Open Access Article
Open Access ArticleHigh-pressure ilmenite-type MnVO3: crystal and spin structures in the itinerant-localized regimes†
Elena
Solana-Madruga
 ab,
Olivier
Mentré
ab,
Olivier
Mentré
 a,
Eugenia P.
Arévalo-López
c,
Dmitry
Khalyavin
a,
Eugenia P.
Arévalo-López
c,
Dmitry
Khalyavin
 d,
Francois
Fauth
d,
Francois
Fauth
 e,
Alexandr
Missiul
e and
Angel M.
Arévalo-López
e,
Alexandr
Missiul
e and
Angel M.
Arévalo-López
 *a
*a
aUMR-8181-UCCS-Unité de Catalyse et Chimie du Solide-Univ. Lille, CNRS, Centrale Lille, ENSCL, Univ. Artois, Lille F-59000, France. E-mail: angel.arevalo-lopez@univ-lille.fr
bDpto. Q. Inorgánica, Facultad CC. Químicas, Universidad Complutense de Madrid, Avda. Complutense sn, 28040, Madrid, Spain
cFacultad de Ciencias, Universidad Nacional Autónoma de Mexico, A.P. 70-399, Cd, Mexico 04510, Mexico
dISIS Facility, Rutherford Appleton, Laboratory, Harwell, Didcot, Oxford OX11 0QX, UK
eCELLS-ALBA synchrotron, Carrer de la Llum 2-26, 08290, Cerdanyola del Vallés, Barcelona, Spain
First published on 15th June 2023
Abstract
Herein we report the Peierls-like transition in the high-pressure ilmenite-type MnVO3. The first-order structural transition from a low-temperature P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) to a high-temperature R
to a high-temperature R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) occurs at 475 K, accompanied by a semiconductor to metal-like transition. The triclinic distorted phase presents a V–V dimerization with a short ∼2.85 Å bond formation at 300 K. Below 80 K MnVO3 orders antiferromagnetically with a small ferromagnetic component. Neutron diffraction reveals that both Mn and V cations contribute to the magnetic ordering, contrary to what expected on the V–V dimer formed. Extended Hückel and DFT calculations expose that the formation of covalent pairing is only partially achieved, contrary to the MgVO3 and CoVO3 counterparts.
occurs at 475 K, accompanied by a semiconductor to metal-like transition. The triclinic distorted phase presents a V–V dimerization with a short ∼2.85 Å bond formation at 300 K. Below 80 K MnVO3 orders antiferromagnetically with a small ferromagnetic component. Neutron diffraction reveals that both Mn and V cations contribute to the magnetic ordering, contrary to what expected on the V–V dimer formed. Extended Hückel and DFT calculations expose that the formation of covalent pairing is only partially achieved, contrary to the MgVO3 and CoVO3 counterparts.
Introduction
Transition metal oxides with ABO3 stoichiometry are intensively studied due to their chemical, structural and functional versatility. A wide variety of crystal structures accepting different degrees of distortion can be stabilized, mainly depending on the relative ionic radii of A- and B-site cations, as defined by the Goldschmidt relation.1 Synthesis conditions can also induce notable effects on the preferred crystallization of the compounds with different structures. In particular, high-pressure and high-temperature synthesis provides accessibility to metastable compounds with unusual oxidation states and metal coordinations. Small cations can also be stabilised into large voids.2,3 This is the case, for instance, of A-site manganites, where perovskite and corundum related structures are reported with a rich variety of cation ordered superstructures.4Two high pressure polymorphs of MnVO3 were first reported in 1970. The initial structural characterization revealed a triclinic distortion P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) of an ilmenite-type structure (MnVO3-I) and an orthorhombic (Pnma) perovskite (MnVO3-II) for the low- and the high-pressure phases below and above 4 GPa respectively.5 It was not until 2011 when MnVO3-II, the only quenchable simple perovskite with Mn2+ in the A site, was fully understood. It showed a complex behaviour with spin polarized conduction electrons and an incommensurate magnetic structure in a half-metallic state.6
of an ilmenite-type structure (MnVO3-I) and an orthorhombic (Pnma) perovskite (MnVO3-II) for the low- and the high-pressure phases below and above 4 GPa respectively.5 It was not until 2011 when MnVO3-II, the only quenchable simple perovskite with Mn2+ in the A site, was fully understood. It showed a complex behaviour with spin polarized conduction electrons and an incommensurate magnetic structure in a half-metallic state.6
MnVO3-I however, has not been fully understood. It belongs to the AVO3 with A = Mg, Mn, Co, Ni and Cu high-pressure triclinic ilmenites.7–9 MgVO3 and CoVO3 exhibit a ladder-like V–V dimerization and non-magnetic singlet ground states with a concomitant triclinic to rhombohedral phase transformations at 500 and 550 K respectively.10,11 NiVO3 and CuVO3 have not been fully studied.9,12,13
MnVO3-I has been recently obtained in single-crystal form from a solid-state recrystallization under high-pressure and high-temperature conditions.14 The ladder-like V–V dimerization pattern is also observed in this phase. Here we present a complete characterization of bulk MnVO3-I, including the thermal evolution of its physical properties and ground-state magnetic structure. A comparative study against the related MgVO3 and CoVO3 is performed to discuss the observed behaviour. Unprecedented magnetic order into the V sublattice is observed despite the presence of V–V dimers, which is justified in terms of a combination of correlation- (Mott) and band-effects (Peierls) in the low temperature triclinic semiconducting phase against the metallic-like high temperature rhombohedral polymorph, similar to the VO2 scenario.15–19
Experimental
Pollycrystalline MnVO3-I was obtained at 4 GPa and 1273 K for 30 minutes using a Walker-type multianvil press, further details concerning this technique are given in the ESI.† Stoichiometric amounts of MnO (99.9% Sigma-Aldrich) and VO2 were placed in a Pt capsule and into the press. VO2 was prepared from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 V2O3
1 V2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V2O5 mixture in vacuum at 700 °C for 24 h, and V2O3 was previously obtained via reduction of V2O5 (99.9% Sigma-Aldrich) under hydrogen at 700 °C. Synchrotron X-ray radiation powder diffraction (SXRPD) was performed at the BL04 MSPD beamline of ALBA synchrotron in Spain. Data were collected using a λ = 0.4142 Å wavelength in the 0° < 2θ < 40° angular range with a 0.006° effective step size.20,21 High-resolution neutron powder diffraction (NPD) data were collected in a combination of several high-pressure experiment runs (63 mg in total) in the WISH diffractometer at the ISIS facility.22 Long scans were obtained at 2 K and 100 K and shorter scans were collected every 3 K on warming. Rietveld refinements were performed using Fullprof Suite.23
V2O5 mixture in vacuum at 700 °C for 24 h, and V2O3 was previously obtained via reduction of V2O5 (99.9% Sigma-Aldrich) under hydrogen at 700 °C. Synchrotron X-ray radiation powder diffraction (SXRPD) was performed at the BL04 MSPD beamline of ALBA synchrotron in Spain. Data were collected using a λ = 0.4142 Å wavelength in the 0° < 2θ < 40° angular range with a 0.006° effective step size.20,21 High-resolution neutron powder diffraction (NPD) data were collected in a combination of several high-pressure experiment runs (63 mg in total) in the WISH diffractometer at the ISIS facility.22 Long scans were obtained at 2 K and 100 K and shorter scans were collected every 3 K on warming. Rietveld refinements were performed using Fullprof Suite.23
Single-crystal data from isolated small crystals was collected on a Bruker DUO diffractometer (Mo Kα radiation) and treated using the JANA 2006 software.24
X-ray photoelectron spectroscopy (XPS) analyses were carried out in an ultra-high vacuum (UHV) system Scanning XPS microprobe PHI 5000 VersaProbe II, with an Al Kα X-ray source (hν = 1486.6 eV) monochromatic with 200 μm beam diameter, and a Multi-Channel Detector (MCD) analyzer. EDX analysis were also performed in a FLEXSEM 1000, see ESI† for further details.
Magnetic properties were measured using a PPMS 9T Dynacool from Quantum Design. Magnetization was measured in ZFC-FC mode under 0.1 T and hysteresis loops collected at several temperatures. Heat capacity was measured on an as-obtained pellet between 2 and 300 K and corrected with an addendum. Resistivity measurements were performed using a 4-probe system with a Keithley 6221 source and a 2182A Nanovoltmeter installed in a Linkam high temperature station.
Extended Hückel tight-binding calculations (EHTB) have been performed using the SAMOA package.25 DFT+U calculations were performed with the general gradient approximation (GGA) using the popular Perdew–Burke–Ernzerhof (PBE) approach in the Vienna ab initio simulation package (VASP).26 Extra on-site electron–electron Coulomb interactions were also considered with the GGA+U approach implemented in the code. Crystal structures were optimized by minimizing the total energy, phonon dispersions calculations were not performed to test dynamical stability of the optimized structures. The lattices and atomic positions were relaxed starting from the 300 K and the 560 K MnVO3-I refined models, within a convergence force criterion of 3 × 10−2 eV Å−1. The relaxed lattice contraction ΔV/V0 are 6.5% and 4.9% for the rhombohedral and triclinic cells respectively, as commonly found for DFT. It was checked that the experimental low temperature triclinic phase is most stable by ∼40 meV per formula unit, compared to the rhombohedral form. For electronic calculations, we assume the most stable AFM state with U = 1 eV for Mn and V atoms. The electrons Mn, V (3s, 3p, 3d, 4s) and O (2s, 2p) were treated as valence states. The cutoff energy for the plane-wave-basis is 400 eV, and the total energy of the system was converged with respect to the plane-wave cutoff energy and reciprocal space samplings. The free-energy convergence criterion was set to10−10 eV using a Γ-centered 8 × 8 × 8 Monkhorst-Pack k-point mesh. A systematic revision of all possible spin configurations was not pursued, but can be performed in future theoretical studies aiming a further understanding of the transition mechanism.
Results and discussion
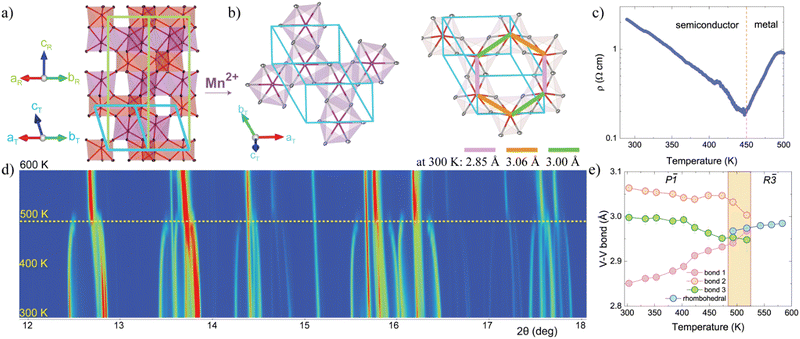
MnVO3-I was synthesized under high-pressure and high-temperature conditions as described in the experimental section above. Small single crystals were successfully isolated for structural determination at room temperature (CCDC 2259432†) in good agreement with the recently reported MnVO3 crystal structure.14 The model obtained by single crystal (Table S1, ESI†) was subsequently used in the Rietveld refinements of SXRPD and NPD data and results are summarized in ESI.† MnVO3-I crystallizes in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with a = 5.0177(7) Å, b = 5.0513(7) Å, c = 5.5210(8) Å, α = 116.679(6)°, β = 90.044(6)° and γ = 118.924(5)° cell parameters at 300 K. The structure is shown in Fig. 1a and b. It presents a distorted ilmenite-type structure, as the MgVO3 and CoVO3.10,11 The low symmetry in MnVO3-I arises from the distortion of the Mn and V honeycomb lattices. Three different V–V lengths are observed, two long (3.025(1) Å and 3.00(1) Å) and one short (2.849(1) Å) at 300 K. The short length of the V–V pair is still comparable to those of the related MgVO3 (∼2.78 Å) and CoVO3 (∼2.74 Å). This agrees with the ionic radius of the different A cations, 0.72, 0.745 and 0.83 Å for Mg, Co and Mn respectively. Moreover, the short V–V bond is below the critical interatomic distance (Rc ∼2.94 Å) for which direct metal–metal bond may be formed in vanadium oxides,27,28 thus structural clues for V–V dimerization exist in MnVO3-I. The Mn sublattice shows no formation of dimerized features exhibiting a narrow distribution of Mn–Mn distances (3.065(1), 3.017(1) and 2.964(1) Å at 300 K). The calculated bond valence sum (BVS)29 results in ∼2.08+ for Mn and ∼3.47+ for V. This reflects the stressed structure obtained in a high-pressure metastable phase. EDX and XPS measurements respectively support the expected composition and formal oxidation states Mn2+V4+O32−, see ESI.†
space group with a = 5.0177(7) Å, b = 5.0513(7) Å, c = 5.5210(8) Å, α = 116.679(6)°, β = 90.044(6)° and γ = 118.924(5)° cell parameters at 300 K. The structure is shown in Fig. 1a and b. It presents a distorted ilmenite-type structure, as the MgVO3 and CoVO3.10,11 The low symmetry in MnVO3-I arises from the distortion of the Mn and V honeycomb lattices. Three different V–V lengths are observed, two long (3.025(1) Å and 3.00(1) Å) and one short (2.849(1) Å) at 300 K. The short length of the V–V pair is still comparable to those of the related MgVO3 (∼2.78 Å) and CoVO3 (∼2.74 Å). This agrees with the ionic radius of the different A cations, 0.72, 0.745 and 0.83 Å for Mg, Co and Mn respectively. Moreover, the short V–V bond is below the critical interatomic distance (Rc ∼2.94 Å) for which direct metal–metal bond may be formed in vanadium oxides,27,28 thus structural clues for V–V dimerization exist in MnVO3-I. The Mn sublattice shows no formation of dimerized features exhibiting a narrow distribution of Mn–Mn distances (3.065(1), 3.017(1) and 2.964(1) Å at 300 K). The calculated bond valence sum (BVS)29 results in ∼2.08+ for Mn and ∼3.47+ for V. This reflects the stressed structure obtained in a high-pressure metastable phase. EDX and XPS measurements respectively support the expected composition and formal oxidation states Mn2+V4+O32−, see ESI.†
Fig. 1d shows a small angular range for the thermal evolution of the SXRPD patterns between 300 and 600 K. A reversible first order structural phase transition from triclinic (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) to rhombohedral (R
) to rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) ilmenite-type structure occurs at 475 K with a coexistence of both phases up to 525 K. Refined atomic positions confirm the presence of V–V dimers as revealed by interatomic distances in Fig. 1e. In the rhombohedral phase the dimerization is vanished by symmetry, resulting in a V–V and Mn–Mn distances of 2.984(9) Å and 2.960(6) Å respectively at 560 K. The transformation matrix between the triclinic P
) ilmenite-type structure occurs at 475 K with a coexistence of both phases up to 525 K. Refined atomic positions confirm the presence of V–V dimers as revealed by interatomic distances in Fig. 1e. In the rhombohedral phase the dimerization is vanished by symmetry, resulting in a V–V and Mn–Mn distances of 2.984(9) Å and 2.960(6) Å respectively at 560 K. The transformation matrix between the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and rhombohedral R
and rhombohedral R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) polymorphs used in here is: (a′, b′, c′)R = (a,b,c)T [1 0 1, 0 1 2, 0 0 3].
polymorphs used in here is: (a′, b′, c′)R = (a,b,c)T [1 0 1, 0 1 2, 0 0 3].
The cell parameters and the volume (ESI†) exhibit a change at the transition and both structures coexist in the 475–525 K temperature range proving that it is of first order. Fig. 1c shows the electrical resistivity measurements at high temperatures with a clear change from a semiconductor to a metal-like transition at 450 K on heating. However, one should be careful, since this could just mean that the carrier mobility is strongly affected by scattering with phonons and/or defects as temperature increases. It is however 25 K below the structural transition, suggesting that the electronic correlations (Mott) may need to be lost before the structural change can occur (Peierls). This somehow resembles the dynamical dimer state in MgVO3, where a difference of ∼100 K between the magnetic and the structural transition was reported.10 Moreover, MgVO3 shows a change from a Pauli-paramagnetic (metal) to a non-magnetic dimerized state (semiconductor). Thus, by analogy, suggesting the observed electronic transition at 450 K in MnVO3-I involves metallization. It is also similar to the metal–insulator transition in VO2, where the dimerization and the electron correlations break at the transition.16
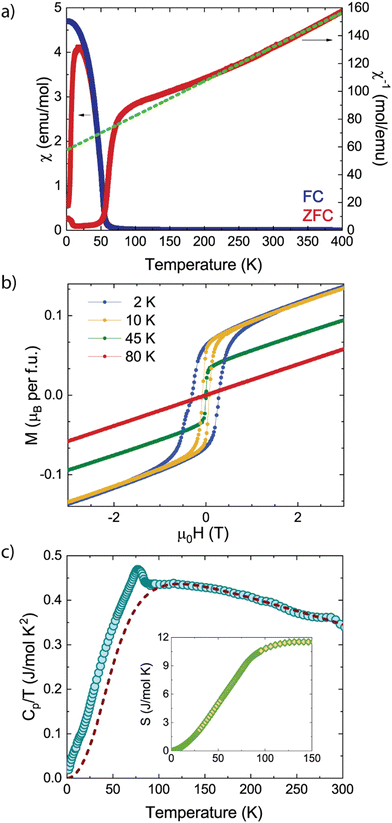
Low temperature dependent magnetization measurements for MnVO3-I in 0.01 T external field (Fig. 2a) show a ferrimagnetic transition at TN = 77(1) K. The Curie–Weiss fit of the inverse susceptibility above 250 K shows a paramagnetic moment of 5.7(1) μB f.u.−1, close to the theoretical value of 5.92 μB when considering only Mn2+. Weiss temperature of θW = –233(1) K demonstrates that antiferromagnetic spin–spin interactions are dominant but partially frustrated (|θ|/TN ≈ 3). Magnetic hysteresis loops below TN developed a weak ferromagnetic (WFM) component with a remnant magnetization of 0.07μB at 2 K. Heat capacity measurements present a lambda-like peak at 77 K in agreement with the WFM transition, see Fig. 2c. Assuming the vanadium atoms are in a dimerized singlet state and after the phonon contribution was subtracted, the magnetic entropy released of 11(1) J mol−1 K−1 accounts for 3/4 of the theoretical value S = R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (2S + 1) = 14.9 J mol−1 K−1 for MnVO3-I.
(2S + 1) = 14.9 J mol−1 K−1 for MnVO3-I.
Mn3V2O8 minority phase presents a transition at 21 K,30 which appears below the ferrimagnetic transition of MnVO3-I (TN = 77 K). Thus, the observed features in our experimental data are intrinsic to our sample. Refinements of neutron diffraction data consider the impurity phase, thus ensuring its presence does not affect the interpretation of the magnetic properties.
The temperature dependence of the MnVO3-I NPD data reveals the appearance of magnetic peaks below TN = 77 K, see ESI.† These extra reflections coincide with the crystal structure and can be indexed with propagation vector k0 = [0 0 0] (Γ point of the first Brillouin zone). ISODISTORT was used to determine the possible magnetic structures and space group.31,32 The final Rietveld fit was obtained with Fullprof with the mΓ1− irreducible representation with the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ′ magnetic space group (2.6).
′ magnetic space group (2.6).
The magnetic structure, depicted in Fig. 3, can be described as AFM layers of Mn and V atoms. The refined ordered magnetic moments (Rmag = 2.71%, χ2 = 1.49) at 2 K converge to 3.87(2) and 0.44(5) for Mn and V respectively. These are confined in the bc plane with refined components mMn = (0, 3.27(2), 3.85(1)) μB and mV = (0, 0.18(1), –0.17(1)) μB. Refinement of the magnetic structure considering only Mn moments was attempted, but resulted in fits of much lower quality (Rmag = 8.32%, χ2 = 3.04), thus proving the necessity to include the vanadium contribution into the model. The slightly reduced moment values obtained for both sublattices reflect the discussed frustration in the system, previously observed from susceptibility data. Moreover, Mn and V spin sublattices are almost perpendicular, with a relative angle of 87.7(3)° between them. The WFM component observed in the M vs. H loops cannot be originated from antisymmetric Dzyaloshinskii-Moriya exchange interaction, since Mn (or V) atoms are centrosymmetric by neighboring pairs. Thus, one needs to consider the mΓ1+ irreducible representation which would result in a ferromagnetic component along the x direction. This may also imply a lower symmetry. However, due to its small value of 0.07 μB no attempts of refinement were pursued.
The temperature variation of the Mn and V moments along with the fit to critical laws μ(T) = μ(0)[1−(T/TM)α]β are shown in Fig. 3c. They result in TM = 84.8(4) K and 31(2) K for Mn and V respectively, and α = 1.68(24) and β = 0.35(3) shared. The critical exponent β is close to the theoretical value of β = 0.34 for a three-dimensional XY magnet, appropriate to S = 5/2 Mn2+ and  spins laying in the bc plane but connected along z by Mn–V super-exchanges. Independent transitions for Mn and V are in good agreement with low temperature features in our bulk magnetic susceptibility measurements.5
spins laying in the bc plane but connected along z by Mn–V super-exchanges. Independent transitions for Mn and V are in good agreement with low temperature features in our bulk magnetic susceptibility measurements.5
The appearance of localized spins in the vanadium site of MnVO3-I is somehow unexpected when compared with the other well studied triclinic distorted ilmenite-type vanadates AVO3 (A = Mg and Co). MgVO3, with a V–V dimer distance of ∼2.78 Å, shows a non-magnetic nature due to the 3dxy orbital pairing below 500 K.10 CoVO3, with a ∼2.74 Å V–V distance, presents dimerized vanadium and is considered to be magnetic solely due to cobalt cations with a S = 3/2 state.11 MnVO3-I has a ∼2.85 Å V–V dimer bond, also below the critical distance for direct exchange (Rc ∼ 2.94 Å).30,31 Along with the metal-like – semiconductor transition observed at 450 K, it is thus expected, by analogy, to be magnetic exclusively due to the manganese cations. However, due to ionic radii size of the Mn these bonds are longer than for MgVO3 and CoVO3, as well as the stronger magnetic interactions between d5–d1 Mn2+–V4+ spins, results in low temperature localization of the V magnetic moments and the idea of a partial V–V dimerized state emerges. A resembling scenario appears in the GaV4O8, where besides the charge ordering behavior, a trimerized state is observed along with reduced spin localization at low temperature in the so-called “hybrid electrons”.33
From extended Hückel tight-binding calculations (EHTB) in distorted V6O24 honeycomb ring clusters isolated from AVO3 (A = Mg, Co and Mn) we can clearly observe a bonding molecular orbital formation for the Mg and Co compounds. However, this is not visible for MnVO3-I and it thus supports the conclusion that although there is a shortening in one of the V–V bonds, it is not sufficient to prevent the vanadium to magnetically order at low temperature. This implies that the Rc ∼ 2.94 Å critical value for direct metal-metal bonding should be used as reference only. It also rises the question of whether the simple image of a Peierls-like transition is occurring in MnVO3-I, where the metallic-like state should be ruled by the vanadium cations without intervention of the manganese, like in the MnVO3-II perovskite analogue.6 However, the observation of a first order structural transition at high-temperature and spin ordering in both Mn and V cations points toward an electronic correlation scenario. Besides these, the difference of 25 K between the metal-like – semiconductor and the structural transitions may indicate all together that a combination of both effects is taking part.
The transition is in fair agreement with the calculated density of states (DOS) for the high- (trigonal) and low-temperature (triclinic) MnVO3-I using our experimental models in the AFM (k0 = [0 0 0] state), see Fig. 4b. The high spin local Mn2+ is well observed by half-occupied t2g and eg 3d bands split by ∼1 eV. They show large exchange splitting between the majority and minority spins (∼3 eV) for both structures. The calculated moment of ∼4 μB for Mn (U = 1 eV) suggest a partial delocalization and Mn–O valence effects of the Mn d electrons. Moreover, the majority V4+ 3d bands (t2g orbitals) mainly participate to the upper valence and lower conduction bands, with weak Mn–O–V hybridization. In the low temperature structure, a narrow gap of 0.2 eV opens in the t2g manifold, in agreement with the semiconducting behavior observed. The V–V pairing accounts for the reduced localized moment on V site (∼0.66 μB /V). At high-temperature in the rhombohedral structure, the vanadium t2g manifold degenerates and crosses the Fermi level within a metal-like state. However, an important localized moment of ∼1 μB/V is calculated, which picture the main contribution of V atoms compared to O and Mn atoms in the narrow band crossing at the Fermi level. The different electronic behaviour of the triclinic and the trigonal phases show important coulombic effects, thus supporting the mixed scenario between electronic correlation and band effects discussed above, somehow similar to VO2. Further studies of the accurate electronic structure should clarify what is the exact mechanism of the transition in MnVO3-I. Moreover, the recognition of new systems similar to VO2 is of current interest through the realization of thin film devices that may stabilize the high-pressure phase of MnVO3-I.34,35
Conclusions
A metal-like – semiconductor transition was observed in the MnVO3-I high pressure phase at 450 K. This is accompanied by a R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) to P
to P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) structural transition where a Peierls-like V–V bond shortening occurs resembling that of VO2. MnVO3-I orders antiferromagnetically at TN = 77(1) K with a small ferromagnetic component of 0.07 μB at 2 K. NPD reveals a k0 = [0 0 0] magnetic propagation vector. The magnetic structure exhibits Mn and V spin components of 3.87(2) and 0.44(5) μB respectively. Contrary to the MgVO3 and CoVO3 analogues, EHTB calculations highlight that the dimerization is not full in MnVO3-I and point towards a more complex electronic behaviour that may be clarified by RIXS experiments and more detailed calculations.
structural transition where a Peierls-like V–V bond shortening occurs resembling that of VO2. MnVO3-I orders antiferromagnetically at TN = 77(1) K with a small ferromagnetic component of 0.07 μB at 2 K. NPD reveals a k0 = [0 0 0] magnetic propagation vector. The magnetic structure exhibits Mn and V spin components of 3.87(2) and 0.44(5) μB respectively. Contrary to the MgVO3 and CoVO3 analogues, EHTB calculations highlight that the dimerization is not full in MnVO3-I and point towards a more complex electronic behaviour that may be clarified by RIXS experiments and more detailed calculations.
Author contributions
The study was designed by AMAL. Synthesis was performed by ESM and AMAL. Bulk magnetism and electric conductivity data were measured and analysed by ESM and AMAL. XPS experiments and analysis were performed by EPAL. Synchrotron diffraction data were collected and analysed by AM, FF, ESM and AMAL. Neutron diffraction data were collected and analysed by DK, ESM and AMAL. EHTB and DFT calculations were done by OM and AMAL. The paper was written with contributions from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank support from ANR AMANTS project (19-CE08-0002-01). The ALBA synchrotron (ID proposal 2022025657) and the ISIS muon and neutron source are acknowledged for access to beam time. The Chevreul Institute (FR 2638), Region Hauts-de-France, and FEDER are acknowledged for funding the X-ray diffractometers, the “LEGO” multianvil-press and the PPMS magnetometer. E. P. A. L. thanks financial support from DGAPA-UNAM Project IA103923 and L. Huerta for technical support in XPS measurements.Notes and references
- V. M. Goldschmidt, Mat-Nature KI, 1926, 2, 117 Search PubMed
.
- G. Demazeau, H. Huppertz, J. A. Alonso, R. Pottgen, E. Moran and J. P. Attfield, Z. Naturforsch., 2006, 61b, 1457 CrossRef
.
- A. Belik and W. Yi, J. Phys.: Condens. Matter, 2014, 26, 163201 CrossRef PubMed
.
- E. Solana-Madruga and A. M. Arévalo-López, J. Solid State Chem., 2022, 315, 123470 CrossRef CAS
.
- Y. Syono, S. Akimoto and Y. Endoh, J. Phys. Chem. Solids, 1971, 32, 243 CrossRef CAS
.
- M. Markkula, A. M. Arévalo-López, A. Kusmartseva, J. A. Rodgers, C. Ritter, H. Wu and J. P. Attfield, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 094450 CrossRef
.
- B. L. Chamberland, J. Solid State Chem., 1970, 1, 138 CrossRef CAS
.
- B. L. Chamberland, P. S. Danielson and C. W. Moeller, J. Solid State Chem., 1978, 26, 377 CrossRef CAS
.
- B. L. Chamberland, J. Solid State Chem., 1970, 2, 521 CrossRef CAS
.
- H. Yamamoto, S. Kamiyama, I. Yamada and H. Kimura, J. Am. Chem. Soc., 2022, 144, 1082 CrossRef CAS PubMed
.
- S. Kamiyama, I. Yamada, M. Fukuda, Y. Okazaki, T. Nakamura, T. Nishikubo, M. Azuma, H. Kimura and H. Yamamoto, Inorg. Chem., 2022, 61, 7841 CrossRef CAS PubMed
.
- J. R. Rea, P. W. Bless and E. Kostiner, J. Solid State Chem., 1972, 5, 446 CrossRef CAS
.
- J. R. Rea and E. Kostiner, J. Solid State Chem., 1973, 7, 163 CrossRef CAS
.
- S. Kamiyama, T. Sakakura, H. Kimura, H. Sagayama, S. Kishimoto, I. Yamada and H. Yamamoto, Cryst. Growth Des., 2023, 23, 2295 CrossRef CAS
.
- S. Biermann, A. Poteryaev, A. I. Lichtenstein and A. Georges, Phys. Rev. Lett., 2005, 94, 026404 CrossRef CAS PubMed
.
- M. W. Haverkort, Z. Hu, A. Tanaka, W. Reichelt, S. V. Streltsov, M. A. Korotin, V. I. Anisimov, H. H. Hsieh, H. J. Lin, C. T. Chen, D. I. Khomskii and L. H. Tjeng, Phys. Rev. Lett., 2005, 95, 196404 CrossRef CAS
.
- D. Shiga, B. E. Yang, N. Hasegawa, T. Kanda, R. Tokunaga, K. Yoshimatsu, R. Yukawa, M. Ktamura, K. Horiba and H. Kumigashira, Phys. Rev. B., 2020, 102, 115114 CrossRef CAS
.
- J. M. Tomczak, F. Aryasetiawan and S. Biermann, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 115103 CrossRef
.
- R. Zhang, Q. S. Fu, C. Y. Yin, C. L. Li, X. H. Chen, G. Y. Qian, C. L. Lu, S. L. Yuan, X. J. Zhao and H. Z. Tao, Sci. Rep., 2018, 8, 17093 CrossRef CAS
.
- F. Fauth, I. Peral, C. Popescu and M. Knapp, Powder Diffr., 2013, 28, S360 CrossRef CAS
.
- F. Fauth, R. Boer, F. Gil-Ortiz, C. Popescu, O. Vallcorba, I. Peral, D. Fulla, J. Benach and J. Juanhuix, Eur. Phys. J. Plus, 2015, 130, 160 CrossRef
.
- L. C. Chapon, P. Manuel, P. G. Radaelli, C. Benson, L. Perrott, S. Ansell, N. J. Rhodes, D. Raspino, D. Duxbury, E. Spill and J. Norris, Neutron News, 2011, 22, 22 CrossRef
.
- J. Rodriguez-Carvajal, Physica B, 1993, 192, 55 CrossRef CAS
.
- V. Petricek, M. Dusek and L. Palatinus, Z. Kristallogr., 2014, 229, 345 CAS
.
- D. Dai, J. Ren, W. Lian and H. Whangbo, SAMOA (Structure and Molecular Orbital Analyzer), http://primec.com/products/samoa/samoa.htm.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558 CrossRef CAS
.
- J. B. Goodenough, G. Dutta and A. Manthiram, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 43, 10170 CrossRef CAS PubMed
.
-
J. B. Goodenough, Magnetism and the chemical bond. Wiley Interscience, New York, 1963 Search PubMed
.
- I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 41, 244 CrossRef
.
- E. Morosan, J. Fleitman, T. Klimczuk and R. J. Cava, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 144403 CrossRef
.
-
H. T. Stokes, D. M. Hatch and B. J. Campbell, ISODISTORT, ISOTROPY Software Suite, http://iso.byu.edu Search PubMed
.
- B. J. Campbell, H. T. Stokes, D. E. Tanner and D. M. Hatch, J. Appl. Cryst., 2006, 39, 607 CrossRef CAS
.
- C. Aguilar-Maldonado, O. Mentré, A. A. Tsirlin, C. Ritter, A. Missiul, F. Fauth and A. M. Arévalo-López, Mater. Horiz., 2021, 8, 2325 RSC
.
- J.-P. Pouget, C. R. Phys., 2021, 22, 37 CAS
.
- J. del Valle, N. M. Vargas, R. Rocco, P. Salev, Y. Kalcheim, P. N. Lapa, C. Adda, M.-H. Lee, P. Y. Wang, L. Fratino, M. J. Rozenberg and I. K. Schuller, Science, 2021, 373(6557), 907 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Tables and figures for the low and high temperature polymorphs are included. CCDC 2259432. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3tc01541f |
| This journal is © The Royal Society of Chemistry 2023 |