Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Color tuning of multi-resonant thermally activated delayed fluorescence emitters based on fully fused polycyclic amine/carbonyl frameworks†‡
John Marques
dos Santos
 a,
Chin-Yiu
Chan
a,
Chin-Yiu
Chan
 c,
Shi
Tang
c,
Shi
Tang
 d,
David
Hall
d,
David
Hall
 ab,
Tomas
Matulaitis
ab,
Tomas
Matulaitis
 a,
David B.
Cordes
a,
David B.
Cordes
 a,
Alexandra M. Z.
Slawin
a,
Alexandra M. Z.
Slawin
 a,
Youichi
Tsuchiya
a,
Youichi
Tsuchiya
 c,
Ludvig
Edman
c,
Ludvig
Edman
 *d,
Chihaya
Adachi
*d,
Chihaya
Adachi
 *c,
Yoann
Olivier
*c,
Yoann
Olivier
 *b and
Eli
Zysman-Colman
*b and
Eli
Zysman-Colman
 *a
*a
aOrganic Semiconductor Centre, EaStCHEM School of Chemistry, University of St Andrews, St Andrews, KY16 9ST, UK. E-mail: eli.zysman-colman@st-andrews.ac.uk
bLaboratory for Computational Modeling of Functional Materials, Namur Institute of Structured Matter, University of Namur, Rue de Bruxelles, 61, 5000 Namur, Belgium. E-mail: yoann.olivier@unamur.be
cCenter for Organic Photonics and Electronics Research (OPERA), Kyushu University, Motooka 744, Nishi-ku, Fukuoka 819-0395, Japan. E-mail: adachi@cstf.kyushu-u.ac.jp
dThe Organic Photonics and Electronics Group, Department of Physics, Umea University, SE-90187 Umea, Sweden. E-mail: ludvig.edman@umu.se
First published on 2nd May 2023
Abstract
Two novel π-extended amine/carbonyl-based multi-resonance thermally activated delayed fluorescence (MR-TADF) emitters have been designed and synthesized. The two emitters are isomeric, composed of nine fused rings and show green-yellow emission. Sym-DiDiKTa and Asym-DiDiKTa possess tert-butyl groups distributed in a symmetrical and asymmetrical fashion, respectively, which significantly impact the single-crystal packing structure. The two compounds possess similar singlet–triplet energy gaps, ΔEST, of around 0.23 eV, narrowband emission characterized by a full-width at half-maximum, FWHM, of 29 nm and a photoluminescence quantum yield, ΦPL, of 70% and 53% for the symmetric and asymmetric counterparts, respectively, in toluene. Investigation in OLEDs demonstrated that the devices with Sym-DiDiKTa and Asym-DiDiKTa displayed electroluminescence maxima of 543 and 544 nm, and maximum external quantum efficiencies (EQEmax) of 9.8% and 10.5%, respectively. The maximum EQE was further improved to 19.9% by employing a hyperfluorescence strategy. We further present the first example of a neutral MR-TADF emitter incorporated in a LEC device where Sym-DiDiKTa acts as the emitter. The LEC shows a λEL at 551 nm and FWHM of 60 nm with luminance of 300 cd m−2 and a fast turn-on time of less than 2 s to 100 cd m−2.
10th Anniversay statementThe journal of Materials Chemistry C has been one of our favourite journals for electroluminescent materials and device research. The quality and breadth of the science covered in the journal particularly in the area of emitter development for electroluminescent devices has been excellent. It is always a pleasure to peruse each week's table of contents and to then read exciting and new science in optoelectronic materials. We look forward to the next 10 years and beyond and will continue to support JMCC. |
Introduction
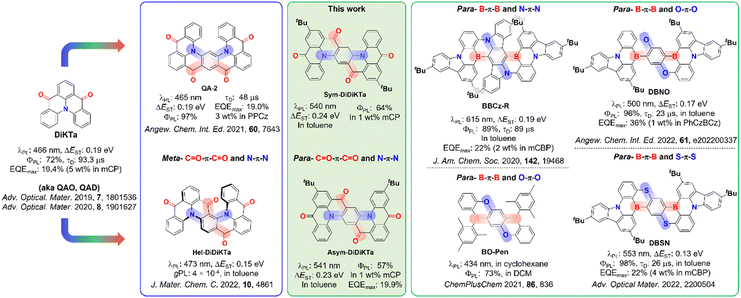
Organic light-emitting diodes (OLEDs) have now matured as a technology and are now integrated in efficient, flexible and ultra-high contrast next-generation displays.1,2 Organic thermally activated delayed fluorescence (TADF) materials are increasingly viewed as an attractive alternative emitter class to phosphorescent complexes that contain noble metals as they do not contain scarce elements yet can likewise harvest both singlet and triplet excitons in the device to generate light at comparable efficiencies.3–7 Unlike phosphorescent OLEDs that funnel the emitting excitons via the lowest-lying triplet excited state, TADF compounds convert triplet excitons into singlets via reverse intersystem crossing (RISC),8,9 which is possible at ambient temperatures due to the small S1–T1 energy gap, ΔEST.10 Compounds that possess a small ΔEST show a small overlap of the electron density between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). To adhere to this requirement, the great majority of organic TADF emitters are based on a highly twisted donor-acceptor architecture and emit from a long-range charge transfer (LRCT) state.7 This strategy, however, leads to molecules for which the conformational space is large in the excited state11,12 that manifests in broadband emission with full width half maximum (FWHM) values of around 70–100 nm.13 This is unattractive to the display industry as the color purity of these devices is poor and color filters must therefore be used to meet the industry standards for red, green and blue pixels. Hence, significant effort has been devoted in recent years to suppress vibrational and conformational relaxation within the emitter through the development of narrowband emitters.14–20An exciting molecular design strategy to achieve these requirements was introduced by Hatakeyama and co-workers and relies typically on p- and n-doped nanographenes.21,22 Termed multiple resonance (or multiresonant) TADF (MR-TADF) emitters, these compounds possess small-to-moderate ΔEST values, narrowband emission (with FWHM typically < 30 nm) and limited positive solvatochromism due to the short-range charge transfer (SRCT) nature of the emissive S1.22 Maximum external quantum efficiencies (EQEmax) as high as 34% for deep blue,22 40% for sky-blue,23 35%24 for green and 36% for red25 have been achieved for OLEDs containing MR-TADF emitters comprised of boron–nitrogen (B/N) doped polycyclic aromatic frameworks. Recently, the library of MR-TADF emitters has been expanded to contain boron–oxygen (B/O)-based emitters26 and carbonyl–nitrogen (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/N)-based emitters14–19 as well as C
O/N)-based emitters14–19 as well as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/N/O-,17,27,28 C
O/N/O-,17,27,28 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/N/S-17, 29 and C
O/N/S-17, 29 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/N/SO2-based29 emitters.
O/N/SO2-based29 emitters.
While the recent conceptual progress in MR-TADF emitter design has delivered a burgeoning number of efficient B/N-doped compounds that emit across the entirety of the visible spectrum,30,31 few tactics have been advanced to exploit the promising class of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/N-based MR-TADF emitters, which are represented by the emitter DiKTa (Fig. 1).14–19,32,33 Recently, Yasuda et al. reported a linearly extended C
O/N-based MR-TADF emitters, which are represented by the emitter DiKTa (Fig. 1).14–19,32,33 Recently, Yasuda et al. reported a linearly extended C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/N-based emitter, QA-2 (Fig. 1) displaying TADF in 3 wt% PPCz doped film, with a ΔEST of 0.19 eV.27 Similar to the design strategy used for v-DABNA, where the central ring is functionalized with meta-disposed B–π–B and N–π–N groups, QA-2 also contains meta-disposed C
O/N-based emitter, QA-2 (Fig. 1) displaying TADF in 3 wt% PPCz doped film, with a ΔEST of 0.19 eV.27 Similar to the design strategy used for v-DABNA, where the central ring is functionalized with meta-disposed B–π–B and N–π–N groups, QA-2 also contains meta-disposed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–π–C
O–π–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–π–N, which leads to an improvement of the TADF properties over the parent compound DiKTa, with a faster delayed lifetime (48 μs in 3 wt% PPCz and 93.3 μs in 5 wt% mCP, respectively). Despite the apparent increased conjugation length, a slightly blue-shifted λPL of 465 nm in toluene was observed for QA-2, compared to that of DiKTa (ΔEST of 0.15 eV and λPL of 473 nm in toluene).15 The OLED with QA-2 showed an EQEmax of 19.0%. Our group has recently reported a helically chiral isomer of QA-2 that contains meta-disposed C
O and N–π–N, which leads to an improvement of the TADF properties over the parent compound DiKTa, with a faster delayed lifetime (48 μs in 3 wt% PPCz and 93.3 μs in 5 wt% mCP, respectively). Despite the apparent increased conjugation length, a slightly blue-shifted λPL of 465 nm in toluene was observed for QA-2, compared to that of DiKTa (ΔEST of 0.15 eV and λPL of 473 nm in toluene).15 The OLED with QA-2 showed an EQEmax of 19.0%. Our group has recently reported a helically chiral isomer of QA-2 that contains meta-disposed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–π–C
O–π–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–π–N skeleton. Enantiomers of Hel-DiDiKTa (Fig. 1)34 display circularly polarized luminescence (CPL), with a |gPL| of 4 × 10−4, but likewise, its λPL (473 nm) is similar to that of DiKTa.
O and N–π–N skeleton. Enantiomers of Hel-DiDiKTa (Fig. 1)34 display circularly polarized luminescence (CPL), with a |gPL| of 4 × 10−4, but likewise, its λPL (473 nm) is similar to that of DiKTa.
 | ||
| Fig. 1 Schematical representation of the molecular design discussed in this work.15,27,31,34,36,37,42 | ||
The exploitation of para-disposed functional groups to generate π-extended skeletons is a powerful strategy to tune the photophysical properties of MR-TADF emitters to the red.25,35 Yasuda and co-workers have adopted this strategy where both the B–π–B and N–π–N-based groups are para-linked to construct red MR-TADF emitters such as BBCz-R (Fig. 1) that exhibits a λPL of 615 nm and a narrow FWHM of 21 nm (0.07 eV) in dilute toluene solution.31 This strategy has also been used to generate B/O and B/S derivatives as shown in Fig. 1.36,37 Remarkably, among the many MR-TADF emitters reported, comprehensive studies on compounds containing para-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–π–C
O–π–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–π–N frameworks have not as of yet been presented. In this context, expanding the chemical space explored in ketone-based MR-TADF emitters is desired.
O and N–π–N frameworks have not as of yet been presented. In this context, expanding the chemical space explored in ketone-based MR-TADF emitters is desired.
Herein, we present two new isomeric π-extended C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/N-based polycyclic aromatic compounds based on the annellation of two triangulene DiKTa units to form tetraketone structures. Differently from QA-2 and Hel-DiDiKTa, the central ring in each of Sym-DiDiKTa and Asym-DiDiKTa is functionalized with para-C
O/N-based polycyclic aromatic compounds based on the annellation of two triangulene DiKTa units to form tetraketone structures. Differently from QA-2 and Hel-DiDiKTa, the central ring in each of Sym-DiDiKTa and Asym-DiDiKTa is functionalized with para-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–π–C
O–π–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–π–N groups, these molecules represent the first ketone-based MR-TADF examples of their kind (Fig. 1).17–19,33Sym-DiDiKTa is composed of nine fully fused rings with symmetrically arranged tert-butyl substituents while the tert-butyl groups are disposed asymmetrically in Asym-DiDiKTa. Both emitters show moderate ΔEST of around 0.23 eV, red-shifted emission at ∼540 nm compared to DiKTa, a small FWHM of 29 nm and delayed lifetime, τd, of 4.6 ms and 3.0 ms for Sym-DiDiKTa and Asym-DiDiKTa, respectively.
O and N–π–N groups, these molecules represent the first ketone-based MR-TADF examples of their kind (Fig. 1).17–19,33Sym-DiDiKTa is composed of nine fully fused rings with symmetrically arranged tert-butyl substituents while the tert-butyl groups are disposed asymmetrically in Asym-DiDiKTa. Both emitters show moderate ΔEST of around 0.23 eV, red-shifted emission at ∼540 nm compared to DiKTa, a small FWHM of 29 nm and delayed lifetime, τd, of 4.6 ms and 3.0 ms for Sym-DiDiKTa and Asym-DiDiKTa, respectively.
Owing to their exciting properties, these compounds were employed as emitters in OLED devices, and to assess improvements in efficiency roll-off, they were also investigated as terminal emitters in hyperfluorescence (HF) OLEDs, which combine a narrowband emitter as terminal dopant with a co-deposited TADF emitter acting as an assistant dopant in a host matrix. Although MR-TADF OLEDs have achieved extremely high EQEmax, an issue generally encountered is the severe efficiency roll-off that is mainly caused by quenching mechanisms such as singlet–triplet annihilation (STA) and triplet–triplet annihilation (TTA) due to the accumulation of triplet excitons as a result of their typically slow reverse intersystem-crossing rate (kRISC).17,38 Therefore, a potential solution to circumvent this issue is to use MR-TADF compounds in combination with an efficient exciton harvesting assistant dopant in a HF device.39–41 In these devices, exciton harvesting is managed by the donor–acceptor TADF assistant dopant and these excitons are transferred to the terminal MR-TADF emitter via a Förster resonant energy transfer (FRET) mechanism. Emission from the terminal emitter then occurs, benefiting from the typically fast radiative decay and narrowband emission of the MR-TADF compound.39 To ensure efficient FRET, there must be an effective overlap between the emission spectrum of the assistant dopant and the absorption spectrum of the emitter.
Results and discussion
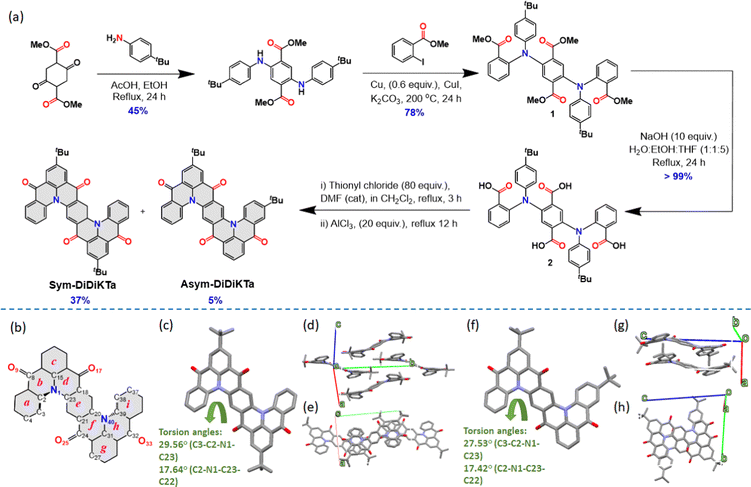
The two emitters were obtained via a four-step linear sequence (Fig. 2a). Compound 1 was obtained in good yield following a copper-catalysed Ullman coupling between the previously reported dimethyl 2,5-bis((4-(tert-butyl)phenyl)amino)terephthalate43 and methyl 2-iodobenzoate. Quantitative saponification yielded the key intermediate 2, which then underwent a four-fold intramolecular Friedel–Crafts acylation of the in situ-prepared acyl chloride derivative in the presence of the Lewis acid AlCl3 to afford a mixture of two isomers, Sym-DiDiKTa and Asym-DiDiKTa, in 36% and 5% yield, respectively, after isolation by column chromatography. The compounds were further purified by gradient-temperature vacuum sublimation and their structure and purity were confirmed by a combination of NMR spectroscopy, high-resolution mass spectrometry, melting point determination and HPLC and elemental analysis.Single crystals were isolated following the gradient-temperature vacuum sublimation. Crystallographic data of Sym-DiDiKTa and Asym-DiDiKTa revealed that both molecules display very similar structures with each DiKTa unit showing a helical-like structure. The torsion angles generated by this helical section are similar (torsion angles C3–C2–N1–C23): 29.6(5)° for Sym-DiDiKTa and 27.9(3) and 28.1(3)° for Asym-DiDiKTa (Fig. 2c and f). Both compounds pack following a one-dimensional slipped π–π stacking motif with interplanar chains along the c-axis for Sym-DiDiKTa [centroid⋯centroid distance 3.5876(18) Å] and along the a-axis for Asym-DiDiKTa [centroid⋯centroid distances 3.4697(13)–3.7685(13) Å]. The disposition of the tert-butyl groups in the compounds influences their packing. In the case of Sym-DiDiKTa, adjacent molecules are partially superimposed, forming π-stacked chain along the c-axis with the molecules stacked in a slipped manner (Fig. 2d and e). In contrast, for Asym-DiDiKTa, adjacent molecules display a greater degree of overlap and more π-stacking (Fig. 2g), resulting in symmetric pairs of adjacent molecules with the tert-butyl groups pointing in the opposite direction (Fig. 2h). Additional π-stacking interactions link these to form π-stacked chains along the a-axis. The distance between the central ring (e) between two nearest molecules is 9.1422(9) Å for Sym-DiDiKTa, whereas for Asym-DiDiKTa it is only 3.4699(17) Å. We also obtained single crystals of Sym-DiDiKTa from slow evaporation of a CH2Cl2:MeOH solution (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio). Under these conditions, the crystal structure is comprised π-stacked columns of molecules along the crystallographic a-axis (Fig. S13a and b, ESI‡), with greater superimposition than in the previous case (distance between the central rings (e) of 3.8500(2) Å). This is achieved with the aid of MeOH molecules forming both strong and weak hydrogen bonds between and within the columns.
2 ratio). Under these conditions, the crystal structure is comprised π-stacked columns of molecules along the crystallographic a-axis (Fig. S13a and b, ESI‡), with greater superimposition than in the previous case (distance between the central rings (e) of 3.8500(2) Å). This is achieved with the aid of MeOH molecules forming both strong and weak hydrogen bonds between and within the columns.
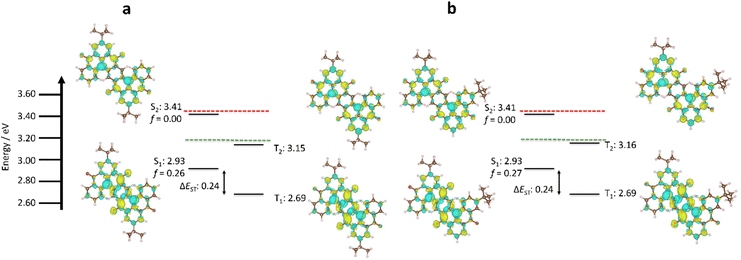
We have previously demonstrated that DFT methods are not appropriate for accurately predicting the excited state properties of MR-TADF materials.44 Explicit inclusion of second-order electronic correlation effects using wavefunction-based methods such as spin component scaling second-order approximate Coupled–Cluster (SCS-CC2) calculations with the cc-pVDZ basis set addresses this problem, resulting in accurate ΔEST prediction for MR-TADF materials.45 The two isomers possess identical S1 and T1 energies of 2.93 eV and 2.69 eV, respectively, and thus a ΔEST of 0.24 eV (Fig. 3), which is similar to previously reported ketone-containing MR-TADF materials.46 Compared to the parent compound DiKTa (ΔEST = 0.27 eV and S1 = 3.45 eV), there is a modest decrease in ΔEST and significant stabilization of S1. The smaller computed ΔEST in Sym-DiDiKTa to Asym-DiDIKTa relative to DiKTa is due to the increase in the π-conjugation of these materials compared to DiKTa resulting in a lowering of the exchange energy, as has been documented in other extended MR-TADF systems, such as v-DABNA and OAB-ABP-1.26,46 The predicted stabilization of S1 results from the nitrogen atoms being positioned para to each other, which positively reinforce their electron-donating character, as has been previously reported.18,41 The oscillator strength associated with the S0–S1 transition is significant at 0.26 and 0.27 for Sym-DiDiKTa and Asym-DiDIKTa, respectively. The difference density plots of S1 and T1 of each emitter highlight the alternating pattern of increasing and decreasing electronic density on neighbouring atoms that produce the emissive short-range charge transfer (SRCT) excited state, which is characteristic of MR-TADF materials.46
Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) recorded in degassed dichloromethane (Fig. 4a and b) document a reversible reduction wave at Ered = −0.96 and −0.94 V vs. SCE for Sym-DiDiKTa and Asym-DiDiKTa, respectively. The oxidation wave of Sym-DiDiKTa is more reversible than that of Asym-DiDiKTa (both at 1.53 V vs. SCE), revealing the impact of the regiochemistry that the tert-butyl groups play in terms of electrochemical stability. The corresponding HOMO and LUMO levels are nearly identical, at −5.87 eV for both compounds and −3.38 eV for Sym-DiDiKTa and −3.40 eV for Asym-DiDiKTa, respectively, which align well with the gas-phase DFT calculations at the PBE0/6-31G(d,p) level of theory (−5.94 eV and −2.69 eV, respectively, Table S11 and Fig. S20, ESI‡). The experimentally determined HOMO levels of Sym-DiDiKTa and Asym-DiDiKTa are similar to that of DiKTa (−5.93 eV), while the LUMO levels are significantly stabilized (LUMO of DiKTa = −3.11 eV).15 The electrochemical data are summarized in Table S9 (ESI‡).
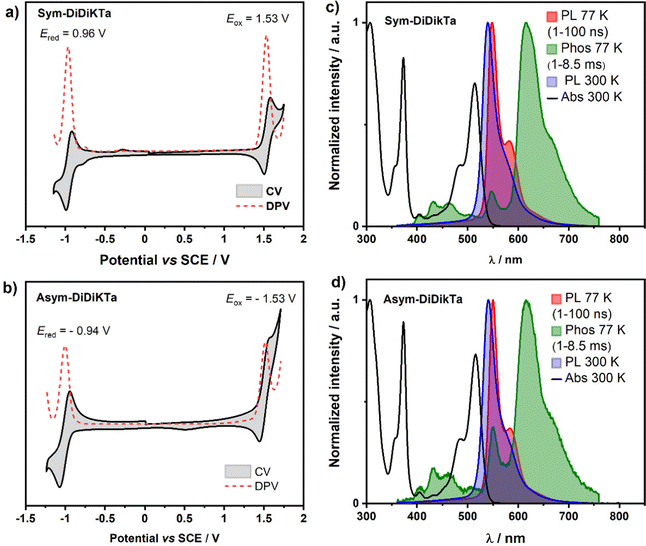 | ||
| Fig. 4 Optoelectronic characterization of Sym-DiDiKTa and Asym-DiDiKTa: (a and b) cyclic and differential pulse voltammograms of Sym-DiDiKTa and Asym-DiDiKTa, respectively, in degassed CH2Cl2 with 0.1 M [nBu4N]PF6 as the supporting electrolyte and Fc/Fc+ as the internal reference (Fc/Fc+ = 0.46 V vs SCE).47 (c and d) Absorption (black line), steady-state PL spectra obtained in toluene at 300 K (blue line) and 77 K (red line; delay: 1 ns; gate time: 100 ns, λexc = 343 nm), and phosphorescence (Phos.; delay: 1 ms; gate time: 8.5 ms, λexc = 343 nm) spectra in toluene glass at 77 K (green olive line) of Sym-DiDiKTa and Asym-DiDiKTa, respectively. | ||
The monomolecular photophysical properties of Sym-DiDiKTa and Asym-DiDiKTa were first studied in dilute toluene solution (Fig. 4c, d and Table 1). The UV-vis absorption spectra of both emitters in toluene are expectedly nearly identical, yet show significant differences compared to that of DiKTa in terms of the SRCT absorption band's wavelength (around 435 for DiKTa14,15 but around 515 nm for Sym-DiDiKTa and Asym-DiDiKTa). The molar absorptivity, ε, of the SRCT band for Sym-DiDiKTa and Asym-DiDiKTa is 26,325 M−1 cm−1 and 23,458 M−1 cm−1, respectively, assigned to the transition to S1 according to the SCS-CC2 calculations (Fig. 3). The ε is somewhat larger than reported for DiKTa (ε of DiKTa = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1),15 which is consistent with the trends in the calculated oscillator strength where f = 0.20 for DiKTa, compared to 0.26 and 0.27 for Sym-DiDiKTa and Asym-DiDiKTa, respectively. The absorption spectra of both Sym-DiDiKTa and Asym-DiDiKTa also show a high-energy shoulder at 485 nm (ε = 11
000 M−1 cm−1),15 which is consistent with the trends in the calculated oscillator strength where f = 0.20 for DiKTa, compared to 0.26 and 0.27 for Sym-DiDiKTa and Asym-DiDiKTa, respectively. The absorption spectra of both Sym-DiDiKTa and Asym-DiDiKTa also show a high-energy shoulder at 485 nm (ε = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 and 10
680 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 238 M−1 cm−1 for Sym-DiDiKTa and Asym-DiDiKTa, respectively), which likely originates from a vibronic band and not a transition to a higher-lying singlet state given that computed energies of the S2 state in each of these two compounds is ca. 0.48 eV higher in energy and the S0–S2 transition possesses negligible oscillator strength.16 There is also a high-energy, high-intensity band at 373 nm (ε = 31
238 M−1 cm−1 for Sym-DiDiKTa and Asym-DiDiKTa, respectively), which likely originates from a vibronic band and not a transition to a higher-lying singlet state given that computed energies of the S2 state in each of these two compounds is ca. 0.48 eV higher in energy and the S0–S2 transition possesses negligible oscillator strength.16 There is also a high-energy, high-intensity band at 373 nm (ε = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1 and 28
900 M−1 cm−1 and 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 527 M−1 cm−1 for Sym-DiDiKTa and Asym-DiDiKTa, respectively, Fig. 4c and d), which is assigned to transitions to S4 and S5, for Sym-DiDiKTa and Asym-DiDiKTa, respectively (Table S12, ESI‡) based on the comparison with the SCS-CC2 simulated absorption spectra (Fig. S21 and Table S12, ESI‡). The difference density plots of these states (Fig. S21, ESI‡) show that there is only minimal density situated on the central phenyl ring and more density situated on the carbonyl groups compared with the difference density pattern of the S1 state (vide supra).
527 M−1 cm−1 for Sym-DiDiKTa and Asym-DiDiKTa, respectively, Fig. 4c and d), which is assigned to transitions to S4 and S5, for Sym-DiDiKTa and Asym-DiDiKTa, respectively (Table S12, ESI‡) based on the comparison with the SCS-CC2 simulated absorption spectra (Fig. S21 and Table S12, ESI‡). The difference density plots of these states (Fig. S21, ESI‡) show that there is only minimal density situated on the central phenyl ring and more density situated on the carbonyl groups compared with the difference density pattern of the S1 state (vide supra).
| In toluene | In film | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| λ abs /nm | ε a/M−1 cm−1 | λ PL /nm | Φ PL in N2 (air)c/% | FWHMd/nm (eV) | S 1 /eV | T 1 /eV | ΔESTf/eV | λ PL/nm | Φ PL/% | FWHMd/nm (eV) | |
| a UV–vis absorption of CT transition. b Prompt emission in toluene degassing with N2. c Photoluminescence quantum yield in toluene relative to quinine sulfate in 1N H2SO4 (ΦPL = 54.6%) d Full-width at half-maximum. e Obtained using an integrating sphere under N2. f Energy gap between S1 and T1 calculated from the difference of the peaks of the fluorescence and phosphorescence spectra in toluene glass at 77 K. g 1 wt% Sym-DiDiKTa and Asym-DiDiKTa doped in mCP. | |||||||||||
| Sym-DiDiKTa | 373/485/515 | 31900/11680/26325 | 540 | 69 (65) | 29 (0.12) | 2.26 | 2.02 | 0.24 | 542g | 64 | 35 (0.14)g |
| Asym-DiDiKTa | 373/485/516 | 28527/10238/23458 | 541 | 53 (50) | 29 (0.12) | 2.25 | 2.02 | 0.23 | 547g | 57 | 35 (0.14)g |
Both compounds display green emission in dilute toluene with emission maxima, λPL, of 540 nm and 541 nm for Sym-DiDiKTa and Asym-DiDiKTa, respectively, which are ca. 90 nm red-shifted from that of DiKTa (λPL = 453 nm). This bathochromic shift is corroborated by the SCS-CC2 calculations.19,20,31,48 Both compounds display narrow PL spectra at room temperature (FWHM = 29 nm), and small Stokes shifts of ca. 25 nm, which confirms the small degree of geometrical reorganization in the excited state owing to their conformationally rigid structure (Fig. 2c and d). The small degree of positive solvatochromism (Fig. S15 and Tables S2, S3, ESI‡) reflects the SRCT character of the emissive excited state.15 The S1 excited state energy levels were determined to be 2.26 and 2.25 eV, respectively, for Sym-DiDiKTa and Asym-DiDiKTa and the T1 excited state energy levels are identical at 2.02 eV, each obtained from the λPL of the respective prompt fluorescence and phosphorescence spectra in toluene glass at 77 K. We note that the nature of T1 and S1 are identical based on the difference density plots (Fig. 3). The corresponding ΔEST values are 0.24 eV and 0.23 eV, respectively, for Sym-DiDiKTa and Asym-DiDiKTa. These values match with those predicted by SCS-CC2 calculations (ΔEST = 0.24 eV for both compounds). The PL quantum yield, ΦPL, in toluene is 70% under N2, which decreases to 64% in air for Sym-DiDiKTa and 53% under N2 and 50% in air for Asym-DiDiKTa. The ΦPL values were next measured for vacuum-deposited 1 wt% doped thin films in 1,3-bis(N-carbazolyl)benzene (mCP). The ΦPL values were determined to be 64% and 57% for Sym-DiDiKTa and Asym-DiDiKTa, respectively (Table S5, ESI‡). The ΦPL values closely resemble those obtained in dilute toluene, which implies that non-radiative decay due to vibrations is not significant in these compounds.
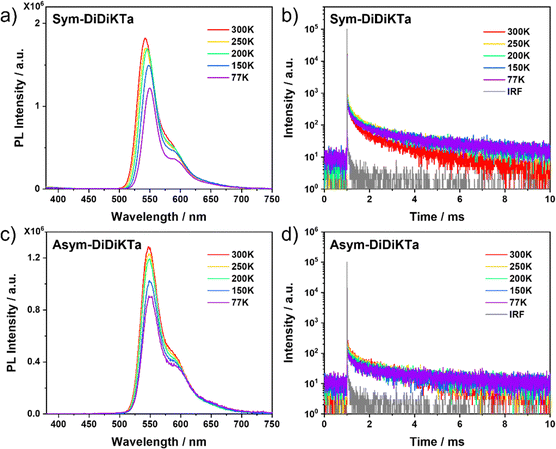
We next investigated the solid-state photophysical properties of spin-coated 1 wt% Sym-DiDiKTa and Asym-DiDiKTa doped thin films in mCP. The low doping concentration was selected to mitigate the potential for undesired aggregation in the films. As shown in Fig. 5a and c, at 300 K, Sym-DiDiKTa and Asym-DiDiKTa show a similar emission profile with λPL at 542 and 547 nm, respectively, values that are close to the prompt emission maximum in toluene. A small FWHM of 35 nm (0.13 eV) was calculated for both compounds in the films, which is slightly broader than the FWHM of 29 nm (0.12 eV) determined in toluene, indicating the influence of the matrix on the conformational stabilization of the excited states. Another aspect to consider are concentration-dependent aggregation effects since the concentration of emitters at 1 wt% doping in the film is higher than that of the 106 M solution. The average τd values are 4.6 ms and 3.0 ms for Sym-DiDiKTa and Asym-DiDiKTa, respectively (Fig. 5 and Table S7, ESI‡). We then compared the oxygen dependence of the PL spectrum at room temperature. The PL spectrum in air shows a small decrease in intensity compared to the spectrum under vacuum (Fig. S17a and b, ESI‡), indicating a weak involvement of triplet excited states. The temperature-dependent PL spectra (Fig. 5a and c) and temperature-dependent time-resolved PL decays (Fig. 5b and d) of both emitters document the characteristic decrease in intensity and in the contribution of the delayed emission, respectively, with decreasing temperature. This could be due to the MR-TADF behavior only being apparent in a suitable host matrix due to exciplex-like host-emitter interactions.29
OLED characterization
We fabricated vacuum-deposited OLEDs using Sym-DiDiKTa and Asym-DiDiKTa as the emitters. The following device configuration was used: indium tin oxide (ITO)-coated glass (100 nm)/TAPC (35 nm)/TCTA (10 nm)/mCP: 3 wt% of Sym-DiDiKTa or Asym-DiDiKTa (20 nm)/TmPyPB (30 nm)/Liq (2 nm)/Al (100 nm). 1,1-Bis[(di-4-tolylamino)phenyl]cyclohexane (TAPC) is the hole-transporting layer, 4,4′,4-tris(carbazol-9-yl)triphenylamine (TCTA) is used for electron-blocking and host layers, 1,3,5-tris(3-pyridyl-3-phenyl)benzene (TmPyPB) is the electron-transporting layer, and lithium 8-hydroxyquinolinolate (Liq) and aluminum are the electron injection and cathode layers, respectively. Fig. 6 and Table 2 summarize all the device characteristics. TADF-only devices based on Sym-DiDiKTa (Device I) and Asym-DiDiKTa (Device II) emitted green narrowband electroluminescence, λEL, at 543 and 544 nm, respectively, values that are consistent to their corresponding λPL. The FWHMs of both Devices I and II are 36 nm, which resulted in CIE coordinates of (0.362, 0.623) and (0.376, 0.613), respectively. The EQEmax of Device I is 9.8%, which is slightly lower than the 10.5% measured for Device II. We hypothesized that the moderate ΦPL in the mCP-doped films limits the EQEmax values in Devices I and II. The moderate ΦPL may be due to the long-lived delayed fluorescence that permits a greater probability that non-radiative decay processes such as triplet-polaron quenching and triplet–triplet annihilation will contribute to the decay of the excitons. Both devices suffered from severe efficiency roll-off issues, which in part are due to the long delayed fluorescence lifetimes of Sym-DiDiKTa and Asym-DiDiKTa. Moreover, the linear decrease in the EQE – current density curve indicates possible exciton-polaron annihilation processes, which can be ascribed to trap formation by the emitters.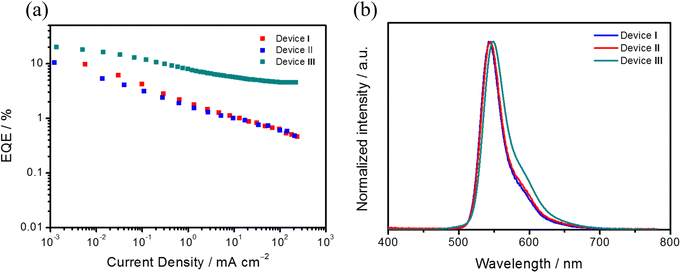 | ||
| Fig. 6 (a) EQE versus current density curves of Devices I–III. (b) Electroluminescence spectra of Devices I–III at 500 cd m−2. | ||
| Device | Dopant | V on/Va | EQE/%b | λ EL/nmc | Max. brightness (cd m−2)d | FWHM/nmc | CIE (x, y)c |
|---|---|---|---|---|---|---|---|
| a Voltage at 1 cd m−2. b Value at maximum, 100 cd m−2 and 1000 cd m−2. c Value at 500 cd m−2. d Maximum brightness. | |||||||
| I | 3 wt% Sym-DiDiKTa | 3.5 | 9.8/1.8/0.8 | 543 | 4310 | 36 | (0.359, 0.624) |
| II | 3 wt% Asym-DiDiKTa | 4 | 10.5/1.3/0.8 | 544 | 3989 | 36 | (0.369, 0.616) |
| III | 3 wt% Asym-DiDiKTa: 20 wt% 4CzIPN | 3.2 | 19.9/9.9/6.4 | 548 | 53625 | 56 | (0.397, 0.592) |
To improve the device performance, we implemented a hyperfluorescence (HF) strategy in Device III with the following device structure: indium tin oxide (ITO)-coated glass (100 nm)/HAT-CN (10 nm)/Tris-PCz (30 nm)/mCBP (5 nm)/mCBP: 20 wt% 4CzIPN: 3 wt% Asym-DiDiKTa (30 nm)/T2T (10 nm)/BPy-TP2 (40 nm)/Liq (2 nm)/Al (100 nm), where 1,4,5,8,9,11-hexaazatriphenyl-enehexacarbonitrile (HAT-CN) is the hole-injection layer, 9-phenyl-3,6-bis(9-phenyl-9Hcarbazol-3-yl)-9H-carbazole (Tris-PCz) is the hole-transporting layer, mCBP is used in the exciton-blocking and host layers, 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) is a green TADF assistant dopant, 2,4,6-tris(biphenyl-3-yl)-1,3,5-triazine (T2T) is the hole-blocking layer and 2,7-di(2,2′-bipyridin-5-yl)triphenylene (BPy-TP2) is the electron-transporting layer, and lithium 8-hydroxyquinolinolate (Liq) and Al are the electron injection and cathode layers, respectively. Gratifyingly, the device performance improved and an EQEmax of 19.9% was achieved along with an enhanced maximum brightness of 53625 cd m−2. The HF device not only resulted in a better EQE, but also reduced the efficiency roll-off when compared to Device II. The λEL is red-shifted to 548 nm and there is a slightly larger FWHM of 56 nm. The broader FWHM may originate from an incomplete energy transfer from 4CzIPN to Asym-DiDiKTa.
LEC devices
The light-emitting electrochemical cell (LEC) can be comprised of solely air-stable materials and feature a very simple and robust device structure in the form of a single-layer active material sandwiched between two electrodes. This renders the LEC technology highly fit for scalable and cost-efficient printing and coating fabrication under ambient air.49,50 The characteristic feature of LEC devices is the combination of mobile ions with the emissive organic semiconductor within the emissive layer.51,52 These mobile ions redistribute when a voltage is applied, and enable p-type electrochemical doping of the organic semiconductor at the anode and n-type doping at the cathode. With time, these doping regions grow in size and make contact under the formation of a p–n junction. The fact that Sym-DiDiKTa displays highly reversible electrochemical oxidation and reduction behaviour in the cyclic voltammetry experiments (Fig. 4a) suggests that it could be fit for the task of the emissive organic semiconductor in a LEC device.We fabricated and characterized LEC devices with the following configuration: indium tin oxide (140 nm)/poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (40 nm)/26DCzPPy (44 wt%):POT2T (44 wt%):Sym-DiDiKTa (4 wt%):THABF4 (8 wt%) (100 nm)/Al (100 nm), where THABF4 (tetrahexylammonium tetrafluoroborate) is the ionic liquid electrolyte that contributes the mobile ions, and 6-bis(3-carbazol-9-yl)pyridine (26DCzPPy):(1,3,5-triazine-2,4,6-triyl)tris(benzene-3,1-diyl)tris(diphenylphosphine oxide) (POT2T) is a blend-host matrix, which was introduced to enable for the formation of a uniform solution-processed thin film and to suppress losses by exciton-polaron53 and exciton-exciton quenching.
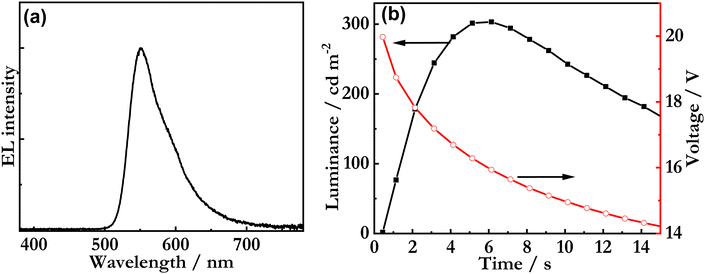
Fig. 7a presents the steady-state EL spectrum recorded during the driving with a constant current density of 77 mA cm−2, with the λEL at 551 nm and the FWHM being 60 nm. The broadening of the EL spectrum of the LEC device in comparison to the PL spectra in Fig. 5a indicates the formation of exciplexes with the blend host. This conclusion is supported by the fact that the FWHM of the EL spectrum is essentially independent of the guest concentration for a guest concentration range of 1 to 8 wt% (Fig. S22, ESI‡). Fig. 7b details the voltage and luminance transients recorded during the early stages of the constant-current driving. The observed initial decrease of the voltage is a characteristic indicator of conductivity-enhancing electrochemical doping, whereas the increase in luminance is in line with the gradual formation of a p-n junction, where electrons and holes can recombine efficiently into excitons. Importantly, these observations imply that the Sym-DiDiKTa emitter can be in situ electrochemically p- and n-type doped during LEC operation. This conclusion is further supported by the fact that the LEC device delivered a significant luminance of 300 cd m−2 despite being equipped with an air-stable Al cathode in direct contact with the active emissive material. Finally, the luminance turn-on time is a direct indicator of the ion mobility in the active material,54,55 and the comparatively fast turn-on time of less than 2 s to 100 cd m−2 demonstrates that the ion mobility in the active material is high.
Conclusion
In summary, new amine/carbonyl-based MR-TADF materials were developed by an approach that expands the π-conjugated backbone to show green-yellow emission. The regio-functionalization of the tert-butyl groups was shown to significantly impact the single-crystal packing structure. With a ΔEST of around 0.23 eV in toluene, the narrowband emitters, FWHM, of 29 nm, display TADF activity with a τd of 4.6 ms and 3.0 ms for Sym-DiDiKTa and Asym-DiDiKTa, respectively. Application in OLEDs provided devices with electroluminescence maxima at around 544 nm, and an EQEmax of circa 10% which low value is attributed to the long delayed lifetimes of this class. Upon further studies in hyperfluorescence devices, an EQE of 19.9% was finally obtained, showing the promise of this class of molecules towards this strategy. We also show the first examples of a LEC device incorporating an MR-TADF emitter. With a λEL at 551 nm and a larger FWHM of 60 nm due to formation of exciplexes with the host, the device based on Sym-DiDiKTa delivered a significant luminance of 300 cd m−2 and high ion mobility with a fast turn-on time of less than 2 s to 100 cd m−2.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The St Andrews team would like to thank EPSRC (EP/P010482/1) and the Leverhulme Trust (RPG-2016-047) for financial support. Computational resources have been provided by the Consortium des Équipements de Calcul Intensif (CÉCI), funded by the Fonds de la Recherche Scientifiques de Belgique (F. R. S.-FNRS) under Grant no. 2.5020.11. Y. O. acknowledges funding by the Fonds de la Recherche Scientifique-FNRS under Grant no. F.4534.21 (MIS-IMAGINE). The authors also acknowledge Ms N. Nakamura and Ms K. Kusuhara for their technical assistance with this research. This work was supported financially by the JSPS Core-to-Core Program (grant number: JPJSCCA20180005), Kyulux Inc, the Swedish Research Council, the Swedish Energy Agency, and Bertil & Britt Svenssons stiftelse för belysningsteknik.References
- X. K. Chen, D. Kim and J. L. Brédas, Thermally Activated Delayed Fluorescence (TADF) Path toward Efficient Electroluminescence in Purely Organic Materials: Molecular Level Insight, Acc. Chem. Res., 2018, 51(9), 2215–2224 CrossRef CAS PubMed.
- T. Tsujimura, OLED display fundamentals and applications, 2017 Search PubMed.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492(7428), 234–238 CrossRef CAS PubMed.
- G. Méhes, H. Nomura, Q. Zhang, T. Nakagawa and C. Adachi, Enhanced electroluminescence efficiency in a spiro-acridine derivative through thermally activated delayed fluorescence, Angew. Chem., Int. Ed., 2012, 51(45), 11311–11315 CrossRef PubMed.
- T. Nakagawa, S. Y. Ku, K. T. Wong and C. Adachi, Electroluminescence based on thermally activated delayed fluorescence generated by a spirobifluorene donor-acceptor structure, Chem. Commun., 2012, 48(77), 9580–9582 RSC.
- S. Hirata, Y. Sakai, K. Masui, H. Tanaka, S. Y. Lee, H. Nomura, N. Nakamura, M. Yasumatsu, H. Nakanotani, Q. Zhang, K. Shizu, H. Miyazaki and C. Adachi, Highly efficient blue electroluminescence based on thermally activated delayed fluorescence, Nat. Mater., 2015, 14(3), 330–336 CrossRef CAS PubMed.
- M. Y. Wong and E. Zysman-Colman, Purely Organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes, Adv. Mater., 2017, 29(22), 1–54 CrossRef PubMed.
- A. Endo, M. Ogasawara, A. Takahashi, D. Yokoyama, Y. Kato and C. Adachi, Thermally activated delayed fluorescence from Sn4+-porphyrin complexes and their application to organic light-emitting diodes -A novel mechanism for electroluminescence, Adv. Mater., 2009, 21(47), 4802–4806 CrossRef CAS PubMed.
- A. Endo, K. Sato, K. Yoshimura, T. Kai, A. Kawada, H. Miyazaki and C. Adachi, Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes, Appl. Phys. Lett., 2011, 98(8), 2011–2014 CrossRef.
- C. A. Parker and C. G. Hatchard, Triplet–singlet emission in fluid solutions, Trans. Faraday Soc., 1961, 57, 1894–1904 RSC.
- F. Santoro, A. Lami, R. Improta, J. Bloino and V. Barone, Effective method for the computation of optical spectra of large molecules at finite temperature including the Duschinsky and Herzberg-Teller effect: The Qx band of porphyrin as a case study, J. Chem. Phys., 2008, 128(22), 224311 CrossRef PubMed.
- X. Qiu, G. Tian, C. Lin, Y. Pan, X. Ye, B. Wang, D. Ma, D. Hu, Y. Luo and Y. Ma, Narrowband Emission from Organic Fluorescent Emitters with Dominant Low-Frequency Vibronic Coupling, Adv. Opt. Mater., 2020, 9(4), 2001845 CrossRef.
- H. W. Chen, J. H. Lee, B. Y. Lin, S. Chen and S. T. Wu, Liquid crystal display and organic light-emitting diode display: present status and future perspectives, Light: Sci. Appl., 2018, 7(3), 17168 CrossRef CAS PubMed.
- Y. Yuan, X. Tang, X. Y. Du, Y. Hu, Y. J. Yu, Z. Q. Jiang, L. S. Liao and S. T. Lee, The Design of Fused Amine/Carbonyl System for Efficient Thermally Activated Delayed Fluorescence: Novel Multiple Resonance Core and Electron Acceptor, Adv. Opt. Mater., 2019, 7(7), 1801536 CrossRef.
- D. Hall, S. M. Suresh, P. L. dos Santos, E. Duda, S. Bagnich, A. Pershin, P. Rajamalli, D. B. Cordes, A. M. Z. Slawin, D. Beljonne, A. Köhler, I. D. W. Samuel, Y. Olivier and E. Zysman-Colman, Improving Processability and Efficiency of Resonant TADF Emitters: A Design Strategy, Adv. Opt. Mater., 2020, 8(2), 1901627 CrossRef CAS.
- X. Li, Y. Z. Shi, K. Wang, M. Zhang, C. J. Zheng, D. M. Sun, G. L. Dai, X. C. Fan, D. Q. Wang, W. Liu, Y. Q. Li, J. Yu, X. M. Ou, C. Adachi and X. H. Zhang, Thermally Activated Delayed Fluorescence Carbonyl Derivatives for Organic Light-Emitting Diodes with Extremely Narrow Full Width at Half-Maximum, ACS Appl. Mater. Interfaces, 2019, 11(14), 13472–13480 CrossRef CAS PubMed.
- S.-n Zou, C.-c Peng, S.-y Yang, Y.-k Qu, Y.-j Yu, X. Chen, Z.-q Jiang and L.-s Liao, Fully Bridged Triphenylamine Derivatives as Color-Tunable Thermally Activated Delayed Fluorescence Emitters, Org. Lett., 2021, 23(3), 958–962 CrossRef CAS PubMed.
- Y. Tsuchiya, Y. Ishikawa, S. H. Lee, X. K. Chen, J. L. Brédas, H. Nakanotani and C. Adachi, Thermally Activated Delayed Fluorescence Properties of Trioxoazatriangulene Derivatives Modified with Electron Donating Groups, Adv. Opt. Mater., 2021, 9(14), 2002174 CrossRef CAS.
- F. Huang, K. Wang, Y. Z. Shi, X. C. Fan, X. Zhang, J. Yu, C. S. Lee and X. H. Zhang, Approaching Efficient and Narrow RGB Electroluminescence from D-A-Type TADF Emitters Containing an Identical Multiple Resonance Backbone as the Acceptor, ACS Appl. Mater. Interfaces, 2021, 13(30), 36089–36097 CrossRef CAS PubMed.
- Y. Zhang, D. Zhang, T. Huang, A. J. Gillett, Y. Liu, D. Hu, L. Cui, Z. Bin, G. Li, J. Wei and L. Duan, Multi-Resonance Deep-Red Emitters with Shallow Potential-Energy Surfaces to Surpass Energy-Gap Law, Angew. Chem., Int. Ed., 2021, 60(37), 20498–20503 CrossRef CAS PubMed.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Ultrapure Blue Thermally Activated Delayed Fluorescence Molecules: Efficient HOMO-LUMO Separation by the Multiple Resonance Effect, Adv. Mater., 2016, 28(14), 2777–2781 CrossRef CAS PubMed.
- Y. Kondo, K. Yoshiura, S. Kitera, H. Nishi, S. Oda, H. Gotoh, Y. Sasada, M. Yanai and T. Hatakeyama, Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter, Nat. Photonics, 2019, 13(10), 678–682 CrossRef CAS.
- Q. Wang, Y. Xu, T. Yang, J. Xue and Y. Wang, Precise Functionalization of a Multiple-Resonance Framework: Constructing Narrowband Organic Electroluminescent Materials with External Quantum Efficiency over 40, Adv. Mater., 2023, 35(3), e2205166 CrossRef PubMed.
- Y. Xu, Q. Wang, J. Wei, X. Peng, J. Xue, Z. Wang, S. J. Su and Y. Wang, Constructing Organic Electroluminescent Material with Very High Color Purity and Efficiency Based on Polycyclization of the Multiple Resonance Parent Core, Angew. Chem., Int. Ed., 2022, 61(30), e202204652 CAS.
- Y. Zou, J. Hu, M. Yu, J. Miao, Z. Xie, Y. Qiu, X. Cao and C. Yang, High-Performance Narrowband Pure-Red OLEDs with External Quantum Efficiencies up to 36.1% and Ultralow Efficiency Roll-Off, Adv. Mater., 2022, 34(29), 2201442 CrossRef CAS PubMed.
- N. Ikeda, S. Oda, R. Matsumoto, M. Yoshioka, D. Fukushima, K. Yoshiura, N. Yasuda and T. Hatakeyama, Solution-Processable Pure Green Thermally Activated Delayed Fluorescence Emitter Based on the Multiple Resonance Effect, Adv. Mater., 2020, 32, 2004072 CrossRef CAS PubMed.
- H. Min, I. S. Park and T. Yasuda, cis-Quinacridone-Based Delayed Fluorescence Emitters: Seemingly Old but Renewed Functional Luminogens, Angew. Chem., Int. Ed., 2021, 60(14), 7643–7648 CrossRef CAS PubMed.
- S. Wu, A. K. Gupta, K. Yoshida, J. Gong, D. Hall, D. B. Cordes, A. M. Z. Slawin, I. D. W. Samuel and E. Zysman-Colman, Highly Efficient Green & Red Narrowband Emissive Organic Light-Emitting Diodes Employing Multi-Resonant Thermally Activated Delayed Fluorescence Emitters, Angew. Chem., Int. Ed., 2022, 61(52), e202213697 CAS.
- X. Wu, B. K. Su, D. G. Chen, D. Liu, C. C. Wu, Z. X. Huang, T. C. Lin, C. H. Wu, M. Zhu, E. Y. Li, W. Y. Hung, W. Zhu and P. T. Chou, The role of host–guest interactions in organic emitters employing MR-TADF, Nat. Photonics, 2021, 15(10), 780–786 CrossRef CAS.
- S. Madayanad Suresh, D. Hall, D. Beljonne, Y. Olivier and E. Zysman-Colman, Multiresonant Thermally Activated Delayed Fluorescence Emitters Based on Heteroatom-Doped Nanographenes: Recent Advances and Prospects for Organic Light-Emitting Diodes, Adv. Funct. Mater., 2020, 30(33), 1908677 CrossRef CAS.
- M. Yang, I. S. Park and T. Yasuda, Full-Color, Narrowband, and High-Efficiency Electroluminescence from Boron and Carbazole Embedded Polycyclic Heteroaromatics, J. Am. Chem. Soc., 2020, 142(46), 19468–19472 CrossRef CAS PubMed.
- D. Sun, M. Suresh, D. Hall, M. Zhang, C. Si, D. B. Cordes, A. M. Z. Slawin, Y. Olivier, X. Zhang and E. Zysman-colman, The design of an extended multiple resonance TADF emitter based on a polycyclic amine/carbonyl system, Mater. Chem. Front., 2020, 4, 2018–2022 RSC.
- S. Wu, W. Li, K. Yoshida, D. Hall, S. Madayanad Suresh, T. Sayner, J. Gong, D. Beljonne, Y. Olivier, I. D. W. Samuel and E. Zysman-Colman, Excited-State Modulation in Donor-Substituted Multiresonant Thermally Activated Delayed Fluorescence Emitters, ACS Appl. Mater. Interfaces, 2022, 14(19), 22341–22352 CrossRef CAS PubMed.
- J. M. dos Santos, D. Sun, J. M. Moreno-Naranjo, D. Hall, F. Zinna, S. T. J. Ryan, W. Shi, T. Matulaitis, D. B. Cordes, A. M. Z. Slawin, D. Beljonne, S. L. Warriner, Y. Olivier, M. J. Fuchter and E. Zysman-Colman, An S-shaped double helicene showing both multi-resonance thermally activated delayed fluorescence and circularly polarized luminescence, J. Mater. Chem. C, 2022, 10, 4861–4870 RSC.
- X. Lv, J. Miao, M. Liu, Q. Peng, C. Zhong, Y. Hu, X. Cao, H. Wu, Y. Yang, C. Zhou, J. Ma, Y. Zou and C. Yang, Extending the π-Skeleton of Multi-Resonance TADF Materials towards High-Efficiency Narrowband Deep-Blue Emission, Angew. Chem., Int. Ed., 2022, 61(29), e202201588 CrossRef CAS PubMed.
- X. Cai, J. Xue, C. Li, B. Liang, A. Ying, Y. Tan, S. Gong and Y. Wang, Achieving 37.1% Green Electroluminescent Efficiency and 0.09 eV Full Width at Half Maximum Based on a Ternary Boron-Oxygen-Nitrogen Embedded Polycyclic Aromatic System, Angew. Chem., Int. Ed., 2022, 61(23), e202200337 CrossRef CAS PubMed.
- X. F. Luo, H. X. Ni, A. Q. Lv, X. K. Yao, H. L. Ma and Y. X. Zheng, High-Efficiency and Narrowband OLEDs from Blue to Yellow with Ternary Boron/Nitrogen-Based Polycyclic Heteroaromatic Emitters, Adv. Opt. Mater., 2022, 10(16), 2200504 CrossRef CAS.
- Y. Zhang, D. Zhang, J. Wei, Z. Liu, Y. Lu and L. Duan, Multi-Resonance Induced Thermally Activated Delayed Fluorophores for Narrowband Green OLEDs, Angew. Chem., Int. Ed., 2019, 58(47), 16912–16917 CrossRef CAS PubMed.
- Y. Zhang, J. Wei, D. Zhang, C. Yin, G. Li, Z. Liu, X. Jia, J. Qiao and L. Duan, Sterically Wrapped Multiple Resonance Fluorophors for Suppression of Concentration Quenching and Spectrum Broadening, Angew. Chem., Int. Ed., 2021, 61(2), 202113206 Search PubMed.
- C. Y. Chan, M. Tanaka, Y. T. Lee, Y. W. Wong, H. Nakanotani, T. Hatakeyama and C. Adachi, Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission, Nat. Photonics, 2021, 15(3), 203–207 CrossRef CAS.
- J. Wei, C. Zhang, D. Zhang, Y. Zhang, Z. Liu, Z. Li, G. Yu and L. Duan, Indolo[3,2,1-jk]carbazole Embedded Multiple-Resonance Fluorophors for Narrowband Deep-blue Electroluminescence with EQE ≈ 34.7% and CIEy ≈ 0.085, Angew. Chem., Int. Ed., 2021, 60(22), 12269–12273 CrossRef CAS PubMed.
- J. J. C. Lee, C. Chi and J. Wu, Synthesis of a Highly Fluorescent Bis(1,4-oxaborine)pentacene, ChemPlusChem, 2021, 86(6), 836–839 CrossRef CAS PubMed.
- M. Shimizu, Y. Asai, Y. Takeda, A. Yamatani and T. Hiyama, Twisting strategy applied to N,N-diorganoquinacridones leads to organic chromophores exhibiting efficient solid-state fluorescence, Tetrahedron Lett., 2011, 52(32), 4084–4089 CrossRef CAS.
- A. Pershin, D. Hall, V. Lemaur, J. C. Sancho-Garcia, L. Muccioli, E. Zysman-Colman, D. Beljonne and Y. Olivier, Highly emissive excitons with reduced exchange energy in thermally activated delayed fluorescent molecules, Nat. Commun., 2019, 10(1), 597 CrossRef CAS PubMed.
- D. Hall, K. Stavrou, E. Duda, A. Danos, S. Bagnich, S. Warriner, A. M. Z. Slawin, D. Beljonne, A. Köhler, A. Monkman, Y. Olivier and E. Zysman-Colman, Diindolocarbazole – achieving multiresonant thermally activated delayed fluorescence without the need for acceptor units, Mater. Horiz., 2022, 9, 1068–1080 RSC.
- D. Hall, J. Carlos Sancho-Garcia, A. Pershin, D. Beljonne, E. Zysman-Colman and Y. Olivier, Modelling of multi-resonant thermally activated delayed fluorescence emitters-properly accounting for electron correlation is key!, J. Chem. Theory Comput., 2022, 18(8), 4903–4918 CrossRef CAS PubMed.
- N. G. Connelly and W. E. Geiger, Chemical redox agents for organometallic chemistry, Chem. Rev., 1996, 96(2), 877–910 CrossRef CAS PubMed.
- M. Yang, S. Shikita, H. Min, I. S. Park, H. Shibata, N. Amanokura and T. Yasuda, Wide-Range Color Tuning of Narrowband Emission in Multi-resonance Organoboron Delayed Fluorescence Materials through Rational Imine/Amine Functionalization, Angew. Chem., Int. Ed., 2021, 60(43), 23142–23147 CrossRef CAS PubMed.
- A. Sandström, H. F. Dam, F. C. Krebs and L. Edman, Ambient fabrication of flexible and large-area organic light-emitting devices using slot-die coating, Nat. Commun., 2012, 3, 1002 CrossRef PubMed.
- G. Hernandez-Sosa, S. Tekoglu, S. Stolz, R. Eckstein, C. Teusch, J. Trapp, U. Lemmer, M. Hamburger and N. Mechau, The Compromises of Printing Organic Electronics: A Case Study of Gravure-Printed Light-Emitting Electrochemical Cells, Adv. Mater., 2014, 3235–3240 CrossRef CAS PubMed.
- Q. B. Pei, G. Yu, C. Zhang, Y. Yang and A. J. Heeger, Polymer Light-Emitting Electrochemical-Cells, Science, 1995, 269(5227), 1086–1088 CrossRef CAS PubMed.
- S. Y. Hu and J. Gao, Shaping Electroluminescence with a Large, Printed Bipolar Electrode Array: Solid Polymer Electrochemical Cells with Over a Thousand Light-Emitting p–n Junctions, ChemElectroChem, 2020, 7(7), 1748–1751 CrossRef CAS.
- S. Tang, A. Sandström, P. Lundberg, T. Lanz, C. Larsen, S. van Reenen, M. Kemerink and L. Edman, Design rules for light-emitting electrochemical cells delivering bright luminance at 27.5 percent external quantum efficiency, Nat. Commun., 2017, 8(1), 1190 CrossRef PubMed.
- J. Mindemark and L. Edman, Illuminating the electrolyte in light-emitting electrochemical cells, J. Mater. Chem. C, 2016, 4(3), 420–432 RSC.
- E. Zysman-Colman, J. D. Slinker, J. B. Parker, G. G. Malliaras and S. Bernhard, Improved turn-on times of light-emitting electrochemical cells, Chem. Mater., 2008, 20(2), 388–396 CrossRef CAS.
Footnotes |
| † The research data supporting this publication can be accessed at https://doi.org/10.17630/f844ce22-cf6b-413d-ae79-70e2e38a1f74 |
| ‡ Electronic supplementary information (ESI) available: A summary of prior examples of carbonyl-containing MR-TADF emitters, experimental details, synthesis procedures and characterization data, NMR spectra, HRMS, HPLC, X-ray crystallographic data, supplemental photophysical data, electrochemical data, xyz coordinate file for computations. CCDC 2225501–2225503. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3tc00641g |
| This journal is © The Royal Society of Chemistry 2023 |