triplet excitons in OLEDs via TADF; indeed, the device exhibited a maximum external quantum efficiency (EQEmax) of 24.1% and sky-blue emission with λEL of 486 nm. The close-to-resonant T3/S1 states in 3CzBPz allow the RISC process occurring via the higher-energy Tn channels. The OLED with this compound as the emitter shows pure blue emission with λEL of 464 nm and an EQEmax 9.6%. However, the too large ΔEST for 2CzBPz precludes either of these two exciton-harvesting mechanisms from being operational and the OLED based on 2CzBPz exhibits an EQEmax of only 3.2% but the device is deep blue with λEL of 446 nm.
Results and discussion
Synthesis
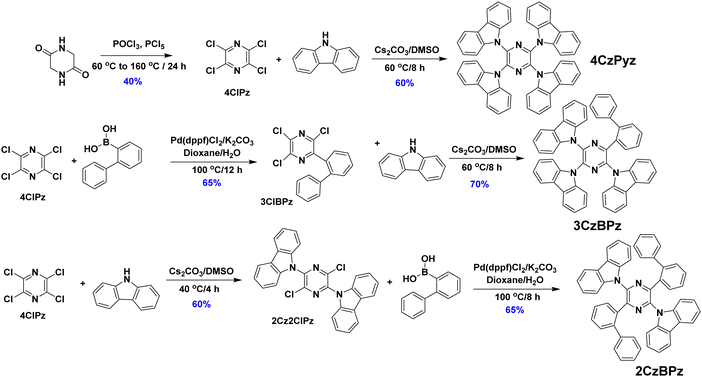
The synthesis of the three emitters is outlined in Scheme 1. The key intermediate, tetrachloropyrazine, (4ClPz) was synthesized in 40% yield from dioxopiperazine upon reaction with phosphorus pentachloride.254ClPz was reacted with excess carbazole under SNAr conditions to obtain 4CzPyz in a yield of 60%, which is significantly improved compared to the 2.5% previously reported using a Suzuki–Miyaura cross-coupling strategy.24 For the synthesis of 3CzBPz, 4ClPz was converted to the biphenyl-substituted intermediate (3ClBPz) via a Suzuki–Miyaura reaction, which was then reacted with carbazole to obtain 3CzBPz in a 70% yield.26 For the synthesis of 2CzBPz, 4ClPz was reacted first with carbazole to obtain the dicarbazole intermediate 2Cz2ClPz, which was then directly reacted with 2-biphenylboronic acid via a Suzuki–Miyaura cross-coupling reaction to obtain 2Cz2BPz in a yield of 65%.26 The three emitters were characterized by combination of 1H and 13C NMR spectroscopy, X-ray diffraction (XRD), high-resolution mass spectrometry, melting point determination, high performance liquid chromatography (HPLC), and elemental analysis. The three emitters have melting points of over 380 °C and high degradation temperatures (Td for 5 wt% weight loss), measured by thermogravimetric analysis (TGA), of 371, and 420 °C for 2CzBPz and 4CzPyz (Fig. S7, ESI‡), respectively. For 3CzBPz sublimation was, however, observed during the TGA, and the temperature responsible for 5 wt% weight loss is 407 °C.Single crystals of 4CzPyz were grown via slow evaporation of a mixed solution of chloroform/methanol, while single crystals of 3CzBPz and 2CzBPz were obtained following gradient-temperature vacuum sublimation. The structures are illustrated in Fig. 2. The three compounds show similar degrees of twist of the carbazole rings away from the plane of the central pyrazine, 4CzPyz and 3CzBPz being the most similar. In 4CzPyz the carbazole groups are twisted slightly differently depending on which side of the pyrazine ring they are on. In the first independent molecule, one side shows dihedral angles between the pyrazine and the mean plane of the carbazole of 34.67° and 43.18°, the other of 49.68° and 54.20°. The second independent molecule shows a less extreme difference, one side showing angles of 42.91° and 43.45°, the other of 55.09° and 56.73°. For 3CzBPz, the carbazole at the ortho position to the biphenyl is twisted with a dihedral angle of 47.39°, while the dihedral angles for the carbazoles at the meta and para positions are larger at 56.01° and 52.71°, respectively. The dihedral angle between the carbazoles and the pyrazine in 2CzBPz are at the higher end of the range seen, at 55.06°, rather different to those of a previously reported green-emissive pyrazine-containing TADF emitter pDTCz-DPzS [38.20(8)°].7 As was the case for the previously reported structure of the DMF solvate of 4CzPyz,27 the crystal structure of 4CzPyz shows several intermolecular C–H⋯π interactions with H⋯centroid distances between 2.53 and 2.67 Å and corresponding C⋯centroid distances between 3.425(9) and 3.593(10) Å (Fig. S8, ESI‡). The structure of 3CzBPz also showed some C–H⋯π interactions, with H⋯centroid distances of 2.69 Å and corresponding C⋯centroid distances of 3.6002(18) Å. This close packing mode is not observed in 2CzBPz.
Theoretical calculations
Optimization of the ground state geometries of the three emitters was carried out by DFT calculation at the PBE0/6-31G(d,p) level of theory, starting from the X-ray crystal structures.28,29 The Tamm–Dancoff approximation (TDA) to TD-DFT was applied to investigate the character of both the excited singlet and triplet states as well as to estimate ΔEST.28 The spin–orbital coupling matrix element (SOCME) values between excited states were obtained using PySOC based on the optimized triplet state geometries.30 The HOMO of 4CzPyz is distributed across the whole molecule while the LUMO is mainly localized on the pyrazine, whereas for 3CzBPz and 2CzBPz, the HOMOs are located on the carbazole moieties and the pyrazine, while LUMOs are delocalized on pyrazine and the biphenyl substituent. The HOMOs for the three emitters are predicted to be around −5.50 eV, while the LUMOs for 3CzBPz and 2CzBPz are destabilized to −1.62 eV and −1.60 eV, respectively, compared to −1.72 eV for 4CzPyz, which hints that the delocalization of the pyrazine weakens the electron-withdrawing character of the acceptor. The TDA-DFT calculations predict the three emitters to have similar, moderately large ΔEST values of around 0.40 eV. For 4CzPyz, the S1 and T1 are 3.09 eV and 2.56 eV, respectively, and the intermediate T2 (2.99 eV) is energetically close to S1 (Fig. 3). Moreover, the nature of T2 is of HLCT character and different from the CT dominant character of S1 and T1, which leads to a much higher SOCME value (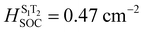 ) between T2 and S1 than 2 × 10−4 cm−2 for direct SOC between T1 and S1 (Fig. S1, ESI‡). The S1 and T1 of 3CzBPz are destabilized, respectively, to 3.17 eV and 2.65 eV, both of which show dominant CT character, while the T2 is 2.99 eV and T3 is degenerated to S1 at 3.19 eV, both possessing HLCT character. T3 and S1 exhibits the highest SOC with
) between T2 and S1 than 2 × 10−4 cm−2 for direct SOC between T1 and S1 (Fig. S1, ESI‡). The S1 and T1 of 3CzBPz are destabilized, respectively, to 3.17 eV and 2.65 eV, both of which show dominant CT character, while the T2 is 2.99 eV and T3 is degenerated to S1 at 3.19 eV, both possessing HLCT character. T3 and S1 exhibits the highest SOC with  of 1.21 cm−2 compared to 1.18 cm−2 and 0.45 cm−2 for
of 1.21 cm−2 compared to 1.18 cm−2 and 0.45 cm−2 for  and
and  , respectively (Fig. S2, ESI‡). The high SOC and small energy gap (0.02 eV) between S1 and T3 indicate that RISC in 3CzBPz can occur between T3 and S1via high-energy triplet state mechanism.18 The S1 and T1 of 2CzBPz are 3.18 eV and 2.67 eV, respectively, both of which show dominant CT character. The T2 and T3 of 2CzBPz are 3.01 eV and 3.28 eV, and the energy offset between S1/T2 (0.17 eV) or S1/T3 (0.10 eV) seems to reduce RISC processes involving these states greatly, with the ΔEST also too large, this compound is predicted to be fluorescent.
, respectively (Fig. S2, ESI‡). The high SOC and small energy gap (0.02 eV) between S1 and T3 indicate that RISC in 3CzBPz can occur between T3 and S1via high-energy triplet state mechanism.18 The S1 and T1 of 2CzBPz are 3.18 eV and 2.67 eV, respectively, both of which show dominant CT character. The T2 and T3 of 2CzBPz are 3.01 eV and 3.28 eV, and the energy offset between S1/T2 (0.17 eV) or S1/T3 (0.10 eV) seems to reduce RISC processes involving these states greatly, with the ΔEST also too large, this compound is predicted to be fluorescent.
Electrochemistry
Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements of 4CzPyz, 3CzBPz, and 2CzBPz were carried out in DCM with tetrabutylammonium hexafluorophosphate (n-Bu4NPF6) as the supporting electrolyte to estimate the HOMO and LUMO energies (Fig. 4). The CV traces of 4CzPyz, 3CzBPz, and 2CzBPz show irreversible oxidation waves with Epas of 1.16 V, 1.08 V and 1.07 V, respectively, which align with the corresponding oxidation potentials obtained from the DPV of Eox = 1.12 V, 1.07 V, and 1.08 V, respectively. 4CzPyz possesses a pseudo-reversible reduction wave with Epc of −1.80 V which aligns with the reduction potential Ered = −1.76 V obtained from the DPV, while no reduction waves are observed for 3CzBPz and 2CzBPz within the DCM solvent window. The corresponding HOMO levels of 4CzPyz, 3CzBPz and 2CzBPz inferred from the Eox values in the DPVs are −5.46 eV, −5.41 eV and −5.42 eV, respectively, which are slightly stabilized than those predicted from the DFT calculations yet match the calculated trend. The HOMO value of 4CzPyz (−5.46 eV) is slightly destabilized compared to the reported value of −5.59 eV in DCM.24 The LUMO level of 4CzPyz is −2.58 eV, which is moderately destabilized compared to the reported value of −2.83 eV in DCM,24 while the LUMO levels for 3CzBPz and 2CzBPz are calculated to be −2.47 eV and −2.45 eV, respectively, where these are inferred from the HOMO energies and the optical gaps (Eg), which themselves were determined from the intersection of the normalized absorption and fluorescence spectra in toluene (2.94 eV and 2.97 eV, Fig. S5d, ESI‡). The LUMOs values of 4CzPyz, 3CzBPz and 2CzBPz are moderately more stabilized than the values obtained from DFT calculations, yet follow the calculated trend.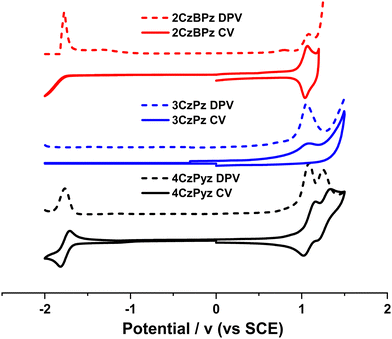 | ||
| Fig. 4 CV (solid lines) and DPV (dashed lines) for 4CzPyz, 3CzBPz, and 2CzBPz in degassed DCM solution containing [nBu4N]PF6 as the supporting electrolyte and using Fc/Fc+ as an external standard (Fc/Fc+ = 0.46 eV versus SCE,31 scan rate = 100 mV s−1). | ||
Photophysics
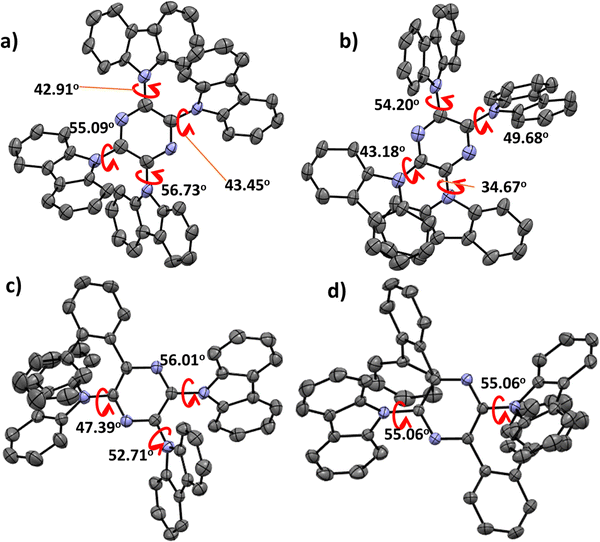
The UV-vis absorption spectra of 4CzPyz, 3CzBPz, and 2CzBPz in toluene are shown in Fig. 5. The profiles match closely to the TDA-DFT calculated absorption spectra (Fig. S4, ESI‡). The TDA-DFT calculations predict that the S1 of the three emitters should exhibit charge-transfer dominated hybrid transitions from HOMO to LUMO (Fig. S1–S3, ESI‡). 4CzPyz, 3CzBPz, and 2CzBPz all exhibit structured absorption around 450–380 nm. 4CzPyz showed the strongest hybrid CT absorption with molar absorptivity (ε) values of 2.4 × 104 M−1 cm−1 (425 nm) and 2.3 × 104 M−1 cm−1 (413 nm), while for 3CzBPz the absorption band is less intense and blue-shifted to 1.8 × 104 M−1 cm−1 (408 nm) and 1.7 × 104 M−1 cm−1 (398 nm). The absorption of 2CzBPz is further attenuated and blue-shifted, with ε of 0.9 × 104 M−1 cm−1 (401 nm) and 1.1 × 104 M−1 cm−1 (388 nm). The tendency of the ε values match the TDA-DFT results where the oscillator strengths (f) reduce from 0.37 for 4CzPyz to 0.22 and 0.05 for 3CzBPz and 2CzBPz, respectively. The three compounds exhibit structureless emission in toluene with λPL of 473 nm, 450 nm, and 440 nm for 4CzPyz, 3CzBPz, and 2CzBPz, respectively. The blue-shifted emission of 3CzBPz, and 2CzBPz are ascribed to the weaker character of the acceptors. The three emitters exhibit a moderately high ΦPL in degassed toluene, of 71%, 65%, and 64% for 4CzPyz, 3CzBPz, and 2CzBPz, respectively, which are slightly reduced to 66%, 63%, and 61% after exposure to oxygen. The S1 and T1 values were extracted from the onsets of the fluorescence and phosphorescence spectra, respectively, in 2-methyltetrahydrofuran (2-MeTHF) glass at 77 K (Fig. 5(b)–(d)). At 77 K, the three emitters exhibit broad and featureless fluorescence as well as phosphorescence spectra, which suggest that both of these states possess mainly CT character. The S1/T1 states for 4CzPyz, 3CzBPz, and 2CzBPz are measured to be 2.94/2.67 eV, 3.06/2.72 eV, and 3.10/2.72 eV, respectively, which match the tendency of TDA-DFT calculation, and the corresponding ΔEST values are 0.27 eV, 0.36 eV, and 0.38 eV, respectively.The time-resolved PL decays of these materials were measured in 10−5 M degassed toluene solution (Fig. 6(a)). 4CzPyz, 3CzBPz, and 2CzBPz exhibit prompt lifetime (τp) of 6.4 ns, 4.3 ns, and 6.8 ns, respectively; however, no delayed component was detected. The PL decay and oxygen insensitivity behavior of 4CzPyz, 3CzBPz and 2CzBPz in toluene indicate that in toluene these compounds behave as fluorophores only. As the TDA-DFT calculations indicated that a HLCT triplet exciton harvesting mechanism may be possible in 4CzPyz and 3CzBPz, we examined the effect of solvent polarity on the nature of the excited state (Fig. S5, ESI‡) by undertaking a Lippert–Mataga study for each of the three emitters. All materials showed emission that was insensitive to polarity changes in low-polarity solvents, such as cyclohexane, and showed broadened emission in high-polarity solvents such as acetonitrile and methanol. The behaviour is a hallmark of compounds with low-lying HLCT excited states. The Stokes shift versus the orientation polarizability (Δf) plots as well as the electric dipole moment (μe) of three emitters are presented in Fig. 6(b)–(d). Notably, the Lippert–Mataga plots of three emitters each show two regions where there is a linear relationship between solvent polarity and Stokes shift, indicative of compounds possessing excited states of mixed LE/CT character. The Lippert–Mataga plots reveal that the three emitters show increased CT character in high polarity solvents while in low polarity solvents the S1 states possess more significant LE character. The smaller degree of red-shifting of the emission spectra and smaller μe in 2CzBPz suggest that there is greater LE character to the S1 state in this compound than in 3CzBPz and 4CzPyz.
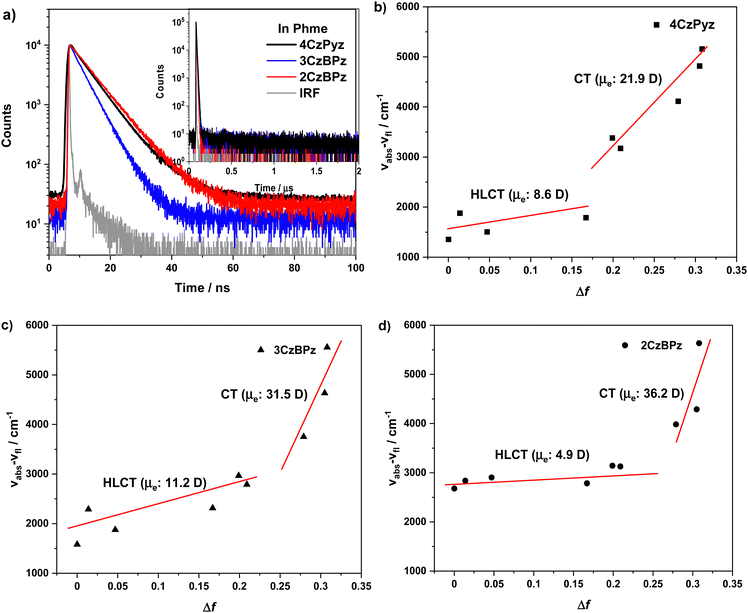 | ||
| Fig. 6 (a) transient PL decay curves of three emitters in toluene (λexc = 379 nm), and solvatochromic Lippert–Mataga plots for (b) 4CzPyz, c() 3CzBPz, and 2CzBPz. | ||
With a view to using these compounds as emitters in OLEDs, we then investigated the photophysics of the three emitters in solid-state matrices. The photoluminescence spectra of three emitters are insensitive to the medium, with λPL of 486 nm, 463 nm, and 446 nm for 4CzPyz, 3CzBPz and 2CzBPz (Fig. 7(a)), which are only modestly red-shifted compared to the corresponding λPL of 473 nm, 450 nm, and 440 nm in toluene, respectively. The highest ΦPL value for 4CzPyz was achieved in 10 wt% doped PPT film at 75%, which is significantly higher than the 36% previously reported in the 10 wt% doped DPEPO film.24 The ΦPL values for 3CzBPz and 2CzBPz in PPT are 56%, and 38%, respectively, which are slightly lower than corresponding values in DPEPO (Fig. 7(b)). The tendency of the ΦPL of the three emitters match the TDA-DFT calculated trend in oscillator strength.
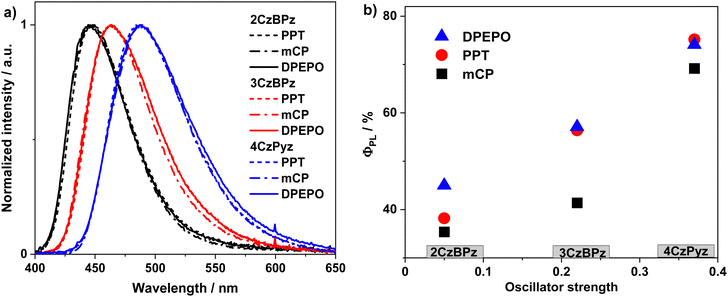 | ||
| Fig. 7 (a) Emission spectra and (b) ΦPL of 4CzPyz, 3CzBPz, and 2CzBPz in 10 wt% doped hosts (λexc = 310 nm). | ||
The three emitters in 10 wt% doped PPT films exhibit unstructured emission, with FWHM of 64 nm, 63 nm and 44 nm for 4CzPyz, 3CzBPz and 2CzBPz, respectively, and the phosphorescence is of dominant CT character with broader (FWHM ∼ 120 nm) and featureless emission (Fig. 7). The calculated ΔEST values of the three emitters determined from the onsets of the prompt fluorescence and phosphorescence spectra, are 0.23 eV (same value as previously reported in DPEPO),24 0.29 eV, and 0.34 eV for 4CzPyz, 3CzBPz and 2CzBPz (Fig. 8), respectively.
The room temperature time-resolved PL decays in 10 wt% doped PPT films are shown in Fig. 9. The prompt fluorescence decays of the three emitters show bi-exponential kinetics with τavg of 5.9, 5.1, and 7.6 ns for 4CzPyz, 3CzBPz and 2CzBPz (Fig. 9(a)), respectively. The doped films of 4CzPyz and 3CzBPz show delayed emission, with delayed lifetimes, τd (Fig. 9(b)) of 150 μs for 4CzPyz (the lifetime reported in the literature was 174 μs),24 and 929 μs for 3CzBPz. The intensity of the delayed emission increases with increasing temperature (Fig. S6, ESI‡). The smaller ΔEST and the temperature dependence of the delayed component from 4CzPyz indicates that the compound in a 10 wt% doped film in PPT shows TADF. The weak TADF of the 3CzBPz doped film in PPT film can also be triggered with the assistance of higher-energy triplet excited states despite the larger ΔEST of 0.29 eV. However, there is no indication of TADF in 2CzBPz due to the too large ΔEST of 0.34 eV.
 | ||
| Fig. 9 Transient PL decay curves of 4CzPyz, 3CzBPz, and 2CzBPz in 10 wt% doped PPT films in (a) 100 ns time window and (b) 8 ms time window (λexc = 378 nm). | ||
Electroluminescence properties
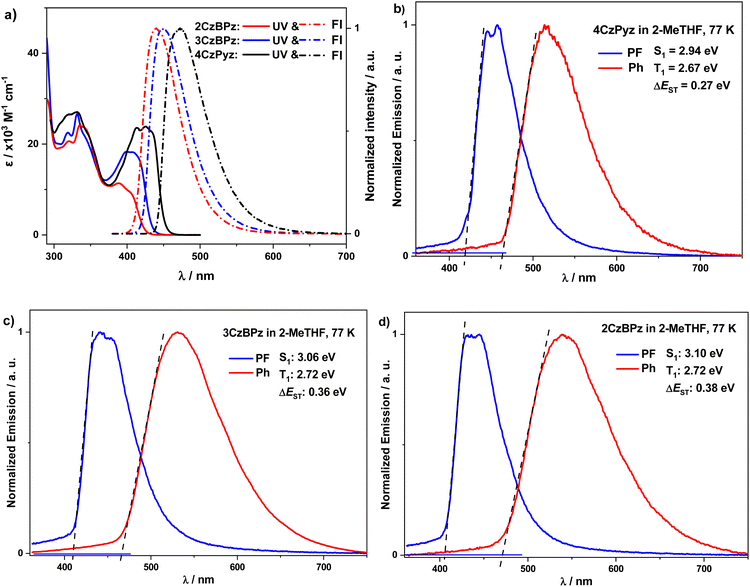
Next, we investigated the electroluminescence properties of these three emitters. Multilayer OLEDs were fabricated using these materials as dopants within the emissive layer, with the following device structures: ITO/HATCN (5 nm)/TAPC (40 nm)/TCTA (10 nm)/mCP (10 nm)/PPT: emitter (10 wt%) (30 nm)/PPT (10 nm)/TmPyPB (40 nm)/LiF (0.8 nm) Al (100 nm). Here, HATCN acts as the hole injection layer, TAPC and TCTA are used as hole transporting layers, and mCP is the exciton blocking layer. PPT was selected as the host material due to the good carrier transport ability and the high ΦPL values of the three emitters in 10 wt% doped films, and TmPyPB is the electron-transporting material. The performance of the devices is summarized in Fig. 10. The devices based on the three emitters exhibit turn on voltage (Von) at 3.0 V, and the maximum luminance (Lummax) exceeded 104 cd m−2. The OLEDs based on 4CzPyz exhibit sky blue emission with λEL of 486 nm, and CIE coordinates of (0.20, 0.36). Thanks to the high ΦPL and TADF character of 4CzPyz in PPT host (Fig. 7(b) and 9(b)), a maximum EQE (EQEmax) of 24.1% is achieved at 3 cd m−2, which is much higher than our previously reported pyrazine-based TADF emitter DTCz-Pz (EQEmax: 11.6% at 2 cd m−2). The EQE reduced to 7.0% and 2.6% at 100 cd m−2 and 1000 cd m−2, respectively. For OLEDs with 3CzBPz, blue emission is achieved with λEL of 464 nm and CIE coordinates of (0.16, 0.22). The EQEmax for the device with 3CzBPz reached 9.6% at 1 cd m−2 and this reduced to 3.1% and 2.0% at 100 cd m−2 and 1000 cd m−2, respectively. The severe efficiency roll-off of the devices with 4CzPyz and 3CzBPz are ascribed to the relatively larger ΔEST values and thus longer triplet exciton lifetimes, a correlation that has also been noted in previously reported pyrazine- or pyrimidine-based blue emitters (Table S3, ESI‡).12,32–34The EQE is a product of four parameters and can be expressed as:
| EQE = γΦFηS/Tηout | (1) |
Conclusions
We investigated the excited state dynamics, photophysical properties, and exciton utilization mechanism in OLEDs of three pyrazine-based multi-carbazole containing emitters through a combined, detailed theoretical and experimental study. When four carbazoles are connected to the pyrazine core, the emitter 4CzPyz exhibits a moderate ΔEST of 0.23 eV, high ΦPL of 74% and TADF character (τp: 5.9 ns, τd: 150 μs) in PPT film. The device based on 4CzPyz shows sky-blue emission (λEL = 486 nm) with EQEmax of 24.1% thanks to the highly efficient exciton utilization by TADF mechanism. When one carbazole is replaced by an ortho-biphenyl, the ΔEST of 3CzBPz is increased to 0.29 eV, ΦPL decreases to 57%, and the TADF character is lost in PPT film. However, the photophysical properties and DFT calculations support that 3CzBPz can harvest triplet excitons via a higher-energy triplet state channel mechanism. This is reflected also in the OLED performance where the device shows an EQEmax of 9.6% at λEL of 464 nm; indeed, 86% of the excitons are estimated to be converted into light in the OLED based on 3CzBPz. When two ortho-biphenyl groups are connected to the pyrazine core, the ΔEST of 2CzBPz is further increased to 0.34 eV and ΦPL is reduced to 45% in PPT film. The calculations and photophysics indicate 2CzBPz to be a deep blue fluorescence emitter, and the OLEDs based on 2CzBPz exhibits an EQEmax 3.2% with λEL of 446 nm. These results show the critical role of a suitably aligned LE triplet state in modulating the nature of the exciton harvesting mechanism, leading to dramatically different efficiencies in the OLEDs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The St Andrews team would also like to thank EPSRC (EP/P010482/1 and EP/L017008/1) for financial support. EZ-C is a Royal Society Leverhulme Trust Senior Research Fellow (SRF\R1\201089). D.C thanks the China Postdoctoral Science Foundation (No. 2022TQ0227).References
- C. M. Marian, Annu. Rev. Phys. Chem., 2020, 72, 617–640 CrossRef PubMed.
- T. J. Penfold, E. Gindensperger, C. Daniel and C. M. Marian, Chem. Rev., 2018, 118, 6975–7025 CrossRef CAS PubMed.
- R. Ieuji, K. Goushi and C. Adachi, Nat. Commun., 2019, 10, 13044 Search PubMed.
- Y.-Z. Shi, H. Wu, K. Wang, J. Yu, X.-M. Ou and X.-H. Zhang, Chem. Sci., 2022, 13, 3625–3651 RSC.
- J. Teng, Y. Wang, C. Chen, J. Teng and Y. Wang, J. Mater. Chem. C, 2020, 8, 11340–11353 RSC.
- X. K. Chen, D. Kim and J. L. Brédas, Acc. Chem. Res., 2018, 51, 2215–2224 CrossRef CAS PubMed.
- P. L. dos Santos, D. Chen, P. Rajamalli, T. Matulaitis, D. B. Cordes, A. M. Z. Slawin, D. Jacquemin, E. Zysman-Colman and I. D. W. Samuel, ACS Appl. Mater. Interfaces, 2019, 11, 45171–45179 CrossRef CAS PubMed.
- D. Sun, E. Duda, X. Fan, R. Saxena, M. Zhang, S. Bagnich, X. Zhang, A. Köhler and E. Zysman-Colman, Adv. Mater., 2022, 34, 2110344 CrossRef CAS PubMed.
- P. Xiao, J. Huang, Y. Yu, J. Yuan, D. Luo, B. Liu and D. Liang, Appl. Sci., 2018, 8, 1–27 CAS.
- F. B. Dias, K. N. Bourdakos, V. Jankus, K. C. Moss, K. T. Kamtekar, V. Bhalla, J. Santos, M. R. Bryce and A. P. Monkman, Adv. Mater., 2013, 25, 3707–3714 CrossRef CAS PubMed.
- Q. Zhang, J. Li, K. Shizu, S. Huang, S. Hirata, C. Adachi, P. C. Adachi, Q. Zhang, J. Li, K. Shizu, S. Hirata and H. Miyazaki, J. Am. Chem. Soc., 2012, 134, 14706–14709 CrossRef CAS PubMed.
- P. Rajamalli, D. Chen, S. M. Suresh, Y. Tsuchiya, C. Adachi and E. Zysman-Colman, Eur. J. Org. Chem., 2021, 2285–2293 CrossRef CAS.
- L. S. Cui, H. Nomura, Y. Geng, J. U. K. Kim, H. Nakanotani and C. Adachi, Angew. Chem., Int. Ed., 2017, 56, 1571–1575 CrossRef CAS PubMed.
- G. Sun, X. H. Wang, J. Li, B. T. Yang, Y. Gao and Y. Geng, Sci. Rep., 2021, 11, 17686–17688 CrossRef CAS PubMed.
- T. Liu, X. Chen, J. Zhao, W. Wei, Z. Mao, W. Wu, S. Jiao, Y. Liu, Z. Yang and Z. Chi, Chem. Sci., 2021, 12, 5171–5176 RSC.
- D. Hu, L. Yao, B. Yang and Y. Ma, Philos. Trans. R. Soc., A, 2015, 373, 20140318 CrossRef PubMed.
- Y. Xu, P. Xu, D. Hu and Y. Ma, Chem. Soc. Rev., 2021, 50, 1030–1069 RSC.
- N. Sharma, M. Y. Wong, D. Hall, E. Spuling, F. Tenopala-Carmona, A. Privitera, G. Copley, D. B. Cordes, A. M. Z. Slawin, C. Murawski, M. C. Gather, D. Beljonne, Y. Olivier, I. D. W. Samuel and E. Zysman-Colman, J. Mater. Chem. C, 2020, 8, 3773–3783 RSC.
- M. A. El-Sayed, J. Chem. Phys., 1963, 38, 2834–2838 CrossRef CAS.
- H. Noda, H. Nakanotani and C. Adachi, Sci. Adv., 2018, 4, eaao6910 CrossRef PubMed.
- M. K. Etherington, J. Gibson, H. F. Higginbotham, T. J. Penfold and A. P. Monkman, Nat. Commun., 2016, 7, 13680 CrossRef CAS PubMed.
- H. Noda, X. Chen, H. Nakanotani, T. Hosokai, M. Miyajima, N. Notsuka, Y. Kashima, J. Brédas and C. Adachi, Nat. Mater., 2019, 18, 1084–1090 CrossRef CAS PubMed.
- J. U. Kim, I. S. Park, C. Chan, M. Tanaka, H. Nakanotani and C. Adachi, Nat. Commun., 2020, 11, 1765 CrossRef CAS PubMed.
- L. Salah, M. K. Etherington, A. Shuaib, A. Danos, A. A. Nazeer, B. Ghazal, A. Prlj, A. T. Turley, A. Mallick, P. R. McGonigal, B. F. E. Curchod, A. P. Monkman and S. Makhseed, J. Mater. Chem. C, 2021, 9, 189–198 RSC.
- S. J. Woo, Y. H. Ha, Y. H. Kim and J. J. Kim, J. Mater. Chem. C, 2020, 8, 12075–12084 RSC.
- T. Ishiyama, M. Murata and N. Miyaura, J. Org. Chem., 1995, 60, 7508–7510 CrossRef CAS.
- L. Salah, M. K. Etherington, A. Shuaib, A. Danos, A. A. Nazeer, B. Ghazal, A. Prlj, A. T. Turley, A. Mallick, P. R. McGonigal, B. F. E. Curchod, A. P. Monkman and S. Makhseed, J. Mater. Chem. C, 2021, 9, 189–198 RSC.
- J. A. Pople, J. S. Binkley and R. Seeger, Int. J. Quantum Chem., 1976, 10, 1–19 CrossRef CAS.
- C. Adamo, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- X. Gao, S. Bai, D. Fazzi, T. Niehaus, M. Barbatti and W. Thiel, J. Chem. Theory Comput., 2017, 13, 515–524 CrossRef CAS PubMed.
- N. G. Connelly and W. E. Geiger, Chem. Rev., 1996, 96, 877–910 CrossRef CAS PubMed.
- J. Liu, K. Zhou, D. Wang, C. Deng, K. Duan, Q. Ai and Q. Zhang, Front. Chem., 2019, 7, 1–9 CrossRef CAS PubMed.
- M. Cai, M. Auffray, D. Zhang, Y. Zhang, R. Nagata, Z. Lin, X. Tang, C.-Y. Chan, Y.-T. Lee, T. Huang, X. Song, Y. Tsuchiya, C. Adachi and L. Duan, J. Chem. Eng., 2021, 420, 127591 CrossRef CAS.
- T. Serevičius, J. Dodonova, R. Skaisgiris, D. Banevičius, K. Kazlauskas, S. Juršėnas and S. Tumkevičius, J. Mater. Chem. C, 2020, 8, 11192–11200 RSC.
- M. K. Manna, S. Shokri, G. P. Wiederrecht, D. J. Gosztola and A. J. L. Ayitou, Chem. Commun., 2018, 54, 5809–5818 RSC.
Footnotes |
| † The research data supporting this publication can be accessed at https://doi.org/10.17630/b06557de-c785-4860-bb9e-2d299b2a3eec |
| ‡ Electronic supplementary information (ESI) available. CCDC 2280783–2280785. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3tc02463f |
| This journal is © The Royal Society of Chemistry 2023 |