Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sr2Sb2O7: a novel earth abundant oxide thermoelectric†
Luisa Herring
Rodriguez
 a,
Kieran B.
Spooner
a,
Kieran B.
Spooner
 a,
Maud
Einhorn
a and
David O.
Scanlon
a,
Maud
Einhorn
a and
David O.
Scanlon
 *ab
*ab
aDepartment of Chemistry, University College London, 20 Gordon Street, London, WC1H 0AJ, UK. E-mail: d.scanlon@ucl.ac.uk
bThomas Young Centre, University College London, Gower Street, London, WC1E 6BT, UK
First published on 14th June 2023
Abstract
Thermoelectric devices are increasingly proving to be a viable energy recycling method, with oxide thermoelectrics providing an earth abundant and non-toxic alternative to the materials traditionally used in the field. This study conducts a detailed investigation into the thermoelectric properties of the ternary wide band semiconductor Sr2Sb2O7, which has previously been synthesised under high temperature conditions and shown to be thermally stable. Lattice dynamics calculations predict lattice thermal conductivities below 1 W m−1 K−1 at temperatures above 1125 K. The Seebeck coefficient, electrical conductivity and electronic component to the thermal conductivity were calculated via the explicit calculation of the polar optical phonon scattering, acoustic deformation potential scattering and ionised impurity scattering rates within the AMSET code. The obtained results were combined to obtain a maximum ZT of 0.536 at 1400 K, which when nanostructured to 10 nm was increased to 0.71, showing its predicted potential to perform as a high-performance n-type oxide thermoelectric.
1 Introduction
The world's demand for energy has seen a steep rise since the 1950s; increasing from 28.5 TW h to 173.3 TW h in 2019.1 There has been an increase in the generation of energy through renewable sources, but in the first quarter of 2020, these accounted for only 28% of the total energy generated,2 as most still originated from traditional fossil fuel sources, the limitations of which are well known. A relatively untapped source of renewable energy is the recycling of excess heat energy. Although the efficiency of power plants has been increased, non-renewable energy sources still only operate with efficiencies ranging between 33 and 39%, with the remaining energy being released as heat.3 Moreover, household items and processes such as cement production have efficiencies which range between 12 and 68%.4 The conversion of the released excess heat into electricity is a huge potential source of renewable energy while reducing our energy demands.5Thermoelectric generators convert excess heat energy back into electrical energy through the use of a temperature gradient. This thermal gradient causes the charge carriers to diffuse from the hot side to the cold side, creating an electrical voltage. A thermocouple contains n- and p-type materials which are connected electrically in series and thermally in parallel. The effectiveness of a thermoelectric material is measured by the dimensionless figure of merit, ZT:6
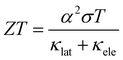 | (1) |
 | (2) |
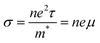 | (3) |
Recent studies have also shown some promising results for n-type thermoelectric materials, including SrTi0.8Nb0.2O3 which obtained a ZT of 0.37 at 1000 K,24 Sr0.92La0.08TiO3 which also obtained a ZT of 0.37 at 1045 K25 and  with x between 0.125 and 0.175 which achieved a ZT of 0.41 at 973 K,26 all of which were obtained experimentally. Effective thermocouples need both the n-type and p-type components to have high ZT values. As seen by the above mentioned recent studies, the ZT for n-type materials are significantly lower, showing the need for further study in this area.
with x between 0.125 and 0.175 which achieved a ZT of 0.41 at 973 K,26 all of which were obtained experimentally. Effective thermocouples need both the n-type and p-type components to have high ZT values. As seen by the above mentioned recent studies, the ZT for n-type materials are significantly lower, showing the need for further study in this area.
Transparent conducting oxides (TCOs), in particular, have been suggested as a subclass of oxides with great potential as thermoelectrics. As their name suggests, they are electrically conducting, fulfilling one of the criteria for a thermoelectric. Studies into TCO thermoelectric devices have shown high PFs, with the lattice thermal conductivities being the main contributors to the ZTs recorded being as low as 0.04.27 In particular, TCO materials which have cations with an empty s-orbital in their conduction band have been shown to have dispersive conduction band minima and thus high electrical conductivities. These dispersive bands indicate the potential low effective mass of electrons, which if doped can lead to n-type behaviour. The result is a high conductivity but a low Seebeck coefficient, highlighting how challenging it is to find materials which balance both properties. Studies have started looking at complex metal oxides as a way of reducing the lattice thermal conductivity while maintaining the advantages presented above. Materials with heavier cations embedded into these complex structures have been of particular interest as they have been shown to reduce phonon lifetimes and hence the material's lattice thermal conductivity.28
To date the most successful experimentally observed n-type TCO thermoelectric is the doubly doped ZnO ceramic Zn0.96Al0.02Ga0.02O with a maximum ZT of 0.65 at 1247 K.29 The main aspect of this material which enhances its properties to make it a successful thermoelectric is the inclusion of dopants in the structure which reduces the lattice thermal conductivity with respect to that of the undoped structure. The structure was also nanostructured with the same purpose. It was reduced from 40 and 7 W m−1 K−1 for Zn0.98Al0.02O to 21 and 5 W m−1 K−1 for Zn0.96Al0.02Ga0.02O at 300 K and 1100 K, respectively. This reduction in the lattice thermal conductivity increased the ZT from 0.36 to 0.65, showing its drastic effect.
The development of Zn0.96Al0.02Ga0.02O is a step in the right direction however it still does not compare to some of the available p-type thermoelectric materials. Examples include Bi0.94Pb0.06Cu0.99Fe0.01SeO which reached a ZT of 1.46 at 873 K and other doped forms of BiCuSeO, highlighting the need for further study into n-type oxide thermoelectrics.30–32
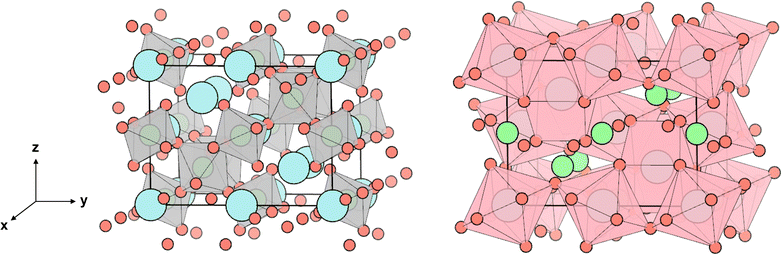
This work focuses on Sr2Sb2O7 as a potential thermoelectric. Sr2Sb2O7 ticks many of the boxes for a good TCO thermoelectric. It is a complex metal oxide with an Imma (74) structure composed of heavy cations (Sb5+ and Sr2+), with the corner and edge sharing Sb–O and Sr–O polyhedra creating a 3D network in the unit cell, shown in Fig. 1. This 3D complex structure should reduce phonon lifetimes while maintaining a good electrical conductivity, as electrons will be conducted via the connectivity of the Sb–O octahedra. The polyhedra can be further divided into two different types for each cation. The Sb–O octahedra can be classified as elongated and compressed octahedra and are shown with their varying bond lengths in Fig. 2. The Sr–O polyhedra differ more significantly between themselves. They are both bonded to eight oxygen atoms, however the first type forms a cube-like structure, whereas the second forms a hexagonal bipyramid structure. Both of these are depicted in Fig. 3. The cube-like polyhedra are connected via edge sharing and the hexagonal bipyramid polyhedra are connected via corner sharing. The two types are connected to each other via edge sharing. Moreover, the Sb5+ cations have empty 5s0 orbitals which should provide a 3-D electronic pathway for a high electrical conductivity.
Some materials with similar electronic structures to Sr2Sb2O7 have been studied recently and have shown great potential. These include ZnSb2O6,33 BaBi2O634 and α-Bi2Sn2O7.35 ZnSb2O6 was studied with a focus on its TCO properties and was shown to have a high conductivity when doped with gallium. BaBi2O6 was recently shown to have potential as an oxide thermoelectric except for its high lattice thermal conductivity.34 BaBi2O6 ticked the necessary criteria for a good thermoelectric, including a cation with an empty s orbital, and a network of Bi–O octahedra. Unfortunately, its structure was not stable at high temperatures and it only yielded a ZT of 0.22 at 600 K. Sr2Sb2O7 has a more complex structure than that of BaBi2O6, and could therefore have a lower lattice thermal conductivity. α-Bi2Sn2O7 also ticked most of the necessary criteria for an effective thermoelectric. The CBM of α-Bi2Sn2O7 was also composed of an empty s orbital and its complex crystal structure gave it a low lattice thermal conductivity. However, it had a low temperature stability, which meant it was predicted to only serve as a room temperature thermoelectric with a ZT of 0.36 at 385 K.
Sr2Sb2O7 has been studied a number of times with a focus on its photocatalytic properties. It was first investigated for its potential as a UV-photocatalyst in a water splitting mechanism; where it was proposed that the distorted SbO6 octahedra, which can be seen in Fig. 2, were the source of its photocatalytic efficiency.36 However, they presented no evidence which specifically indicated the involvement of the octahedra in the electron–hole splitting mechanism.37 Lin et al. investigated the photocataytic ability of Sr2Sb2O7 and Ca2Sb2O7 for the degradation of methyl orange.38 They also pointed at Sr2Sb2O7's more distorted octahedra and open structure as the reason for higher efficiency under UV radiation.
To the best of our knowledge this is the first study into Sr2Sb2O7 with a focus on its potential as an n-type oxide thermoelectric.
2 Computational methodology
This study used Density Functional Theory (DFT) within the Vienna ab initio simulation package (VASP).39–42 The projector augmented-wave (PAW) pseudopotential method was used to take the interaction between the core and valence electrons into account.43,44 The kinetic energy cutoff for the plane-wave basis set and the k-point mesh were converged at 500 eV and a 3 × 3 × 3 Γ-centred k-point grid to within 0.01 eV per atom. The convergence of the plane wave cut off and k-point mesh can be found in Fig. S1 (ESI†). A more dense 7 × 8 × 8 Γ-centred mesh was used for all density of states (DOS) calculations. The Perdew–Burke–Ernzerhof Generalised Gradient Approximation (GGA) for solids (PBEsol)45,46 was used in all lattice dynamics calculations, as it has been shown to be accurate but cheap for these calculations.47 This includes calculations to obtain the lattice thermal conductivity, ionic dielectric constant, piezoelectric coefficient, elastic constant and polar optical phonon frequency. The hybrid functional PBE046,48,49 which uses 25% Hartree–Fock exchange correlation was used for all DOS, band structure (BS), and high-frequency dielectric constant calculations. PBE0 has been proven to accurately calculate the electronic structure properties of solids to match experimental work and eliminates the band gap underestimation seen when GGA functionals are used.50The structure of Sr2Sb2O7 was optimised so the force on each atom was less than 0.01 eV Å−1 and the total electronic energy converged to less than 1 × 10−8 eV, with a larger cutoff energy of 650 eV to avoid basis set errors arising from Pulay stress.51
Computational studies into n-type oxide thermoelectrics have generally been carried out using the constant relaxation time approximation (CRTA) which has now been shown to lead to significantly overestimated ZTs.52 For this reason, the electronic transport properties including the conductivity, Seebeck coefficient and electronic thermal conductivity were calculated using the package AMSET.52 AMSET provides insight into the contributions to the transport properties from four scattering types including; the polar optical phonon scattering (POP), acoustic deformation potential scattering (ADP), ionised impurity scattering (IMP) and the piezoelectric scattering (PIE). These fit into the ZT equation through the electrical conductivity given in eqn (3) where τ−1 is the scattering rate, meaning that lower total scattering rates should lead to higher electrical conductivities and higher ZTs.
The parameters required as input for AMSET were calculated as follows: DFPT (density functional perturbation theory) with the GGA functional PBEsol was used to calculate the polar-optic phonon frequency, elastic constant and ionic dielectric constant. Hybrid first principles calculations were used to obtain the deformation potential and high frequency dielectric constant, which was added to the ionic dielectric constant to obtain the static dielectric constant. These values can be found in Table S1 (ESI†). Once the transport properties were calculated, they were converged with respect to interpolation factor, and this can be seen in Fig. S2 (ESI†).
Lattice dynamics calculations were carried out using the Phonopy package, which uses a supercell approach with finite displacements to calculate the second order force constants.53,54 The supercell size was converged with respect to the phonon dispersions along the high symmetry points of the Brillouin zone of the primitive cell. A 2 × 2 × 2 supercell with 198 atoms, was visually deemed as converged, and can be seen in Fig. S3 (ESI†). The atom contributions to the lattice dynamics were determined from the atom-projected phonon density of states which was calculated using Fourier interpolation. The lattice thermal conductivity was calculated using the Phono3py package which also uses a supercell approach to calculate the third order force constants.55 A 198 2 × 2 × 2 supercell with 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 displacement calculations was also used to calculate the third order force constants. The plots in this paper were plotted with sumo56 and ThermoPlotter57 and the structures are plotted with vesta,58 all of which are open source software.
140 displacement calculations was also used to calculate the third order force constants. The plots in this paper were plotted with sumo56 and ThermoPlotter57 and the structures are plotted with vesta,58 all of which are open source software.
3 Results and discussion
3.1 Structure and geometry
Sr2Sb2O7 has a 22 atom primitive unit cell which crystallises in the Imma (74) space group. It is composed of two types of SbO6 octahedra which create a 3D network across the lattice. The bond lengths for the distorted octahedra are reported in Table 1. The unit cell has elongated octahedra located in the tetrahedral holes, while the compressed octahedra are located on the octahedral holes and the faces of the cell and are all connected via corner sharing. An example of the octahedra with their bond lengths can be seen in Fig. 2. The starting structure was obtained from the MaterialsProject database where the structure was relaxed with PBE.59Table 1 also shows the relaxed lattice parameters obtained using the PBEsol and PBE0 functionals used in this study and in experiment. The lattice parameters obtained using the PBE0 functional are in all cases closer to those obtained in experiment than those calculated with PBEsol.| Parameter (Å) | PBEsol | PBE0 | Experiment38 |
|---|---|---|---|
| a | 7.492 | 7.448 | 7.456 |
| b | 10.401 | 10.366 | 10.371 |
| c | 7.711 | 7.702 | 7.686 |
| Elongated octahedra Sb–O bond lengths | 2.0220 (×2); 1.9912 (×4) | 2.0017 (×2); 1.9712 (×4) | 2.0479 (×2); 2.0091 (×4) |
| Compressed octahedra Sb–O bond lengths | 1.9453 (×2); 2.0384 (×4) | 1.9225 (×2); 2.0197 (×4) | 2.0330 (×2); 2.0219 (×4) |
3.2 Electronic structure
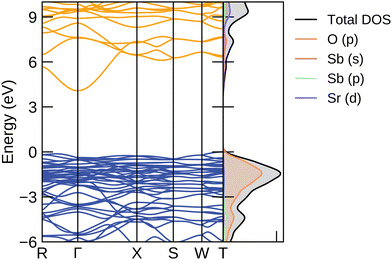
The electronic structure was calculated using the hybrid functional PBE0 from the optimised bulk geometry and plotted with sumo and can be seen in Fig. 4.56 The valence band maximum (VBM) is primarily composed of oxygen p-orbitals, a behaviour typical in oxides. The conduction band minimum (CBM) is dominated by antimony s orbitals, giving the disperse character needed for a good electrical mobility, as seen in other TCO materials.27The PBE0-calculated direct band gap is 4.45 eV at the Γ point and the indirect band gap between the T and Γ point is 4.08 eV, making it transparent, in contrast to the PBEsol results of 1.95 eV and 1.60 eV respectively. Two different experimental values for the optical band gap have been reported, which have both been collected using UV-vis diffuse reflectance spectra. The values range between 4.160 and 3.86 eV.38 The difference between the two reported values stems from the crystallinity of the samples which the spectra were carried out upon. The Sr2Sb2O7 sample carried out by Chen et al. which reported an optical band gap of 4.1 eV, was synthesised via a hydrothermal method and resulted in crystals with an average size of 23.9 nm. The sample tested by Lin et al., on the other hand, was prepared via a solid state reaction method and resulted in an average crystallite size of 300 nm and with a band gap of 3.86 eV. Absorption spectra vary significantly depending on the quality of the sample. Crystallite size and thickness play major roles in the accuracy of the observed results. As such, the larger crystal size obtained by Lin et al. seems to suggest their reported optical band gap to be the more reliable one. By comparison, PBEsol majorly underestimates the band gap, with an indirect band gap of 1.60 eV, a direct band gap of 1.95 eV and an optical band gap of 1.94 eV.
There are a range of reasons for the difference between the optical band gap calculated by Lin et al. and our calculated value of 4.49 eV. Excitonic effects can occur when the band edges are flat and result in the lowering of the adsorption energy onset. Another possibility is that vibrational effects, introduced due to the measurements being carried out at room temperature, lower the optical band gap. Finally, it has recently been shown that the PBE0 functional can slightly overestimate band gaps by 3%.33 All these factors may contribute to the discrepancy observed between our calculated and the reported optical band gaps. As the values are still within a 5% error margin, the PBE0 functional is deemed to provide sufficient detail for this study.
The curvature of the bands gives the inverse of the charge carrier effective mass, which is calculated by sumo using parabolic band fitting with a k-point range of 0.0322. The valence band maximum is flat resulting in a moderate hole effective mass of 1.39 me. Typical transparent conductors have effective masses with values below 1 me. As Sr2Sb2O7 is expected to be n-type in nature, the electron effective mass is of more interest. The bands at the conduction band minimum (CBM) are considerably disperse, with a low mass of 0.34 me, and suggesting that a high conductivity is possible if effectively doped. The R and X point represent points of high symmetry at (0, 0.5, 0) and (0.5, −0.5, 0.5) in the Brillouin zone. This translates to the electron mobility being particularly high between the x and y axis and across the diagonal of the unit cell respectively.
Efficient thermoelectric materials need to display metal-like conduction. A way to determine this is by using the Mott criterion, nMott, a value which gives the carrier concentration above which this requirement will be satisfied. It can be calculated from the electron and hole effective masses via the band structure as follows:61–63
 | (4) |
3.3 Transport properties
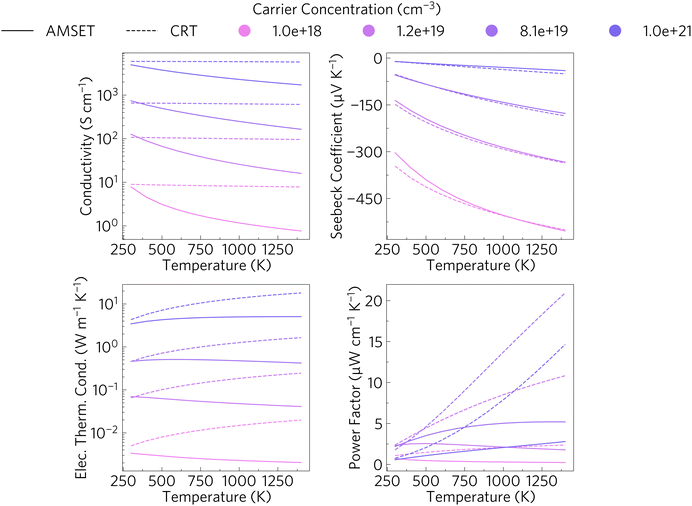
Fig. 5 shows the conductivity, Seebeck coefficient, electrical thermal conductivity and power factor (PF) versus temperature at different carrier concentrations. The PF is the combination of the Seebeck coefficient and the electrical conductivity. The PF is often used for easier comparison between different materials, due to the complex relationship between the Seebeck coefficient and electrical conductivity. The solid lines represent the direction-averaged values obtained when using AMSET and the dashed ones represent those just calculated using the CRTA (constant relaxation time approximation). There is a small degree of anisotropy, with power factor in the z direction being about 8% lower than in the x and y directions at high temperatures due to a small reduction in both electrical conductivity and Seebeck coefficient, as shown in Fig. S4 (ESI†). The discrepancies between the values obtained with AMSET and the CRTA are seen particularly in the bottom right hand graph showing the power factor, highlighting the necessity to explicitly calculate the scattering rates as the CRTA significantly overestimates the conductivity and electrical thermal conductivity, which leads to higher PF values and thus an overestimation of the ZT.To put these results into context with other calculated thermoelectric properties we highlight a study into the thermoelectric response of Sr1−xLaxTiO3 by Okuda et al.64 SrTiO3 obtained a Seebeck coefficient of ∼−350 μV K−1 at 300 K which decreased in magnitude to ∼−75 μV K−1 for Sr0.9La0.1TiO3. It is however easier to compare the power factors, which at room temperature ranged between 28 and 36 μW cm−1 K−2 with carrier concentrations between 0.2 and 2 × 1021 cm−3 for their range of x values. At our target high temperature range of 1000–1400 K, Sr2Sb2O7 obtained values between 1.78 and 1.79 μW cm−1 K−2 for carrier concentrations of 10 × 1019 to 10 × 1021 cm−3. This is significantly lower than that obtained by Okuda et al., however that report calculated the transport properties using the CRTA, which as can be seen by the dashed lines, yields a much higher range of values than those calculated with AMSET.
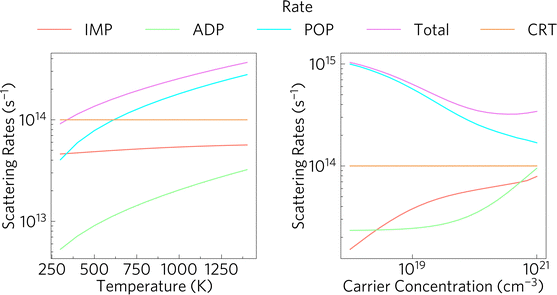
The relationship between the scattering rates and the electrical conductivity can be seen by comparing Fig. 5 and 6. As expected, as the temperature increases, the total scattering rate increases too. The inverse relationship between the scattering rate and the conductivity is highlighted as the conductivity decreases with increasing temperature when calculated with AMSET. This is due to there being increased electron scattering at higher temperatures which reduces the conductivity.
Fig. 6 also provides insight into the contributions from the different types of scattering rates in Sr2Sb2O7. The predominant type of scattering is POP scattering. POP scattering tends to originate from the vibrations of the optical phonon modes creating dipoles which are then scattered across the crystal structure. Optical vibrations result from the out-of-phase motion of atoms in the system. This indicates that the charge carriers are mainly scattered through the out of phase motion of atoms. As previously mentioned, phonon scattering increases with temperature, while the scattering rate decreases with increased carrier concentration, explaining the higher conductivity. At low carrier concentrations, POP is the main scattering type, ADP (acoustic deformation potential) and IMP (ionised impurity scattering) become more significant as the number of charge carriers increases.
Acoustic deformation potential scattering is caused by the coupling of electrons with acoustic phonons. Lattice vibrations cause the displacements of atoms from their lattice sites, modifying the band structure. This modification causes electrons in the conduction band edge to interact with the phonons and scatter them. This explains the steep increase in ADP scattering as the carrier concentration increases, as this will result in a larger number of electrons in the conduction band which can couple with phonons. Similarly, increasing the temperature results in more lattice vibrations and also results in higher quantities of excited electrons due to higher thermal energy in the system. IMP scattering is dominated by the presence of charged defects which, in an n-type material, will scatter electrons in the lattice. Higher carrier concentrations will also lead to more electrons being scattered and to IMP scattering to become more relevant, until it plateaus. IMP is relatively temperature independent.
AMSET also includes contributions from piezoelectric scattering, however, this tends to originate from a lack of a centre of inversion in the unit cell, which Sr2Sb2O7 has, clarifying why it is not present in this system.
3.4 Phonon dispersion and density of states
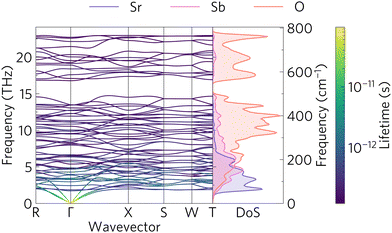
The phonon band structure and PDOS can be seen in Fig. 7 which was calculated with Phonopy and plotted with ThermoPlotter.54 The phonon band structure is calculated via 20 finite displacements on a 2 × 2 × 2 supercell, with 176 atoms, and using the PBEsol functional. The primitive cell contains 22 atoms, resulting in 3N = 66 phonon bands at each wavevector. Imaginary modes, indicating dynamic instability would appear as modes with a negative frequency. There are no such modes, indicating that Sr2Sb2O7 is dynamically stable. | ||
| Fig. 7 Phonon band structure along the high symmetry points of the Brillouin zone of Sr2Sb2O7 and the atom-projected density of states. | ||
Similar to the charge carrier mobilities in the electronic band structure, phonon group velocities are given by the gradient of the bands. Good thermoelectric materials have low phonon group velocities in order to minimise the lattice thermal conductivity. The phonon band structure shows mostly flat modes. The PDOS gives insight into which atoms contribute to the group velocities. The three bottom modes, also known as acoustic modes which at the Γ point correspond to translations in the x, y and z directions, are dominated by the heavy Sr2+ cations. The optical modes correspond to the rest of the higher energy modes. These tend to have significant contribution from oxygen. The plot also shows the phonon lifetimes as a colour gradient with the longer lifetimes appearing as yellow, while the short lifetime phonons are denoted in blue. It is clear that the longer lifetimes are correlated to the phonon group velocities as the larger phonon gradients also correspond to the longer lifetimes. This said, the lifetimes are still comparatively small compared to those seen in other materials, for example, LaZnOP and LaZnOAs have phonon lifetimes which range between 10 × 10−12 and 10 × 10−9 s.65
The phonon band gap between 14.8 and 17 THz originates from the contrast in molecular mass of the oxygen and the heavier strontium and antimony atoms. This band gap has been proven to reduce the thermal conductivity of materials by flattening the phonon modes and reducing the group velocity.9,66 There are also some avoided crossings between the acoustic modes near the Γ and R and X points and the low energy optical modes. These avoided crossings have been shown to also reduce the phonon group velocities and hence reduce the thermal transport abilities of the material.67–69 Avoided crossings are common when heavy atoms are present, and are often described as displaying ‘rattling’ behaviour by scattering phonons.70 They have also been suggested to originate from symmetry-forbidden transitions.71 Finally, the high density of the optical modes between 5 and 14.7 THz, which is often seen in complex crystal structures, has been shown to provide scattering channels for the high velocity acoustic modes; further reducing the lattice thermal conductivity.35
3.5 Lattice thermal conductivity
The lattice thermal conductivity can be seen in Fig. 8. Like Phonopy, Phono3py uses a supercell approach to obtain third order force constants. As described in Section 2, a 2 × 2 × 2 cell, with 176 atoms and 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 displacements was used to obtain the third order force constant. The lattice thermal conductivity was converged with respect to q-point mesh at 1000 K, and this can be seen in Fig. S5 (ESI†). All calculations were carried out with the PBEsol functional. The plot shows that Sr2Sb2O7 has a moderate lattice thermal conductivity. The lattice thermal conductivities are almost the same in the x and y directions whereas it is lower in the z direction.
140 displacements was used to obtain the third order force constant. The lattice thermal conductivity was converged with respect to q-point mesh at 1000 K, and this can be seen in Fig. S5 (ESI†). All calculations were carried out with the PBEsol functional. The plot shows that Sr2Sb2O7 has a moderate lattice thermal conductivity. The lattice thermal conductivities are almost the same in the x and y directions whereas it is lower in the z direction.
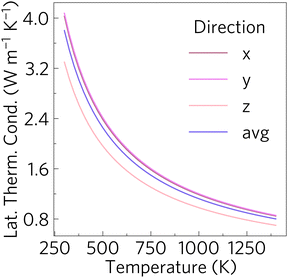 | ||
| Fig. 8 Calculated lattice thermal conductivity with respect to temperature in the x, y and z directions of the lattice, where the values obtained for x and y are almost identical. | ||
Fig. 10 shows the contributions to the phonon projected density of states (PDOS) divided into the different type of cation polyhedra described in the introduction and demonstrates that the first peak at 2 THz corresponds to the cube-like Sr–O polyhedra and is aligned with the very flat bands in the phonon band structure. This suggests that the direction which contains more cube-like Sr–O polyhedra will have a low group velocity and thus a low lattice thermal conductivity. Fig. 9 depicts the polyhedra from the different axis of the conventional unit cell, demonstrating the polyhedra are more densely packed in the z axis, explaining its lower lattice thermal conductivity.
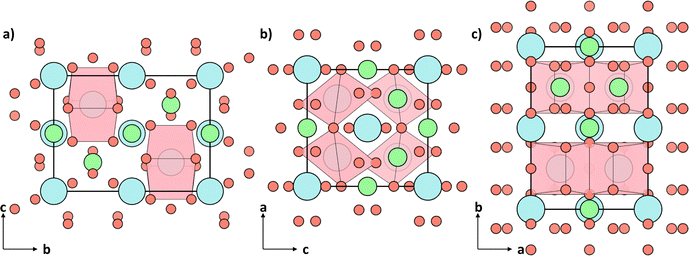 | ||
| Fig. 9 The conventional cell of Sr2Sb2O7 with only the cube-like Sr–O polyhedra shown viewed from the (a) x-axis, (b) y-axis and (c) z-axis. | ||
 | ||
| Fig. 10 Phonon band structure with the projected density of states divided into the different types of cation polyhedra. | ||
The difference between the directions decreases with increasing temperature. This can be attributed to the higher vibrational energy associated with higher temperatures, which will result in the phonons being scattered more equally in all directions. Moreover, the difference between the values obtained for the different directions is small enough to be inconsequential in a real world setting, where the average lattice thermal conductivity dominates. The lattice thermal conductivity reaches values below 1 W m−1 K−1 at temperatures above 1125 K which is much lower than many of the well known n-type oxide thermoelectrics, including Sr1−3x/2LaxTiO3 which obtained a ZT of 0.41 at 973 K with a lattice thermal conductivity of 2.5 W m−1 K−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 and Ca0.97Bi0.03MnCu0.04O3−δ which had a lattice thermal conductivity of 1.5 W m−1 K−1 at 1073 K and obtained a ZT of 0.44.72
26 and Ca0.97Bi0.03MnCu0.04O3−δ which had a lattice thermal conductivity of 1.5 W m−1 K−1 at 1073 K and obtained a ZT of 0.44.72
Fig. 11 highlights the potential reduction in lattice thermal conductivity if Sr2Sb2O7 were nanostructured to 10 nm. This dimension was chosen as it was successfully synthesised to an average crystallite size of 6 nm in 2008.73 This shows the significant effect nanostructuring could have on the lattice thermal conductivity by reducing it to a maximum of 0.72 W m−1 K−1 in the c direction. It is interesting to note however, that although the lattice thermal conductivity in the z direction is lower at higher mean free paths, it is higher than in the x and y directions at mean free paths between ∼1 nm and ∼50 nm. This suggests there are more low mean free path phonons which travel in the z direction than in the x and y directions.
3.6 Thermoelectric properties
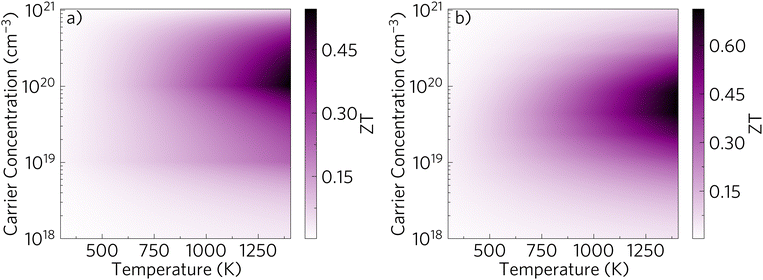
The above properties are combined to obtain the ZT for a range of temperatures and carrier concentrations as seen in Fig. 12(a) in the form of a heatmap. The average ZT is plotted, with a maximum ZT of 0.596 at 1400 K and 8.11 × 1019 cm−3, which breaks down to 0.584, 0.578 and 0.626 in the x, y and z directions respectively. The ZT compares well to the previously mentioned champion Zn0.96Al0.02Ga0.02O with a ZT of 0.65 at 1247 K.29For comparison, the ZT was also calculated with the CRT and can be seen in Fig. S6 (ESI†). The maximum ZT obtained with this method is of 1.6, which would make it the best n-type oxide thermoelectric to date. This highlights the extent to which the CRT overestimates ZTs.
3.7 Nanostructuring potential
Fig. 11 showed the potential of nanostructuring on the lattice thermal conductivity at 1000 K. Nanostructuring reduced the lattice thermal conductivity by 50% at 1000 K and by 45% at 1400 K. It reached values below 1 Wm−1 K−1 in all directions at temperatures above 475 K. The full plot of the lattice thermal conductivity vs. temperature, when nanostructured to 10 nm can be seen in Fig. S7 (ESI†). Moreover, this reduction in lattice thermal conductivity does not take into account the phonon scattering contribution from potential external dopants, which will further reduce it. This means that this is still an upper limit, and further decreases in κlat may be possible. The reduction in lattice thermal conductivity often results in an increase in the maximum ZT.74 To test the effect it would have on the transport properties, AMSET was rerun with nanostructuring to 10 nm included. It was seen that the electronic transport properties reduced, resulting in a power factor reduction of around 15% under optimal doping and temperature (Fig. S8, ESI†), but this is much less than the effect on the thermal conductivity. These transport properties were used to calculate the ZT plot seen in Fig. 12(b). Nanostructuring to 10 nm would lead to a ZT of 0.71 at 1400 K and a carrier concentration of 4.33 × 1019 cm−3. This increase could make Sr2Sb2O7 a world leader in the field of n-type oxide thermoelectrics.4 Conclusions
In this computational thermoelectric study we have shown the necessity of using approaches which go beyond the constant relaxation time to ensure a more realistic estimation of the ZT. The structural, electronic, electronic transport and lattice dynamic properties were calculated to assess the thermoelectric potential of Sr2Sb2O7 using DFT. The electronic properties showed that the material is transparent with a direct and indirect band gap of 4.45 eV and 4.08 eV, respectively. Analysis of the individual scattering rate contributions to the electronic transport properties showed that polar optical phonon scattering is the predominant type of scattering. This was related to the fact that most of the lattice thermal conductivity originates from the Sr2+ cations in the lattice, which also contributed the most to the phonon group velocities. Its lattice thermal conductivity reached a value below 1 W m−1 K−1 at temperatures above 1125 K, which was significantly lower than other materials which obtained ZT of ∼0.4 at similar temperatures. Sr2Sb2O7 has shown huge potential as an oxide thermoelectric with a current predicted maximum ZT of 0.536 at 1400 K and a carrier concentration of 8.11 × 1019 cm−3. When nanostructured to 10 nm, the ZT is predicted to increase to 0.71 at 1400 K and 4.33 × 1019 cm−3, which puts it above the current champion, doubly doped ZnO which has a ZT of 0.65 at 1100 K. If dopable, this could make Sr2Sb2O7 the new leader of n-type oxide thermoelectric materials. The next step is to fully examine Sr2Sb2O7's defect chemistry to determine whether n-type doping is possible and whether the carrier concentrations needed to reach the maximum ZT are achievable.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Via our membership of the United Kingdoms HEC Materials Chemistry Consortium, which is funded by EPSRC (EP/L000202, EP/R029431, EP/T022213), this work used the ARCHER2 U.K. National Supercomputing Service (http://www.archer2.ac.uk) and the U.K. Materials and Molecular Modelling Hub for computational resources, MMM Hub, which is also partially funded by EPSRC (EP/P020194 and EP/T022213). The authors acknowledge the use of the UCL Myriad, and Kathleen and Thomas High Throughput Computing Facilities (Myriad@UCL, Kathleen@UCL and Thomas@UCL) and associated support services in the completion of this work. DOS acknowledges support from the EPSRC (EP/N01572X/1). DOS acknowledges membership of the Materials Design Network.Notes and references
- H. Ritchie and M. Roser, Our World in Data, 2020.
- Global Energy Review, International energy agency technical report, 2020.
- Is the Cooling of Power Plants a Constraint on the Future of Nuclear Power?, Online, https://www.world-nuclear.org/our-association/publications/technical-positions/cooling-of-power-plants.aspx.
- Q. Bian, Environ. Syst. Res., 2020, 9, 8 CrossRef.
- in CRC Handbook of Thermoelectrics, ed. D. Rowe, CRC Press, 2018 Search PubMed.
- A. F. Ioffe, L. S. Stilbans, E. K. Iordanishvili, T. S. Stavitskaya, A. Gelbtuch and G. Vineyard, Phys. Today, 1959, 12, 42 CrossRef.
- A. Volta, Le opere di Alessandro Volta, Edizione Nazionale, Milano, Ulrico Hoepli, 1918 Search PubMed.
- T. J. Seebeck, Ann. Phys., 1826, 82, 253–286 CrossRef.
- G. S. Nolas, G. A. Slack, D. T. Morelli, T. M. Tritt and A. C. Ehrlich, J. Appl. Phys., 1996, 79, 4002 CrossRef CAS.
- S. Parveen, S. V. Vedanayakam and R. P. Suvarna, Int. J. Eng. Technol., 2018, 7, 189 CrossRef CAS.
- C. Goupil, W. Seifert, K. Zabrocki, E. Müller and G. J. Snyder, Entropy, 2011, 13, 1481–1517 CrossRef.
- T. Caillat, J.-P. Fleurial, G. Snyder and A. Borshchevsky, Proceedings ICT 2001. 20 International Conference on Thermoelectrics (Cat. No. 01TH8589), 2001.
- M. H. Francombe, Br. J. Appl. Phys., 1958, 9, 415–417 CrossRef CAS.
- H. J. Goldsmid, Thermoelectric Refrigeration, Springer, US, 1964 Search PubMed.
- Y. Pei, H. Wang and G. J. Snyder, Adv. Mater., 2012, 24, 6125–6135 CrossRef CAS PubMed.
- D. Beretta, N. Neophytou, J. M. Hodges, M. G. Kanatzidis, D. Narducci, M. Martin-Gonzalez, M. Beekman, B. Balke, G. Cerretti, W. Tremel, A. Zevalkink, A. I. Hofmann, C. Müller, B. Dörling, M. Campoy-Quiles and M. Caironi, Mater. Sci. Eng., R, 2019, 138, 100501 CrossRef.
- F. Rosi, Solid-State Electron., 1968, 11, 833–868 CrossRef.
- C. Wood, Rep. Prog. Phys., 1988, 51, 459–539 CrossRef CAS.
- H. Goldsmid, Materials, 2014, 7, 2577–2592 CrossRef CAS PubMed.
- I. Terasaki, Y. Sasago and K. Uchinokura, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, R12685–R12687 CrossRef CAS.
- M. Ito and D. Furumoto, J. Alloys, 2008, 450, 494–498 CrossRef CAS.
- J. Li, J. Sui, Y. Pei, X. Meng, D. Berardan, N. Dragoe, W. Cai and L.-D. Zhao, J. Mater. Chem. A, 2014, 2, 4903 RSC.
- Z. Liu, X. Guo, R. Li, J. Qin, H. Li, X. Chen and X. Zhou, J. Materiomics, 2019, 5, 649–656 CrossRef.
- Y. Chen, J. Liu, Y. Li, X. Zhang, X. Wang, W. Su, J. Li and C. Wang, J. Electron. Mater., 2018, 48, 1147–1152 CrossRef.
- A. Kikuchi, N. Okinaka and T. Akiyama, Scr. Mater., 2010, 63, 407–410 CrossRef CAS.
- Z. Lu, H. Zhang, W. Lei, D. C. Sinclair and I. M. Reaney, Chem. Mater., 2016, 28, 925–935 CrossRef CAS.
- K. B. Spooner, A. M. Ganose and D. O. Scanlon, J. Mater. Chem. A, 2020, 8, 11948–11957 RSC.
- N. V. Nong, N. Pryds, S. Linderoth and M. Ohtaki, Adv. Mater., 2011, 23, 2484–2490 CrossRef PubMed.
- M. Ohtaki, K. Araki and K. Yamamoto, J. Electron. Mater., 2009, 38, 1234–1238 CrossRef CAS.
- B. Feng, G. Li, Z. Pan, X. Hu, P. Liu, Z. He, Y. Li and X. Fan, J. Solid State Chem., 2018, 266, 297–303 CrossRef CAS.
- B. Feng, G. Li, X. Hu, P. Liu, R. Li, Y. Zhang, Y. Li, Z. He and X. Fan, J. Alloys, 2020, 818, 152899 CrossRef CAS.
- Q. Wen, C. Chang, L. Pan, X. Li, T. Yang, H. Guo, Z. Wang, J. Zhang, F. Xu, Z. Zhang and G. Tang, J. Mater. Chem. A, 2017, 5, 13392–13399 RSC.
- A. J. Jackson, B. J. Parrett, J. Willis, A. M. Ganose, W. W. W. Leung, Y. Liu, B. A. D. Williamson, T. K. Kim, M. Hoesch, L. S. I. Veiga, R. Kalra, J. Neu, C. A. Schmuttenmaer, T.-L. Lee, A. Regoutz, T.-C. Lee, T. D. Veal, R. G. Palgrave, R. Perry and D. O. Scanlon, ACS Energy Lett., 2022, 3807–3816 CrossRef CAS PubMed.
- K. B. Spooner, A. M. Ganose, W. W. W. Leung, J. Buckeridge, B. A. D. Williamson, R. G. Palgrave and D. O. Scanlon, Chem. Mater., 2021, 33, 7441–7456 CrossRef CAS.
- W. Rahim, J. M. Skelton and D. O. Scanlon, J. Mater. Chem. A, 2020, 8, 16405–16420 RSC.
- J. Sato, N. Saito, H. Nishiyama and Y. Inoue, J. Phys. Chem. B, 2003, 107, 7965–7969 CrossRef CAS.
- J. Sato, N. Saito, H. Nishiyama and Y. Inoue, J. Photochem. Photobiol., A, 2002, 148, 85–89 CrossRef CAS.
- X. Lin, F. Huang, W. Wang, Y. Wang, Y. Xia and J. Shi, Appl. Catal., A, 2006, 313, 218–223 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. Zhou and K. Burke, Phys. Rev. Lett., 2008, 100, 136406 CrossRef PubMed.
- J. M. Skelton, D. Tiana, S. C. Parker, A. Togo, I. Tanaka and A. Walsh, J. Chem. Phys., 2015, 143, 064710 CrossRef PubMed.
- J. Paier, R. Hirschl, M. Marsman and G. Kresse, J. Chem. Phys., 2005, 122, 234102 CrossRef PubMed.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- J. He and C. Franchini, J. Phys.: Condens. Matter, 2017, 29, 454004 CrossRef PubMed.
- P. Pulay, Mol. Phys., 1969, 17, 197–204 CrossRef CAS.
- A. M. Ganose, J. Park, A. Faghaninia, R. Woods-Robinson, K. A. Persson and A. Jain, Nat. Commun., 2021, 12, 2222 CrossRef CAS PubMed.
- A. Togo, F. Oba and I. Tanaka, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 134106 CrossRef.
- A. Togo and I. Tanaka, Scr. Mater., 2015, 108, 1–5 CrossRef CAS.
- A. Togo, L. Chaput and I. Tanaka, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 094306 CrossRef.
- A. M. Ganose, A. J. Jackson and D. O. Scanlon, J. Open Source Software, 2018, 3, 717 CrossRef.
- K. B. Spooner, M. Einhorn, D. W. Davies and D. O. Scanlon, ThermoPlotter: Streamlined Analysis of Thermoelectric Properties, 2022, https://github.com/SMTG-UCL/ThermoPlotter.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- Materials Data on Sr 2 Sb 2 O 7 by Materials Project , 2020.
- L. Chen, P. Chen, H. Wang, W. Cui, J. Sheng, J. Li, Y. Zhang, Y. Zhou and F. Dong, ACS Appl. Mater. Interfaces, 2021, 13, 5153–5164 CrossRef CAS PubMed.
- N. F. Mott, Rev. Mod. Phys., 1968, 40, 677–683 CrossRef CAS.
- P. P. Edwards and M. J. Sienko, J. Am. Chem. Soc., 1981, 103, 2967–2971 CrossRef CAS.
- D. O. Scanlon, A. B. Kehoe, G. W. Watson, M. O. Jones, W. I. F. David, D. J. Payne, R. G. Egdell, P. P. Edwards and A. Walsh, Phys. Rev. Lett., 2011, 107, 246402 CrossRef PubMed.
- T. Okuda, K. Nakanishi, S. Miyasaka and Y. Tokura, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 113104 CrossRef.
- M. Einhorn, B. A. D. Williamson and D. O. Scanlon, J. Mater. Chem. A, 2020, 8, 7914–7924 RSC.
- S. M. Kauzlarich, A. Zevalkink, E. Toberer and G. J. Snyder, Energy and Environment Series, Royal Society of Chemistry, 2016, pp. 1–26 Search PubMed.
- M. Christensen, A. B. Abrahamsen, N. B. Christensen, F. Juranyi, N. H. Andersen, K. Lefmann, J. Andreasson, C. R. H. Bahl and B. B. Iversen, Nat. Mater., 2008, 7, 811–815 CrossRef CAS PubMed.
- H. Lin, G. Tan, J.-N. Shen, S. Hao, L.-M. Wu, N. Calta, C. Malliakas, S. Wang, C. Uher, C. Wolverton and M. G. Kanatzidis, Angew. Chem., Int. Ed., 2016, 55, 11431–11436 CrossRef CAS PubMed.
- W. Rahim, J. M. Skelton and D. O. Scanlon, J. Mater. Chem. A, 2021, 9, 20417–20435 RSC.
- J. Dong, O. F. Sankey and C. W. Myles, Phys. Rev. Lett., 2001, 86, 2361–2364 CrossRef CAS PubMed.
- K. Brlec, K. B. Spooner, J. M. Skelton and D. O. Scanlon, J. Mater. Chem. A, 2022, 10, 16813–16824 RSC.
- X. Song, S. A. P. Navia, L. Liang, C. Boyle, C.-O. Romo-De-La-Cruz, B. Jackson, A. Hinerman, M. Wilt, J. Prucz and Y. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 39018–39024 CrossRef CAS PubMed.
- H. Xue, Z. Li, H. Dong, L. Wu, X. Wang and X. Fu, Cryst. Growth Des., 2008, 8, 4469–4475 CrossRef CAS.
- Ø. Prytz, E. Flage-Larsen, E. S. Toberer, G. J. Snyder and J. Taftø, J. Appl. Phys., 2011, 109, 043509 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3tc01003a |
| This journal is © The Royal Society of Chemistry 2023 |