Open Access Article
Open Access ArticleAchieving excellent thermostable red emission in singly Mn2+-doped near-zero thermal expansion (NZTE) material Li2Zn3(P2O7)2†
Qin
Liu
abc,
Peipei
Dang
*a,
Guodong
Zhang
ab,
Maxim S.
Molokeev
de,
Sergey P.
Polyutov
 d,
Hongzhou
Lian
a,
Ziyong
Cheng
a,
Guogang
Li
d,
Hongzhou
Lian
a,
Ziyong
Cheng
a,
Guogang
Li
 *cf and
Jun
Lin
*cf and
Jun
Lin
 *ab
*ab
aState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Science, Changchun 130022, P. R. China. E-mail: ppdang@ciac.ac.cn; jlin@ciac.ac.cn
bSchool of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei 230026, P. R. China
cFaculty of Materials Science and Chemistry, China University of Geoscience, Wuhan 430074, P. R. China. E-mail: ggli@cug.edu.cn
dInternational Research Center of Spectroscopy and Quantum Chemistry—IRC SQC, Siberian Federal University, Krasnoyarsk, 660041, Russia
eLaboratory of Crystal Physics, Kirensky Institute of Physics, Federal Research Center KSC SB RAS, Krasnoyarsk, 660036, Russia
fZhejiang Institute, China University of Geosciences, Hangzhou, 311305, P. R. China
First published on 6th July 2023
Abstract
The design of thermostable phosphor is still a pivotal challenge in pc-WLED applications. Herein, an efficient strategy is proposed to design excellent thermostable red emission in the singly Mn2+-doped near-zero thermal expansion (NZTE) material Li2Zn3(P2O7)2. Under the excitation of 412 nm wavelength, the emission could be tuned from 636 to 672 nm by increasing the Mn2+-doping level via synthetic effects among the crystal field, the exchange coupling interaction in Mn–Mn dimers, and energy transfer in different luminescence centers. The PL intensity of LZPO:Mn2+ could be maintained at 97% at 150 °C and 94% at 200 °C of its initial intensity at room temperature. During the heating process, LZPO presented near-zero thermal expansion, contributing to the nearly unaffected PL intensity. The emission loss was also compensated by trap-assisted energy transfer to the luminescent center. This study not only offers a perspective idea for elucidating the correlation between the crystal structure and optical properties, but also opens a new way of designing excellent thermostable luminescent materials based on NZTE materials in self-reduction systems.
1. Introduction
With regard to the exceptional advantages of high color rendering, long service lifetime, low energy consumption, eco-friendliness, fast response, phosphor-converted white-light-emitting diodes (pc-WLEDs) play an important role in many areas, such as solid-state lighting, backlight displays, and information security.1–6 Phosphors are one of the indispensable components in pc-WLED devices, and their photoluminescence (PL) thermal stability plays a crucial role in practical applications, which is usually evaluated by the thermal quenching (TQ) behavior.7–10 Thermal quenching (TQ) is caused by the anabatic non-radiative relaxation of electrons from the excited state to the ground state at high working temperature.11–14 It shortens the service lifetime of pc-WLED devices and restrains the widespread use of phosphors. Hence, it is necessary to explore luminescent materials with excellent thermal stability. However, the design of novel phosphors with excellent thermal stability is still a significant challenge. To date, much effort has been devoted to designing different strategies to solve the emission loss with increasing temperature. For example, defect engineering is a normal approach to achieve excellent PL thermal stability, such as in blue-emitting phosphors Na3Sc2(PO4)3:Eu2+,1 yellow-emitting phosphors LiZnPO4:xMn2+,15 orange-red phosphors β-KMg(PO3)3:Mn2+,16 and red-emitting phosphors BaXP2O7:Mn2+(X = Mg/Zn),17 NaZn(PO3)3:Mn2+.18 However, the precise control of defect position in the matrix lattice is still a challenge. Otherwise, energy transfer between the traps (or sensitizers) and the luminescence centers (activators) helps to gain excellent thermostable emission, such as in SrGa2Si2O8:Mn2+,19 BaMgP2O7: Eu2+, Mn2+,20 Mg3Y2Ge3O12:Eu3+, Mn4+,21 and Ca2LuZr2Al3O12:Cr3+,Yb3+.22 The efficiency of energy transfer is ultimately determined by the crystal structure, whereby the matrix should have suitable sites for both sensitizers and activators. Structural modulation can also be effective for enhancing PL thermal stability, such as in (Zn,Mg)B2O4:Mn2+,23 (Sr,Ca)AlSiN3:Eu2+,24 and Ca(Sc,Mg)(Al,Si)O6:Cr3+.25 Nevertheless, the luminescence properties of luminescent ions are influenced by the radius size, charge, and electronegativity of the matrix cations. In addition, choosing an ideal host with specific performance is an efficient way to achieve high PL thermal stability for luminescent ions. Many cases have been successfully reported, such as the zero thermal expansion (ZTE) material Zn4B6O13:Mn2+ phosphor,26 negative thermal expansion (NTE) materials Sc2W3O12:Eu3+,27 Cs3GdGe3O9:Eu3+,28 and Sc2(MoO4)3:Yb3+/Er3+,29 and layered structural design of MgIn2P4O14:Tm3+,Dy3+,30 and SrIn2P4O14:Tm3+,Dy3+, and so on.31In this study, we synthesized a sequence of red-emitting Li2Zn3−x(P2O7)2:xMn2+ phosphors (abbreviated as LZPO:xMn2+) by a high-temperature solid-state reaction method at ambient atmosphere. The compound Li2Zn3(P2O7)2 was chosen as an ideal matrix material because of its outstanding structural stability. The rigid anionic groups [P2O7]4− were constructed to protect the Mn2+ ions from being oxidized at high temperature, which lay the foundation for the little TQ. At 150 °C, the PL intensity of the LZPO:Mn2+ red phosphor was 97% of that at room temperature, and even at 200 °C, the PL intensity of the LZPO:Mn2+ red phosphor was still 94%, demonstrating its excellent PL thermal stability. The corresponding kinetic mechanisms are discussed in detail via experimental analysis. Based on the Rietveld refinement method, the crystal structure was elucidated. The emission could be tuned from 636 to 672 nm by increasing the Mn2+-doping level, and the corresponding luminescence mechanism was explored. These results indicate that the LZPO:Mn2+ phosphor could be a promising material in applications of high-power (HP) pc-WLEDs. This paper not only offers a perspective idea to reveal the correlation between the crystal structure and optical properties, but also initiates a new avenue of research for designing excellent thermostable luminescent materials based on NZTE materials.
2. Experimental
2.1. Materials and preparation
The as-prepared LZPO:xMn2+ (x = 0–0.5) phosphors were synthesized by a conventional high-temperature solid-state method under an air atmosphere. Li2CO3 (97%, A.R.), ZnO (≥99.0%, BEIJING SHIJI), MnO2 (≥99.9%, Alfa Aesar), MnCO3 (≥99.9%, Alfa Aesar), and NH4H2PO4 (≥99.0%, BEIJING SHIJI) were used as the initial chemicals without further purification according to the stoichiometric ratios. The weighed mixtures were transferred to an agate mortar and ground thoroughly for 30 min. The samples were then put into an alumina crucible and sintered at 700 °C for 10 h, and cooled to room temperature. By an additional grinding, a fine powder was obtained for further characterization. Additionally, a series of LZPO:0.01Mn2+ phosphors samples with different Mn sources (MnCO3, MnO2) were synthesized under an air atmosphere and under a reduced atmosphere of N2 (90%)–H2 (10%) in the same way.2.2. Characterizations
The phase purity was identified using X-ray diffraction (XRD) on a Bruker D8 ADVANCE powder diffractometer with Cu Kα radiation (λ = 1.54 Å) under 40 kV and 40 mA at room temperature. The scanning rate was 0.6° min−1. XRD Rietveld profile refinements of the structural models were performed by using TOPAS 4.2. Diffuse reflectance (DR) measurements were recorded on the UV-visible-diffuse reflectance spectroscopy system UV-3600i Plus (Shimadzu Corporation, Japan). X-Ray photoelectron spectroscopy (XPS) was performed using an XPS microprobe system (Thermo SCIENTIFIC ESCALAB 250Xi). Electron paramagnetic resonance (EPR) spectra of the samples were obtained on a Bruker A300 spectrometer with the X-band (9.84 GHz) at room temperature. Thermoluminescence (TL) glow curves were measured using a TL spectroscopy system (TOSL-3DS) at a heating rate of 0.2 K s−1. Photoluminescence excitation (PLE) and photoluminescence emission (PL) spectra were obtained on an Edinburgh Instruments FLSP-920 fluorescence spectrophotometer equipped with a 150 W xenon lamp as the excitation source. The temperature-dependent PL spectra were evaluated with the same instrument with a temperature controller. The room temperature PL lifetime decay curves of the as-prepared samples were recorded on the same spectrometer with 412 nm wavelength laser radiation as the excitation source. Photoluminescence quantum yield (PLQY) values were collected on an absolute PL quantum yield measurement system (C11347-11, Japan). The LED devices were fabricated by mixing commercial BaMgAl11O17:Eu2+ (BAM:Eu2+) blue phosphor, (Ba,Sr)2SiO4:Eu2+ green phosphor, the as-prepared LZPO:0.1Mn2+ phosphor, and a commercial 365 nm UV chip. Luminescence measurements of the as-fabricated pc-WLED devices were carried out at 3.16 V voltage and at a 20–300 mA driving current. The electroluminescence (EL) performance of the pc-WLED devices were measured on a HAAS 2000 photoelectric measuring system (380–1100 nm, EVERFINE, China) with an integrating sphere.3. Results and discussion
3.1. Phase identification and crystal structure analysis
Fig. 1a shows the crystal structure of Li2Zn3(P2O7)2, which belongs to an orthorhombic phase with the space group Pbcm (57).32 In this structure, there are two symmetry-independent sites for Zn atoms. Each Zn atom coordinates with five O atoms to form [ZnO5] trigonal bipyramids. The Zn1 site is completely occupied, while the Zn2 site is occupied by Zn2+ and Li+ cations, and the Zn2+/Li+ ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Half of the Li+ cations are disordered at the partially occupied Zn2 positions. The other half are coordinated with four O atoms and at the interstitial positions. The two completely occupied [Zn1O5] and the partially occupied [Zn2O5] trigonal bipyramids are connected by a common edge to form [Zn2O8] dimere. These [Zn2O8] dimers alternately share edges to form [ZnO5] chains running along the c axis. The three-dimensional framework structure of Li2Zn3(P2O7)2 is formed by [ZnO5] chains sharing tetrahedra vertices with [P2O7] dimers. In order to analyze the phase structure, the X-ray diffraction (XRD) patterns of LZPO:xMn2+ (x = 0, 0.01, 0.05, 0.1, 0.2, 0.3, 0.5) phosphors and the standard card (ICSD#260280) of Li2Zn3(P2O7) were compared and are shown in Fig. 1b. The as-prepared LZPO:xMn2+ phosphors could be well indexed as pure phases according to the standard card, indicating that the incorporation of Mn2+ into the LZPO lattice had no effect on the phase purity. For getting more detailed information, Rietveld refinements of the LZPO:xMn2+ (x = 0, 0.01, 0.1, 0.2, 0.3) samples were performed (Fig. 1c and Fig S1a–d, ESI†). Tables S1–S3 (ESI†) list the refined structural parameters and cell parameter values. The LZPO host yielded a = 5.18166 (14) Å, b = 13.1903 (3) Å, c = 16.1267 (4) Å, α = γ = β = 90°, V = 1102.22 (5) Å3, and Rp = 4.08%, Rwp = 5.34%, χ2 = 2.5. As for the LZPO:0.1Mn2+ sample, a = 5.18097 (18) Å, b = 13.1996 (4) Å, c = 16.1532 (5) Å, α = γ = β = 90°, V = 1104.66 (6) Å3, and Rp = 4.43%, Rwp = 5.99%, χ2 = 2.51. Owing to having the same valence and a similar ionic radius, the Mn2+ (r = 0.75 Å, CN = 5) ions prefer to occupy the 5-coordination Zn2+ (r = 0.68 Å, CN = 5) sites in the lattice. As shown in Fig. 1d, the cell parameters and lattice volume showed a good linear increase versus the doping level x, which was caused by the replacement of the larger Mn2+ ions for Zn2+ ions. These results imply the synthesis of single Mn2+-doped single-phase LZPO:xMn2+ materials and the successful integration of Mn2+ ions into the lattice.
1. Half of the Li+ cations are disordered at the partially occupied Zn2 positions. The other half are coordinated with four O atoms and at the interstitial positions. The two completely occupied [Zn1O5] and the partially occupied [Zn2O5] trigonal bipyramids are connected by a common edge to form [Zn2O8] dimere. These [Zn2O8] dimers alternately share edges to form [ZnO5] chains running along the c axis. The three-dimensional framework structure of Li2Zn3(P2O7)2 is formed by [ZnO5] chains sharing tetrahedra vertices with [P2O7] dimers. In order to analyze the phase structure, the X-ray diffraction (XRD) patterns of LZPO:xMn2+ (x = 0, 0.01, 0.05, 0.1, 0.2, 0.3, 0.5) phosphors and the standard card (ICSD#260280) of Li2Zn3(P2O7) were compared and are shown in Fig. 1b. The as-prepared LZPO:xMn2+ phosphors could be well indexed as pure phases according to the standard card, indicating that the incorporation of Mn2+ into the LZPO lattice had no effect on the phase purity. For getting more detailed information, Rietveld refinements of the LZPO:xMn2+ (x = 0, 0.01, 0.1, 0.2, 0.3) samples were performed (Fig. 1c and Fig S1a–d, ESI†). Tables S1–S3 (ESI†) list the refined structural parameters and cell parameter values. The LZPO host yielded a = 5.18166 (14) Å, b = 13.1903 (3) Å, c = 16.1267 (4) Å, α = γ = β = 90°, V = 1102.22 (5) Å3, and Rp = 4.08%, Rwp = 5.34%, χ2 = 2.5. As for the LZPO:0.1Mn2+ sample, a = 5.18097 (18) Å, b = 13.1996 (4) Å, c = 16.1532 (5) Å, α = γ = β = 90°, V = 1104.66 (6) Å3, and Rp = 4.43%, Rwp = 5.99%, χ2 = 2.51. Owing to having the same valence and a similar ionic radius, the Mn2+ (r = 0.75 Å, CN = 5) ions prefer to occupy the 5-coordination Zn2+ (r = 0.68 Å, CN = 5) sites in the lattice. As shown in Fig. 1d, the cell parameters and lattice volume showed a good linear increase versus the doping level x, which was caused by the replacement of the larger Mn2+ ions for Zn2+ ions. These results imply the synthesis of single Mn2+-doped single-phase LZPO:xMn2+ materials and the successful integration of Mn2+ ions into the lattice.
3.2. Optical properties of LZPO:xMn2+ (x = 0.01–0.5) phophors
Fig. 2a displays the diffuse reflectance spectra of the LZPO host and LZPO:0.1Mn2+, and photoluminescence excitation (PLE) spectrum of LZPO:0.1Mn2+, respectively. The energy absorption of less than 230 nm came from the ultraviolet (UV) absorption of the matrix.As for LZPO:0.1Mn2+, the absorption of 270 nm was derived from the charge-transfer band of O2−–Mn2+. Another absorption band ranging from 400 to 450 nm was noted, originating from the d–d transition of Mn2+.33,34 In addition, there was a weak valley in the wavelength of about 600 nm in the diffuse reflection spectra of the LZPO:xMn2+ (x = 0.05, 0.1, 0.3) phosphors (Fig. S2, ESI†). All the samples showed the same valley due to the small number of high valence states of Mn, which was caused by the limited self-reduction ability of Mn2+ in the air condition. According to the literature research, this weak valley is similar to the absorption of 4A2 → 4T2 for Mn4+,35,36 and the absorption of 3A2 → 3T1 (3F) for Mn5+.37–39 The optical band gap of the undoped LZPO sample can be calculated by utilizing the Kubelka–Munk equations (see the inset of Fig. 2a):40,41
 | (1) |
| F(R) = (1 − R2)/2R | (2) |
 | (3) |
With increasing the doping level, the PL intensity first increased, and then decreased due to the concentration quenching effect (Fig. 2c). The optimal doping concentration was at x = 0.2. The internal quantum efficiency (IQE) of the LZPO:0.2Mn2+ sample was 53.6% under 416 nm excitation. The emission peak changed from 636 nm at x = 0.01 to 672 nm at x = 0.3, and then remains unchanged at the higher doping level of x = 0.5.
The PL decay curves of LZPO:xMn2+ were tested under 412 nm wavelength excitation to further investigate the luminescence performance. They could be fitted by a second-order exponential function:45–48
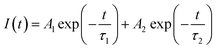 | (4) |
 | (5) |
As the Mn2+ concentration singly increased, the lifetimes of τ1, τ2, and τ for LZPO:xMn2+ continuously decreased from 20.29 to 4.76 ms, 41.72 to 18.35 ms, and 40.02 to 16.12 ms, respectively. The lifetimes of τ1, τ2, and τ showed the same variation trend, which were caused by the enhanced interaction between the Mn2+ ions at a high doping level (Fig. 2d and Table S4, ESI†).
To analyze the luminescence centers of Mn2+ ions, the PL spectra of LZPO:xMn2+ phosphors were fitted by the Gaussian method and the corresponding lifetimes were measured (Table S5, ESI†). The broad-band red emission of LZPO:xMn2+ consisted of two emission peaks (Fig. 3a–c and Fig. S4a–c, ESI†), indicating that the Mn2+ ions occupied two different sites. One peak was in the red region (named P1), while the other was in the deep-red region (named P2). The XPS spectrum of the representative LZPO:0.1Mn2+ phosphor (Fig. S5, ESI†) revealed photoelectron peaks corresponding to Li 1s, P 2p, O 1s, Zn 2p3/2, Zn 2p1/2, and Mn 2p3/2. The detailed high-resolution XPS spectrum and fitting results of the LZPO:0.1Mn2+ phosphor with Mn 2p3/2 are exhibited in Fig. 3d. The peak at 641.5 eV belonged to the 2p3/2 of Mn2+, and peak at 643.5 eV belonged to the 2p3/2 of Mn4+, which further confirmed that the luminescence in this study originated from Mn2+ ions.49 The self-reduction of Mn4+ to Mn2+ in the air condition was confirmed by the XPS analysis. When Mn2+ is doped into the LZPO matrix lattice and sintered in the air condition, it will be oxidized to Mn4+ in the beginning. In order to keep the charge balance, one Mn4+ will replace two Zn2+ sites, leading to the formation of a defect with two positive charges. While a vacancy 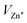 is produced with two negative charges at the nearest position. Finally, the two negative charges will be transferred to the Mn4+ position to reduce it to Mn2+. As discussed before, Mn2+ ions prefer to occupy Zn sites in the lattice. Therefore, P1 in the red region was ascribed to the 4T1(4G) → 6A1(6S) transition of the luminescence center of [MnO5] at Zn sites. From the local structure of the [Zn2O8] dimers in Fig. 3e, the distances between Zn1–Zn1, Zn2–Zn2, Zn1–Zn2 were 3.1995 (4), 2.857 (1), and 3.2500 (7) Å, respectively. When the Mn2+ ions are doped into the lattice, Mn2+ ions will replace Zn2+ ions to form Mn–Mn dimers due to the short Zn–Zn distances, which were less than 5 Å.45 The broad emission P2 should likelyoriginate from the Mn–Mn dimer (named [Mn2O8]) formed by both Mn2+ ions replacing Zn–Zn sites due to the shorter distance.
is produced with two negative charges at the nearest position. Finally, the two negative charges will be transferred to the Mn4+ position to reduce it to Mn2+. As discussed before, Mn2+ ions prefer to occupy Zn sites in the lattice. Therefore, P1 in the red region was ascribed to the 4T1(4G) → 6A1(6S) transition of the luminescence center of [MnO5] at Zn sites. From the local structure of the [Zn2O8] dimers in Fig. 3e, the distances between Zn1–Zn1, Zn2–Zn2, Zn1–Zn2 were 3.1995 (4), 2.857 (1), and 3.2500 (7) Å, respectively. When the Mn2+ ions are doped into the lattice, Mn2+ ions will replace Zn2+ ions to form Mn–Mn dimers due to the short Zn–Zn distances, which were less than 5 Å.45 The broad emission P2 should likelyoriginate from the Mn–Mn dimer (named [Mn2O8]) formed by both Mn2+ ions replacing Zn–Zn sites due to the shorter distance.
The luminescence centers of [MnO5] and [Mn2O8] were further verified by the electron paramagnetic resonance (EPR) spectra (Fig. 3f). The well-resolved sextet hyperfine lines were ascribed to the interaction between the electron spin and nuclear spin of isolated Mn2+ ions at the low doping level of x = 0.01.50,51 However, the EPR spectra consisted of a singlet structure for x = 0.1 and 0.3, and there was less splitting of the sextet hyperfine lines compared to that of the isolated Mn2+ ions due to the spin-relaxation processes in the exchange coupling of Mn2+ ions.52,53 This further demonstrated that the red emission in the LZPO:xMn2+ system originated from the isolated Mn2+ ions and Mn2+–Mn2+ dimers. With increasing the Mn2+-doping level, the PL intensity of P2 became stronger, while the PL intensity of P1 decreased. There are two possible reasons to explain this phenomenon. One is the different site-occupation preference at the different doping levels; whereby the Mn2+ ions are likely to take up Zn–Zn sites rather than isolated Zn sites at a high doping level due to the shorter distance between Zn2+ ions. The other is due to energy transfer from P1 to P2.
The change of full widths at half maximum (FWHMs) as a function of the doping level x is shown in Fig. 3g. At first, the FWHM increased from 106 to 111 nm because of the increase in the PL intensity for P2 emission, reaching its maximum value at x = 0.1. Then the FWHM decreased to 103 nm at x = 0.5 because of the decrease in PL intensity of the narrower P1 emission at the high-energy site [MnO5], caused by the energy transfer from [MnO5] to [Mn2O8]. In short, the relative PL intensity of these two luminescence centers contribute to the values of the FWHM. This was supported by the analysis of the lifetimes. The lifetime of P1 decreased from 40.13 to 16.01, and 38.73 to 16.63 ms for P2 as the doping level increased (Fig. 3h and i). The apparent difference in lifetime further provided evidence that P1 and P2 came from different luminescence centers. When x ≤ 0.05, the lifetime of P1 was longer than that of P2; however, the former became lesser than the latter at x ≥ 0.1, which indicates that there was indeed an energy transfer from P1 to P2.
According to the fitting results, the emissions P1 and P2 were both shifted to longer wavelength with the increase in doping level. P1 was shifted from 628 to 654 nm, and P2 from 680 to 700 nm. To explore the red-shift mechanism of Mn2+ emission, three aspects are considered: crystal field, exchange coupling interaction in Mn–Mn dimers, and energy transfer.54,55 As listed in Table S3 (ESI†), the bond lengths of the nearest neighboring oxygen to Zn were 1.9743, 1.9639, and 1.9142 Å for x = 0, 0.01, and 0.2, respectively. With increasing the doping level, the bond length of the nearest neighboring oxygen to Zn tended to decrease. According to the equation:56
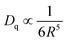 | (6) |
3.3. PL thermal stability of LZPO:xMn2+ phophors
The PL thermal stability is a crucial parameter to ensure the efficient and stable operation of pc-WLED devices at high temperature.57–60 Temperature-dependent PL spectra of the LZPO:xMn2+ phosphors were obtained from 25 °C to 200 °C to evaluate the PL thermal stability. With increasing the ambient temperature, the enhanced thermal motion of molecules strengthened the non-radiative transition, causing a decrease in the PL intensity. Unexpectedly, the PL thermal quenching of the LZPO:0.01Mn2+ sample was not obvious in Fig. 4a. The other two high doping levels for the LZPO:0.1Mn2+ and LZPO:0.3Mn2+ samples were tested to identify if they had excellent PL thermal stability (Fig. 4b and c). Fig. 4d shows a direct-viewing of the emission, including the change in the normalized PL intensity at three doping concentrations with different temperatures. At a low doping level for LZPO:0.01Mn2+, the PL intensity was 97% at 150 °C and 94% at 200 °C of the initial intensity at 25 °C, indicating its superior PL thermostable performance. The PL intensity of LZPO:0.1Mn2+ was 94% at 150 °C and 84% at 200 °C of the initial intensity at 25 °C. Even at a higher doping level for LZPO:0.3Mn2+, the PL intensity was 90% at 150 °C and 70% at 200 °C of the initial intensity at 25 °C. The different TQ behaviors with increasing the doping level were caused by the different luminescence centers of [MnO5] and [Mn2O8]. As for the luminescence of isolated [MnO5], only the interactions between the host lattice and activator would influence the PL thermal stability, but for the [Mn2O8] dimers, the additional interactions between two Mn2+ should be considered.61 The distance between Mn2+–Mn2+ will increase at high temperature, reducing the number of [Mn2O8] dimers.62 Consequently, the PL thermal stability of the emission from the isolated Mn2+ ions is better than that of the [Mn2O8] dimers. The number of [Mn2O8] dimer will increase at a higher doping level, resulting in a decrease in the PL thermal stability at high temperature. Furthermore, the temperature-dependent PL spectra of LZPO:0.01Mn2+ phosphors with different Mn sources and sintered conditions were measured. As shown in Fig. 4e and Fig. S6 (ESI†), the as-prepared LZPO:0.01Mn2+ phosphors exhibited remarkably lower luminescence quenching behaviors, indicating the different Mn sources and sintered conditions had little effect on the PL thermal stability. The above results suggest that the LZPO:Mn2+ phosphor is a PL thermally-stable candidate for pc-WLED devices.To address the lower luminescence quenching behavior, temperature-dependent XRD patterns of LZPO were further measured and are displayed in Fig. 5a. The diffraction peak of the (113) lattice plane shifted from 24.75° at 25 °C to 24.71° at 200 °C. The slight shift of the diffraction peak to the lower angle indicates that the thermal expansion in this lattice was negligible. The corresponding crystallographic parameters and relative values of the volume were almost constant with increasing the temperature (Fig. 5b). The volume expanded by only 0.37% from 1102.22 to 1106.316 Å3, furtherly identifying the near ZTE performance of LZPO, and indicating the structural stability of LZPO against high temperature. This suggests it provides a stable coordination environment for Mn2+ ions, thereby minimizing the emission loss with increasing the temperature. Additionally, thermoluminescence (TL) curves of the LZPO:0.1Mn2+ sample were measured to assess the distribution of the trap levels (Fig. 5c). The LZPO:0.1Mn2+ phosphor presented two obvious TL glow bands in the range from 303 to 573 K, peaking at 340.2 K and 501.4 K. The corresponding trap depths were estimated to be 0.6804 and 1.0028 eV according to the Urbach formula: ET = Em/500.51 Moreover, the intensity of trap A was much stronger than that of trap B, indicating that most trap levels were located in the shallow region in the forbidden band. Thereby the traps-assisted energy transfer to the luminescent center would compensate for the luminous loss due to the non-radiative transition. A schematic diagram of the mechanism of the luminescence process is shown in Fig. 5d. In this mechanism, under 412 nm wavelength excitation, electrons at the 6A1(6S) (for [MnO5]) or 6A1(6S) 6A1(6S) (for [Mn2O8] dimer) ground state are promoted to the excited level [4A1(4G),4E(4G)], then move to the first excited state 4T1(4G) (for [MnO5]) or 6A1(6S)4T1(4G) (for [Mn2O8] dimer) through a non-radiative relaxation process. Then the excited electrons come back to the ground state to generate red and deep-red emission. There is an energy transfer from [MnO5] to the [Mn2O8] dimer. Besides, some excited electrons will be captured by traps formed in the process of self-reduction. Stimulated by thermal activation, these will be released back into conduction band (CB) and will arrive at the first excited state 4T1(4G) or 6A1(6S)4T1(4G) through a non-radiative relaxation process to participate in the radiation transition. As a result, the emission loss is compensated by the process of the traps-assisted energy transfer. The stable coordination environment and traps-assisted energy transfer demonstrate that the LZPO:Mn2+ phosphor is a superior thermostable luminescent material, making it a hopeful candidate for high-power lighting equipment.
3.4. Potential pc-WLED applications
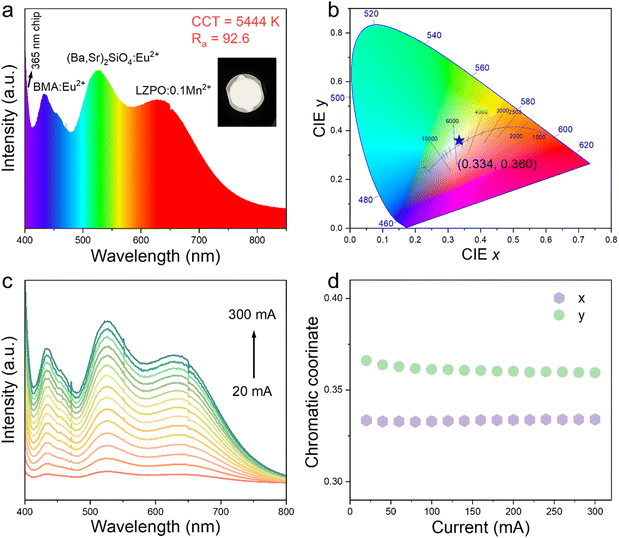
A pc-WLED device was fabricated by mixing a moderate amount of commercial blue phosphor [BaMgAl11O17:Eu2+ (BAM:Eu2+)], green phosphor [(Ba,Sr)2SiO4:Eu2+], and the as-prepared LZPO:0.1Mn2+ phosphor with a commercial 365 nm UV chip. As shown in Fig. 6a and b, the warm pc-WLED presented a low correlated color temperature (CCT) = 5444 K, and high color rendering index (CRI) of 92.6, with the CIE color coordinate of (0.334, 0.360) under a voltage of 3.3 V and current of 300 mA. The luminous efficiency of the pc-WLED was 1.7 Im W−1 under 3.2 V and 60 mA. These values of the pc-WLED are relatively low, and so it would be meaningful to develop new Mn-doped materials with high luminous efficiency in the future. The emission intensity of the as-prepared pc-WLED device gradually increased with increasing the current from 20–300 mA (Fig. 6c). In addition, changes in CIE coordinates for the pc-WLED device were not obvious at different currents (Fig. 6d), indicating the excellent color stability of the fabricated pc-WLED device.4. Conclusion
In summary, a series of red-emitting LZPO:xMn2+ phosphors were successfully synthesized by a high-temperature solid-state reaction process in an ambient atmosphere. Two luminescence centers [MnO5] and [Mn2O8] were identified, which resulted in a broad red emission. Under 412 nm wavelength excitation, the emission peak showed a red-shift from 636 to 672 nm with increasing the doping levels of Mn2+ due to a combined effect of the strengthened crystal field, exchange coupling interaction in Mn–Mn dimers, and energy transfer. Interestingly, the LZPO:xMn2+ phosphors exhibited excellent PL thermal stability. The PL intensity was 97% for LZPO:0.01Mn2+ and 90% for the heavily doped LZPO:0.3Mn2+ at 150 °C compared with their initial intensity at 25 °C, indicating their superior PL thermostable performance. The superior PL thermal stability supported by the structural stability of the near-zero thermal expansion (NZTE) LZPO against high temperature was also certified. Eventually, a pc-WLED device was fabricated to highlight the great application potential (CCT = 5444 K, Ra = 92.6). In light of the above results, the excellent PL thermostable LZPO:Mn2+ phosphor is considered a potential candidate for applications in pc-WLED devices. This study not only provides comprehensive evidence to discuss the relationship between the optical properties and crystal structure, but enriches the choices to develop novel functional optical materials by taking advantage of near-zero thermal expansion matrices.Author contributions
All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Science and Technology Major Project (2022YFB3503800), the Projects for Science and Technology Development Plan of Jilin Province (20210402046GH), the National Natural Science Foundation of China (NSFC No. 51932009, 51929201, 52072349, 52172166), the Natural Science Foundation of Zhejiang Province (LR22E020004), the Project funded by China Postdoctoral Science Foundation (2022TQ0365). M. S. Molokeev and S. P. Polyutov acknowledge the support by the Ministry of Science and High Education of Russian Federation (Project No. FSRZ-2023-0006).Notes and references
- Y. H. Kim, P. Arunkumar, B. Y. Kim, S. Unithrattil, E. Kim, S.-H. Moon, J. Y. Hyun, K. H. Kim, D. Lee, J.-S. Lee and W. B. Im, Nat. Mater., 2017, 16, 543–550 CrossRef CAS.
- Y. Wang, J. Ding, Y. Li, X. Ding and Y. Wang, Chem. Eng. J., 2017, 315, 382–391 CrossRef CAS.
- G. B. Nair, H. C. Swart and S. J. Dhoble, Prog. Mater. Sci., 2020, 109, 100622 CrossRef CAS.
- X. Zhou, J. Qiao and Z. Xia, Chem. Mater., 2021, 33, 1083–1098 CrossRef CAS.
- M.-H. Fang, Z. Bao, W.-T. Huang and R.-S. Liu, Chem. Rev., 2022, 122, 11474–11513 CrossRef CAS.
- N. M. C. H. P. Lan, P. Van Huan, N. H. Thong, D.-H. Nguyen, N. D. T. Kien, C. M. Nhuong, C. X. Thang and V.-H. Pham, Luminescence, 2022, 37, 577–587 CrossRef CAS.
- S. Huang, M. Shang, Y. Yan, Y. Wang, P. Dang and J. Lin, Laser Photonics Rev., 2022, 16, 2200473 CrossRef CAS.
- M. Zhao, Q. Zhang and Z. Xia, Mater. Today, 2020, 40, 246–265 CrossRef CAS.
- M. Wang, X. Ming, J. Cao, L. Yang, Z. Wang, C. Ma, M. Zhang and W. Liu, Inorg. Chem., 2023, 62, 75–86 CrossRef CAS PubMed.
- M. Zhao, H. Liao, M. S. Molokeev, Y. Zhou, Q. Zhang, Q. Liu and Z. Xia, Light Sci. Appl., 2019, 8, 1–9 CrossRef CAS.
- P. Dang, W. Wang, H. Lian, G. Li and J. Lin, Adv. Opt. Mater., 2022, 10, 2102287 CrossRef CAS.
- Y. Wei, H. Yang, Z. Gao, X. Yun, G. Xing, C. Zhou and G. Li, Laser Photonics Rev., 2021, 15, 2000048 CrossRef CAS.
- Y. Wang, J. Ding, Y. Wang, X. Zhou, Y. Cao, B. Ma, J. Li, X. Wang, T. Seto and Z. Zhao, J. Mater. Chem. C, 2019, 7, 1792–1820 RSC.
- R. Bajaj, P. Rohilla, A. Prasad, Ravita, A. Shandilya and A. S. Rao, Luminescence, 2023, 38, 428–436 CrossRef CAS.
- Y. Bai, S. Sun, L. Wu, T. Hu, L. Zheng, L. Wu, Y. Kong, Y. Zhang and J. Xu, J. Mater. Chem. C, 2022, 10, 4317–4326 RSC.
- H. Chen, Y. Lei, J. Li, K. Chen, L. Wu, L. Zheng, T. Sun, Y. Kong, Y. Zhang and J. Xu, Inorg. Chem., 2022, 61, 5495–5501 CrossRef CAS.
- S. Li, W. Hu, M. G. Brik, S. Lian and Z. Qiu, Inorg. Chem. Front., 2022, 9, 3224–3232 RSC.
- L. Wu, S. Sun, Y. Bai, Z. Xia, L. Wu, H. Chen, L. Zheng, H. Yi, T. Sun, Y. Kong, Y. Zhang and J. Xu, Adv. Opt. Mater., 2021, 9, 2100870 CrossRef CAS.
- Z. Long, Y. Wen, J. Qiu, J. Wang, D. Zhou, C. Zhu, J. Lai, X. Xu, X. Yu and Q. Wang, Chem. Eng. J., 2019, 375, 122016 CrossRef CAS.
- R. Shi, L. Ning, Z. Wang, J. Chen, T. Sham, Y. Huang, Z. Qi, C. Li, Q. Tang and H. Liang, Adv. Opt. Mater., 2019, 7, 1901187 CrossRef CAS.
- Y. Wei, H. Yang, Z. Gao, Y. Liu, G. Xing, P. Dang, A. A. A. Kheraif, G. Li, J. Lin and R.-S. Liu, Adv. Sci., 2020, 7, 1903060 CrossRef CAS.
- S. He, L. Zhang, H. Wu, H. Wu, G. Pan, Z. Hao, X. Zhang, L. Zhang, H. Zhang and J. Zhang, Adv. Opt. Mater., 2020, 8, 1901684 CrossRef CAS.
- Q. Dong, J. Cui, Y. Tian, J. Jia, F. Yang, F. Du, J. Peng, X. Ye and S. Yang, CrystEngComm, 2019, 21, 5947–5957 RSC.
- Y.-T. Tsai, C.-Y. Chiang, W. Zhou, J.-F. Lee, H.-S. Sheu and R.-S. Liu, J. Am. Chem. Soc., 2015, 137, 8936–8939 CrossRef CAS.
- D. Wen, H. Liu, Y. Guo, Q. Zeng, M. Wu and R. S. Liu, Angew. Chem., Int. Ed., 2022, 61, e202204411 CAS.
- W. Wang, H. Yang, M. Fu, X. Zhang, M. Guan, Y. Wei, C. C. Lin and G. Li, Chem. Eng. J., 2021, 415, 128979 CrossRef CAS.
- W. Wang, M. Fu, S. Liu, X. Zhang, Y. Wei and G. Li, J. Lumin., 2022, 242, 118536 CrossRef CAS.
- P. Dang, G. Li, X. Yun, Q. Zhang, D. Liu, H. Lian, M. Shang and J. Lin, Light: Sci. Appl., 2021, 10, 29 CrossRef CAS PubMed.
- J. Liao, M. Wang, F. Lin, Z. Han, B. Fu, D. Tu, X. Chen, B. Qiu and H.-R. Wen, Nat. Commun., 2022, 13, 2090 CrossRef CAS.
- J. Zhang, G.-M. Cai, L.-W. Yang, Z.-Y. Ma and Z.-P. Jin, Inorg. Chem., 2017, 56, 12902–12913 CrossRef CAS PubMed.
- Y. Liu, G. Zhang, J. Huang, X. Tao, G. Li and G. Cai, Inorg. Chem., 2021, 60, 2279–2293 CrossRef CAS.
- L. Ji, H. Ma and J. Liang, Acta Crystallogr. C, 2009, 65, 30–32 CrossRef.
- S. Wu, S. Hu, Q. Liu, S. Zhang, D. Jiang, G. Zhang, Y. Le, Y. Xiao, B. Xiao, P. Xiong, Y. Chen and Y. Wang, J. Mater. Chem. C, 2023, 11, 3865–3874 RSC.
- L. Wu, Y. Bai, L. Wu, H. Yi, X. Zhang, L. Zhang, Y. Kong, Y. Zhang and J. Xu, Dalton Trans., 2018, 47, 13094–13105 RSC.
- Y. Wang, Y. Zhou, H. Ming, Y. Zhao, E. Song and Q. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 51255–51265 CrossRef CAS.
- Y. Meng, P. Chen, H. Fan, Z. Lu, X. Zhong, J. Lu, Y. Ou and L. Zhou, Mater. Res. Bull., 2022, 147, 111610 CrossRef CAS.
- M. D. Dramićanin, L. Marciniak, S. Kuzman, W. Piotrowski, Z. Ristić, J. Periša, I. Evans, J. Mitrić, V. Đorđević, N. Romčević, M. G. Brik and C.-G. Ma, Light Sci. Appl., 2022, 11, 279 CrossRef.
- Z. Liao, H. Xu, W. Zhao, H. Yang, J. Zhong, H. Zhang, Z. Nie and Z.-K. Zhou, Chem. Eng. J., 2020, 395, 125060 CrossRef CAS.
- Z. Ristić, W. Piotrowski, M. Medić, J. Periša, Ž. M. Antić, L. Marciniak and M. D. Dramićanin, ACS Appl. Electron. Mater., 2022, 4, 1057–1062 CrossRef.
- Y. Zhu, Y. Liang, S. Liu, H. Li and J. Chen, Adv. Opt. Mater., 2019, 7, 1801419 CrossRef.
- Y. Wei, G. Xing, K. Liu, G. Li, P. Dang, S. Liang, M. Liu, Z. Cheng, D. Jin and J. Lin, Light: Sci. Appl., 2019, 8, 15 CrossRef.
- A. J. van Bunningen, A. D. Sontakke, R. van der Vliet, V. G. Spit and A. Meijerink, Adv. Opt. Mater., 2023, 11, 2202794 CrossRef CAS.
- E. H. Song, Y. Y. Zhou, Y. Wei, X. X. Han, Z. R. Tao, R. L. Qiu, Z. G. Xia and Q. Y. Zhang, J. Mater. Chem. C, 2019, 7, 8192–8198 RSC.
- S.-Y. Zhu, D. Zhao, S.-J. Dai, R.-J. Zhang and L.-Y. Shi, CrystEngComm, 2022, 24, 2966–2975 RSC.
- E. Song, X. Jiang, Y. Zhou, Z. Lin, S. Ye, Z. Xia and Q. Zhang, Adv. Opt. Mater., 2019, 7, 1901105 CrossRef CAS.
- Z. Yang, Y. Zhao, Y. Zhou, J. Qiao, Y.-C. Chuang, M. S. Molokeev and Z. Xia, Adv. Funct. Mater., 2022, 32, 2103927 CrossRef CAS.
- G. Liu, T. Hu, M. S. Molokeev and Z. Xia, iScience, 2021, 24, 102250 CrossRef CAS.
- C. Yang, C. Fan, F. Hussain, W. Sheng, K. Song, J. Wu, Q. Huang, W. Su, J. Xu, S. Sun and D. Wang, J. Rare Earths, 2023, 41, 489–497 CrossRef CAS.
- Y. Chen, J. Chen, J. Liang, J. He, Z.-Q. Liu and Y. Yin, Chem. Mater., 2020, 32, 9551–9559 CrossRef CAS.
- J. Xue, T. Hu, F. Li, F. Liu, H. M. Noh, B. R. Lee, B. C. Choi, S. H. Park, J. H. Jeong and P. Du, Laser Photonics Rev., 2023, 17, 2200832 CrossRef CAS.
- Z.-H. Zuo, Y.-Y. Peng, J. Li, X. Wang, Z.-Q. Liu and Y. Chen, Chem. Eng. J., 2022, 446, 136976 CrossRef CAS.
- E. Song, J. Wang, S. Ye, X.-B. Yang, M. Peng, Q. Zhang and L. Wondraczek, Adv. Opt. Mater., 2017, 5, 1700070 CrossRef.
- X. Li, T. Liu, K. Zhang, Z. Hu, H. An, S. Deng, Y. Kong and B. Wang, J. Mater. Chem. C, 2023, 11, 712–721 RSC.
- L. Shi, Y. Huang and H. J. Seo, J. Phys. Chem. A, 2010, 114, 6927–6934 CrossRef CAS.
- D. Wei and H. J. Seo, J. Lumin., 2021, 229, 117644 CrossRef CAS.
- W. Wang, H. Yang, Y. Liu, X. Yun, Y. Wei and G. Li, CrystEngComm, 2020, 22, 311–319 RSC.
- L. Zhou, W. Wang, D. Xu, Z. Wang, Z. Yi, M. Wang and Z. Lu, Ceram. Int., 2021, 47, 34820–34827 CrossRef CAS.
- Y. Wei, H. Yang, Z. Gao, Y. Liu, G. Xing, P. Dang, A. A. A. Kheraif, G. Li, J. Lin and R. Liu, Adv. Sci., 2020, 7, 1903060 CrossRef CAS PubMed.
- Z. Xia, X. Wang, Y. Wang, L. Liao and X. Jing, Inorg. Chem., 2011, 50, 10134–10142 CrossRef CAS.
- Z. Wang, S. Liang, C. Zhan, K. Xu, J. Hu, D. Chen, L. Song and H. Zhu, J. Rare Earths DOI:10.1016/j.jre.2023.04.014.
- E. Song, M. Chen, Z. Chen, Y. Zhou, W. Zhou, H.-T. Sun, X. Yang, J. Gan, S. Ye and Q. Zhang, Nat. Commun., 2022, 13, 1–9 Search PubMed.
- E. Song, S. Ye, T. Liu, P. Du, R. Si, X. Jing, S. Ding, M. Peng, Q. Zhang and L. Wondraczek, Adv. Sci., 2015, 2, 1500089 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Refined structure parameters, emission peak position fitted by Gaussian method and corresponding lifetime of LZPO:xMn2+ samples. Rietveld refinement XRD pattern, the diffuse reflectance spectra of LZPO:xMn2+ (x = 0.05, 0.1, 0.3) phosphors. PL spectra and XPS spectrum of LZPO:0.1Mn2+. Temperature-dependent PL spectra of LZPO:0.01Mn2+ with different Mn-source sintered in air and N2(90%)-H2(10%) atmosphere. See DOI: https://doi.org/10.1039/d3tc01683h |
| This journal is © The Royal Society of Chemistry 2023 |