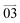Diphenylamine substituted 5,6,12,13-tetraazaperopyrene based polymorphic microcrystals versatile in multi-directional isotropic and anisotropic photon transport†
Di
Tian
a,
Chaofei
Xu
c,
Wei
Yuan
ab,
Xue-Dong
Wang
 *c and
Yulan
Chen
*c and
Yulan
Chen
 *ab
*ab
aDepartment of Chemistry, Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin, 300354, P. R. China. E-mail: yulanchen@jlu.edu.cn
bState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University Changchun, 130012, P. R. China
cInstitute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University Suzhou, 215123, P. R. China. E-mail: wangxuedong@suda.edu.cn
First published on 6th April 2023
Abstract
Geometrically well-defined microcrystals with polymorphic tailorability are in high demand for multi-directional photon flow at the microscale. Herein, diphenylamine (DPA) substituted 5,6,12,13-tetraazaperopyrene c-TAPP-DPA is synthesized and explored as a versatile fluorophore to fabricate polymorphic microcrystals for photonic applications. The flexibly twisted and electron rich DPA groups are verified as a crucial structure element to tune the intermolecular interactions and packing modes of c-TAPP-DPA, resulting in three kinds of crystals, including microrods, and rhomboid and hexagonal microplates. These microcrystals display geometry-dependent fluorescence properties and 1D or 2D waveguide properties with low optical loss. Impressively, the micro-rhomboids show a unique 2D asymmetric waveguide character. Our work enriches the diversity of polymorphic microcrystals, and sheds more light on the structure–property relationship of organic crystals for multi-directional optical waveguide applications.
Introduction
Directional control of photonic waveguides can pave the way to the development of integrated optical circuits.1–4 Organic micro/nano-crystals with ordered structures and excellent optical properties have become ideal candidates for optical waveguides,2,5–9 lasers,10–15 and light-emitting transistors.16–19 A wealth of structures and optical properties of organic fluorophores offer broad opportunities to modulate the resultant crystal structures and functionalities.20–23 Of particular importance is the shape and dimension controls of the crystals for confining and guiding photonic flow with low loss, which greatly rely on the molecular structures and packing modes of the fluorophores.24–28 Notably, polymorphism with two or more crystalline phases stemming from one organic molecule can greatly enrich the types of micro/nano-crystals for excellent photonic properties; meanwhile it provides a sophisticated platform to shed more light on the relationship between molecular stacking patterns and solid-state optical properties.29–35Apart from the polymorphic control of multimodal and low loss optical waveguides, 2D crystals capable of anisotropic propagation of photons, with multiple channels showing different polarization and emission properties, are also in high demand due to their considerable potential in optical planar diodes, logic devices, etc.2,36–42 Although it has been recognized that the fabrication of 2D organic crystals with anisotropic features is pertinent to the molecular-packing modes with controlled transition dipole of molecules in crystals, most reported 2D organic crystals exhibited isotropic photon propagation behaviors.43,44 In this regard, the development of versatile photonic fluorophores with packing modes and transition dipoles suitable for polymorphic micro/nano-crystals and anisotropic 2D photon transport is of great significance, whereby fine-tuning the intermolecular interactions is crucial, yet remains challenging.
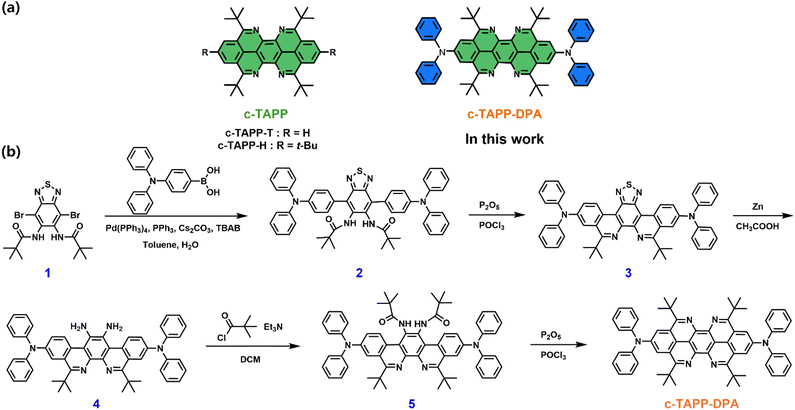
Our previous study has showed that 5,6,12,13-tetraazaperopyrene (TAPP) was a unique photonic fluorophore.45 N-doping and t-Bu substitution enabled a precise control of intermolecular interactions. However, the t-Bu substituted TAPPs can neither fabricate polymorphic micro/nano-crystals, nor feature anisotropic 2D photon propagation. To address this limitation, appropriate substitution of the TAPP core, by introducing steric electron-donating groups, is expected to be a necessity to synergistically tune the intermolecular interactions, frontier orbital energies, and the photophysical and photonic properties of the resultant fluorophores. In this contribution, we therefore synthesized a diphenylamine substituted TAPP for polymorph engineering and photonic applications (c-TAPP-DPA, Scheme 1a). Electronic (electron rich) and conformational (steric and twisting) effects offered by diphenylamine groups endowed c-TAPP-DPA with intrinsic intramolecular charge transfer (ICT) characteristic with the ability to form polymorphic micro/nano-crystals ranging from 1D microrods to 2D rhomboid and hexagonal microplates. These microcrystals exhibited different molecular packing patterns, which eventuate in their different fluorescence and optical waveguide properties. Remarkably, the rhomboid microplates displayed an anisotropic photonic behavior. This work thus enriched the structural and functional diversity of organic photonic fluorophores.
 | ||
| Scheme 1 (a) Chemical structures of substituted 5,6,12,13-tetraazaperopyrenes in previous work and in this work; (b) the synthesis of c-TAPP-DPA. | ||
Results and discussion
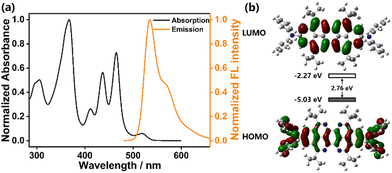
The target molecule c-TAPP-DPA was synthesized via a stepwise four-fold Bischler–Napieralski cyclization as the key step (Scheme 1b), a similar protocol to that for c-TAPP preparation, except for the choice of 4-(diphenylamino)phenylboronic acid as one of the starting reagents. All intermediates and the product were characterized by mass and NMR (1H and 13C) spectroscopy. Good thermal stability of c-TAPP-DPA was confirmed by TGA measurement (Fig. S1, ESI†).In toluene, c-TAPP-DPA showed well-resolved intense UV-visible absorption bands peaks at 411 (ε = 2.3 × 104 M−1 cm−1), 437 (ε = 4.8 × 104 M−1 cm−1) and 465 (ε = 6.1 × 104 M−1 cm−1) nm, respectively, that can typically be seen for the π–π* transition in the peropyrene scaffold (Fig. 1a). Compared to c-TAPP, an additional absorption band at ca. 520 nm was observed, assignable to weak ICT transition in c-TAPP-DPA from the electron-donating DPA moieties to the TAPP core. The toluene solution of c-TAPP-DPA emitted yellow FL with a maximum at 534 nm and an absolute quantum yield of 42% (Table S1, ESI†). Both the characteristic absorption and FL peaks were bathochromically shifted by about 9 nm and 37 nm, respectively, with respect to the t-Bu substituted c-TAPP, which can be attributed to the conjugation between the TAPP core and the two electron-rich substituents. Besides, the solvatochromic effect was verified, in particular, for that in excited state. That was, for the ICT absorption band, and more remarkably for the FL bands, they were red-shifted with solvent polarity increasing, corresponding to classic ICT characteristics. (Fig. S2, ESI†)
Fig. 1b was the calculated frontier orbitals of c-TAPP-DPA based on DFT modelling. The results supported the interpretation that c-TAPP-DPA was an intramolecular donor–acceptor molecule: the HOMO was localized mainly on the peripheral DPA substituents, whereas a more significantly uneven distribution was found for the LUMO that was typically localized at the peropyrene core. The observed charge-transfer band (vide supra) was therefore identified as a result of photo-induced electron transfer from the electron-rich peripheral DPA units (i.e., the HOMO) to the electron-deficient TAPP core (i.e., the LUMO), which differed from the results of c-TAPP-T displaying highly delocalized frontier orbitals. In addition, both the calculated and experimental results verified a narrower band gap concurrent with the elevation of the HOMO level for c-TAPP-DPA as compared to c-TAPP-T (Fig. S3, Table S1, ESI†). These findings were in line with the fact that electron-donating substituents in principle can influence the HOMO to a greater extent than the LUMO.46
Geometrically well-defined single crystals of c-TAPP-DPA, such as 1D microrods, and 2D rhomboid and hexagonal microplates, could be cultivated by solvent diffusion method at different liquid–liquid interfaces (Fig. 2a). For instance, the selection of methanol/dichloromethane and methanol/tetrahydrofuran led to microrods and rhomboid microplates, respectively. They both belonged to the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Table S2 and S3, ESI†). A closer inspection of their packing modes revealed that the microrods and rhomboids possessed a similar lamellar stacking manner yet showed differences in their layer-to-layer spacing, unit cell components and intermolecular interactions. In the two kinds of crystals, the aromatic cores aligned in parallel and far away from each other (9.920 Å, 9.943 Å in Fig. S4a and b, ESI†). The layer-to-layer spacing for microrods and rhomboids was estimated to be 4.814 and 4.935 Å, respectively. Remarkably, as shown in Fig. S4c (ESI†) and Fig. 2c, the unit cell of microrods contained one molecule with multiple intermolecular interactions, such as C–H (DPA)⋯N (2.746 Å) and C–H (t-Bu)⋯C (peropyrene ring) (2.779 Å, 2.827 Å) were the main driving force of the microrods. In contrast, as for rhomboid microplates, each unit cell involved two molecules with more intense intermolecular interactions, including C–H (DPA)⋯N (2.665 Å) and C–H (t-Bu)⋯C (peropyrene ring) (2.857 Å), C–H (DPA)⋯C (peropyrene ring) (2.882 Å), C–H (DPA)⋯C (peropyrene ring) (2.851 Å), C–H (DPA)⋯C–H (t-Bu) (2.367 Å), C (DPA)⋯C–H (t-Bu) (2.856 Å) and C–H (DPA)⋯C (DPA) (2.776 Å, 2.875 Å) (Fig. 2d and Fig. S4d, ESI†).
space group (Table S2 and S3, ESI†). A closer inspection of their packing modes revealed that the microrods and rhomboids possessed a similar lamellar stacking manner yet showed differences in their layer-to-layer spacing, unit cell components and intermolecular interactions. In the two kinds of crystals, the aromatic cores aligned in parallel and far away from each other (9.920 Å, 9.943 Å in Fig. S4a and b, ESI†). The layer-to-layer spacing for microrods and rhomboids was estimated to be 4.814 and 4.935 Å, respectively. Remarkably, as shown in Fig. S4c (ESI†) and Fig. 2c, the unit cell of microrods contained one molecule with multiple intermolecular interactions, such as C–H (DPA)⋯N (2.746 Å) and C–H (t-Bu)⋯C (peropyrene ring) (2.779 Å, 2.827 Å) were the main driving force of the microrods. In contrast, as for rhomboid microplates, each unit cell involved two molecules with more intense intermolecular interactions, including C–H (DPA)⋯N (2.665 Å) and C–H (t-Bu)⋯C (peropyrene ring) (2.857 Å), C–H (DPA)⋯C (peropyrene ring) (2.882 Å), C–H (DPA)⋯C (peropyrene ring) (2.851 Å), C–H (DPA)⋯C–H (t-Bu) (2.367 Å), C (DPA)⋯C–H (t-Bu) (2.856 Å) and C–H (DPA)⋯C (DPA) (2.776 Å, 2.875 Å) (Fig. 2d and Fig. S4d, ESI†).
On the other hand, hexagonal microplates were prepared from methanol and tetrahydrofuran, and the amount of methanol was less than that of preparing rhomboid microplates (details in the ESI†). Single crystal X-ray diffraction analyses verified that these hexagonal microplates adopted a completely different space group and molecular stacking style. In detail, the microplates belonged to the monoclinic Cc space group with three molecules crystalized into an equilateral triangle packing pattern, driven by the multiple intermolecular interactions (C–H (DPA)⋯C–H (t-Bu) (2.197–2.377 Å), C–H (DPA)⋯C (t-Bu) (2.704 Å, 2.857 Å), C–H (t-Bu)⋯C (peropyrene ring) (2.781–2.899 Å), C–H (t-Bu)⋯C–H (t-Bu) (2.324 Å)) (Table S4 and Fig. 2e and Fig. S5a and b, ESI†). The adjacent trimers arranged parallel to each other to form a 3D packing pattern under the intermolecular interactions of C–H (DPA)⋯C (DPA) and C–H (t-Bu)⋯C (peropyrene ring) (Fig. S5c and d, ESI†). Such a novel stacking mode endowed the hexagonal microplates with different FL properties compared to microrods and rhomboids. As presented in Fig. 2b, the two layered crystals displayed yellow FL with an emission maximum at ca. 590 nm. In contrast, a hypsochromic shift of the emission band (20 nm) with brighter green FL (absolute quantum efficiency: 5.41%, Table S1, ESI†) was detected for hexagonal microplates. The excellent and differentiated emission behaviours of polymorphic c-TAPP-DPA crystals were in line with their single crystal structures. As illustrated in Fig. 2, the bulky DPA groups were twisted out of the tetraazaperopyrene plane. Moreover, these crystals have limited or even no π–π overlap areas between the neighbouring TAPP cores. The two factors inhibited intermolecular aggregation of c-TAPP-T-DPA perfectly, which was crucial for their efficient FL in the solid state. In particular, for the hexagonal microplates, the hypsochromic and brighter emission could be attributed to their equilateral triangle stacking mode, which reduces the overlap area between molecules as well as weakens the intermolecular interactions and dipole–dipole interaction. Simultaneously, the looser stacking of hexagonal crystals also endows them with a weaker ability to inhibit the vibration of diphenylamine substituents, making the FWHM of the FL spectrum for hexagonal microplates slightly broader than those of microrod and rhomboid microplates. Collectively, these findings highlighted the ability of c-TAPP-DPA to fabricate polymorphic microcrystals with geometry dependent FL properties, mostly derived from the well controls of multiple intermolecular interactions and twisting effect provided by the DPA substituents on a TAPP core.
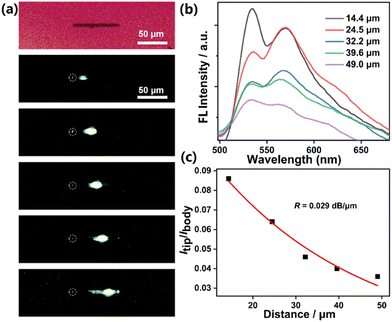
To verify the versatility of these crystals as photonic materials, spatially resolved FL spectra of an individual microrod and microplate were recorded by locally exciting the crystal with a focused laser beam (λ = 375 nm). As for the microrods, when excited by focused laser at different positions, yellow emission was observed at the tip of the crystal (Fig. 3a). The FL spectra of the excitation site (body) and the tip site (tip) are shown in Fig. 3b. The intensity of output light decreased exponentially as a function of the propagation distance between the tip and the excitation point, signifying a characteristic feature of the 1D optical waveguide. Similar to many reported organic microcrystals, a relatively low optical loss coefficient (R) of 0.029 dB μm−1 was estimated according to the emission peaks located at 534 nm with the strongest intensity (Fig. 3c), indicating that FL energy can propagate efficiently along the axial direction of the microrods.
Interestingly, in the case of rhomboid microplates, the photons can propagate within the 2D plane featuring a less explored, asymmetric waveguide behavior. Typically, when the laser beam was focused at the center of the microplate with an edge length of about 20 μm × 48 μm, the FL microscopic images showed asymmetric FL in the four edges, i.e. the out-coupled beams of the two adjacent outcoupled edges (1 and 3) were large and bright, while the opposite ones (2 and 4) were small and obscure (Fig. 4a1). The corresponding FL spectra of the four edges also quantitatively demonstrated that the propagation efficiencies of these microplates were highly sensitive to the propagation direction. That was, the emission intensities of edges 1 and 3 were about 1.5 times stronger than that of 2 and 4 (Fig. 4b and Fig. S6, ESI†). Interestingly, the FL spectra of different axial tips (1,2 and 3,4) also present distinct vibration peaks, which may be related to the slightly different arrangement of molecules within the micro-areas selected for testing. In each direction, the intensity of out-coupled light decreased exponentially as a function of propagation distance by changing the position of the excitation point. Accordingly, the loss coefficient R was determined to be 0.030 dB μm−1, 0.046 dB μm−1, 0.042 dB μm−1 and 0.082 dB μm−1 for the propagation path towards direction  ,
, 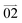 ,
,  and
and  , respectively (Fig. 4a2–a12 and c–f). Smaller R values corresponding to the directions of
, respectively (Fig. 4a2–a12 and c–f). Smaller R values corresponding to the directions of  and
and 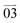 than those for the directions of
than those for the directions of  and
and  confirmed the 2D anisotropic transmission behavior in this rhomboid microcrystal. Very likely, this asymmetric light propagation was due to the suitably aligned transition dipole of the oriented molecules with the incident light in the directions of
confirmed the 2D anisotropic transmission behavior in this rhomboid microcrystal. Very likely, this asymmetric light propagation was due to the suitably aligned transition dipole of the oriented molecules with the incident light in the directions of  and
and  , and vice versa. Such an anisotropic flow of photons occurring in the bright emissive microplate was appealing for the promising application of this kind of microcrystals in optical planar diodes.
, and vice versa. Such an anisotropic flow of photons occurring in the bright emissive microplate was appealing for the promising application of this kind of microcrystals in optical planar diodes.
Lastly, as for the hexagonal microplates, three-directional (DI–DIII) 2D optical waveguide was determined. As shown in Fig. 5a and b1–b15, when the laser beam was focused on the hexagon (edge length of the crystal about 55 μm), photons propagated from center to the edges in three directions, and the out-coupled beams of the two opposite outcoupled edges in each direction showed slight difference, which may be related to the different output angles at two opposite edges.38 Furthermore, in line with the hexagon shape, each propagation vector formed an angle of about 60° with the other two directions (Fig. 5a). The corresponding spatially resolved FL spectra in three directions were obtained by changing the input position on the microplate. In a similar way, RI–III representing the light dissipation during propagation along three directions were calculated as 0.024, 0.029 and 0.030 dB μm−1, respectively (Fig. 5c–e). The approximately identical R values in different directions manifested an isotropic, multi-directional 2D waveguide performance, which implied a homogeneity of the crystal structure with well aligned molecules.
Conclusions
A new D–A type fluorophore consisting of a 5,6,12,13-tetraazaperopyrene core and two DPA substituents at the 2,9-positions has been synthesized (c-TAPP-DPA). Flexibly twisted and electron rich DPA groups were essential to tune the intermolecular interactions and packing modes of c-TAPP-DPA. As a result, three kinds of microcrystals with different geometries (1D microrods, 2D rhomboid microplates and 2D hexagonal microplates) and FL properties (yellow vs. brighter green FL) were obtained. The polymorphism of the three microcrystals was found to be pertinent to different molecular interactions and arrangements of the molecules, and ultimately brought about geometry-dependent multi-dimensional/directional optical waveguide properties with low optical loss coefficient. In particular, the 2D rhomboid microplates displayed interesting asymmetric light propagation. Therefore, the polymorphic c-TAPP-DPA microcrystals have provided an excellent platform to study the relationship between molecular stacking patterns and solid-state optical and photonic properties. Furthermore, the ability to propagate light asymmetrically makes this kind of crystal a promising candidate for optically planar diodes and integrated photonic circuits.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grants 22275068 and 21975178), the Open Project of the State Key Laboratory of Supramolecular Structure and Materials, and the Collaborative Innovation Center of Suzhou Nano Science and Technology (CICNano).References
- C. Zhang, Y. L. Yan, J. N. Yao and Y. S. Zhao, Adv. Mater., 2013, 25, 2854–2859 CrossRef CAS PubMed.
- W. Yao, Y. L. Yan, L. Xue, C. Zhang, G. P. Li, Q. D. Zheng, Y. S. Zhao, H. Jiang and J. N. Yao, Angew. Chem., Int. Ed., 2013, 52, 8713–8717 CrossRef CAS PubMed.
- Y. J. Li, Y. L. Yan, Y. S. Zhao and J. N. Yao, Adv. Mater., 2016, 28, 1319–1326 CrossRef CAS PubMed.
- C. Zhang, Y. L. Yan, Y. S. Zhao and J. N. Yao, Acc. Chem. Res., 2014, 47, 3448–3458 CrossRef CAS PubMed.
- H. Y. Liu, X. Q. Cao, Y. S. Wu, Q. Liao, A. J. Jiménez, F. Würthner and H. B. Fu, Chem. Commun., 2014, 50, 4620–4623 RSC.
- L. Catalano, D. P. Karothu, S. Schramm, E. Ahmed, R. Rezgui, T. J. Barber, A. Famulari and P. Naumov, Angew. Chem., Int. Ed., 2018, 57, 17254–17258 CrossRef CAS PubMed.
- D. Tian and Y. L. Chen, Adv. Opt. Mater., 2021, 9, 2002264 CrossRef CAS.
- M. Luo, J. Zhao, Y. Y. Liu, L. Jiang, S. Wang and Z. G. Chi, Adv. Opt. Mater., 2022, 10, 2101227 Search PubMed.
- S. Q. Wu, B. Zhou and D. P. Yan, Adv. Opt. Mater., 2021, 9, 2001768 CrossRef CAS.
- X. M. Lu, X. D. Wang, Q. Liao and H. B. Fu, J. Phys. Chem. C, 2015, 119, 22108–22113 CrossRef CAS.
- X. D. Wang, Q. Liao, X. M. Lu, H. Li, Z. Z. Xu and H. B. Fu, Sci. Rep., 2014, 4, 7011 CrossRef PubMed.
- X. D. Wang, Q. Liao, Q. H. Kong, Y. Zhang, Z. Z. Xu, X. M. Lu and H. B. Fu, Angew. Chem., Int. Ed., 2014, 53, 5863–5867 CrossRef CAS PubMed.
- W. Zhang, J. N. Yao and Y. S. Zhao, Acc. Chem. Res., 2016, 49, 1691–1700 CrossRef CAS PubMed.
- X. D. Wang, Z. Z. Li, M. P. Zhuo, Y. S. Wu, S. Chen, J. N. Yao and H. B. Fu, Adv. Funct. Mater., 2017, 27, 1703470 CrossRef.
- W. Zhang, Y. L. Yan, J. M. Gu, J. N. Yao and Y. S. Zhao, Angew. Chem., Int. Ed., 2015, 54, 7125–7129 CrossRef CAS PubMed.
- Y. G. Zhen, H. L. Dong, L. Jiang and W. P. Hu, Chin. Chem. Lett., 2016, 27, 1330–1338 CrossRef CAS.
- D. Liu, C. G. Li, S. J. Niu, Y. Li, M. X. Hu, Q. Y. Li, W. G. Zhu, X. T. Zhang, H. L. Dong and W. P. Hu, J. Mater. Chem. C, 2019, 7, 5925–5930 RSC.
- S. Z. Bisri, T. Takenobu, Y. Yomogida, H. Shimotani, T. Yamao, S. Hotta and Y. Iwasa, Adv. Funct. Mater., 2009, 19, 1728–1735 CrossRef CAS.
- J. Li, K. Zhou, J. Liu, Y. G. Zhen, L. Liu, J. D. Zhang, H. L. Dong, X. T. Zhang, L. Jiang and W. P. Hu, J. Am. Chem. Soc., 2017, 139, 17261–17264 CrossRef CAS PubMed.
- Y. S. Zhao, H. B. Fu, A. D. Peng, Y. Ma, D. B. Xiao and J. N. Yao, Adv. Mater., 2008, 20, 2859–2876 CrossRef CAS.
- Y. S. Zhao, H. B. Fu, F. Q. Hu, A. D. Peng, W. S. Yang and J. N. Yao, Adv. Mater., 2008, 20, 79–83 CrossRef CAS.
- M. W. Li, Y. Yuan and Y. L. Chen, Chin. J. Chem., 2021, 39, 3101–3115 CrossRef CAS.
- W. Yuan, X. K. Ren, M. W. Li, H. S. Guo, Y. Han, M. J. Wu, Q. Wang, M. M. Li and Y. Chen, Angew. Chem., Int. Ed., 2018, 57, 6161–6165 CrossRef CAS PubMed.
- C. Wang and Z. Li, Mater. Chem. Front., 2017, 1, 2174–2194 RSC.
- S. Q. Ma, Y. J. Liu, J. B. Zhang, B. Xu and W. J. Tian, J. Phys. Chem. Lett., 2020, 11, 10504–10510 CrossRef CAS PubMed.
- C. Zhang, Y. L. Yan, Y. Y. Jing, Q. Shi, Y. S. Zhao and J. N. Yao, Adv. Mater., 2012, 24, 1703–1708 CrossRef CAS PubMed.
- W. Yao and Y. S. Zhao, Nanoscale, 2014, 6, 3467–3473 RSC.
- Q. Li, Y. Jia, L. R. Dai, Y. Yang and J. B. Li, ACS Nano, 2015, 9, 2689–2695 CrossRef CAS PubMed.
- K. Wang, H. Y. Zhang, S. Y. Chen, G. C. Yang, J. B. Zhang, W. J. Tian, Z. M. Su and Y. Wang, Adv. Mater., 2014, 26, 6168–6173 CrossRef CAS PubMed.
- Y. Yu, Y. C. Tao, S. N. Zou, Z. Z. Li, C. C. Yan, M. P. Zhuo, X. D. Wang and L. S. Liao, Sci. China: Chem., 2020, 63, 1477–1482 CrossRef CAS.
- Z. Z. Xu, Z. W. Zhang, X. Jin, Q. Liao and H. B. Fu, Chem. – Asian J., 2017, 12, 2985–2990 CrossRef CAS PubMed.
- H. Zhang, D. Jin, D. Q. Lin, L. Huang, J. Wang, S. S. Wang and L. H. Xie, Chin. J. Chem., 2022, 40, 832–837 CrossRef CAS.
- X. Ye, Y. Liu, Q. Guo, Q. X. Han, C. Ge, S. Y. Cui, L. L. Zhang and X. T. Tao, Nat. Commun., 2019, 10, 761 CrossRef CAS PubMed.
- X. D. Zhang, J. Y. Gong, W. Tao, X. J. Jiang, C. Chen and P. F. Wei, ACS Mater. Lett., 2022, 4, 1468–1474 CrossRef CAS.
- J. Wang, S. P. Xu, A. S. Li, L. Chen, W. Q. Xu and H. Y. Zhang, Mater. Chem. Front., 2021, 5, 1477–1485 RSC.
- L. P. Heng, X. Y. Wang, D. L. Tian, J. Zhai, B. Z. Tang and L. Jiang, Adv. Mater., 2010, 22, 4716–4720 CrossRef CAS PubMed.
- Z. Q. Li, D. X. Ma, F. F. Xu, T. X. Dan, Z. L. Gong, J. Y. Shao, Y. S. Zhao, J. N. Yao and Y. W. Zhong, Angew. Chem., Int. Ed., 2022, 61, e202205033 CAS.
- Y. Liu, H. P. Hu, L. Xu, B. Qiu, J. Liang, F. Ding, K. Wang, M. M. Chu, W. Zhang, M. Ma, B. Chen, X. Z. Yang and Y. S. Zhao, Angew. Chem., Int. Ed., 2020, 59, 4456–4463 CrossRef CAS PubMed.
- Q. Li, W. Jin, M. M. Chu, W. Zhang, J. M. Gu, B. Shahid, A. B. Chen, Y. F. Yu, S. L. Qiao and Y. S. Zhao, Nanoscale, 2018, 10, 4680–4685 RSC.
- M. P. Zhuo, Y. C. Tao, X. D. Wang, Y. C. Wu, S. Chen, L. S. Liao and L. Jiang, Angew. Chem., Int. Ed., 2018, 57, 11300–11304 CrossRef CAS PubMed.
- J. Wang, S. T. Zhang, S. P. Xu, A. S. Li, B. Li, L. Ye, Y. J. Geng, Y. Tian and W. Q. Xu, Adv. Opt. Mater., 2020, 8, 1901280 CrossRef CAS.
- M. Q. Dai, B. Zhou, X. Y. Fang and D. P. Yan, ACS Appl. Mater. Interfaces, 2022, 14, 40223–40231 CrossRef CAS PubMed.
- N. Chandrasekhar and R. Chandrasekar, Angew. Chem., Int. Ed., 2012, 51, 3556–3561 CrossRef CAS PubMed.
- H. W. Luo, S. J. Chen, Z. T. Liu, C. Zhang, Z. X. Cai, X. Chen, G. X. Zhang, Y. S. Zhao, S. Decurtins, S. X. Liu and D. Q. Zhang, Adv. Funct. Mater., 2014, 24, 4250–4258 CrossRef CAS.
- W. Yuan, J. J. Cheng, X. P. Li, M. J. Wu, Y. Han, C. M. Yan, G. Zou, K. Mullen and Y. L. Chen, Angew. Chem., Int. Ed., 2020, 59, 9940–9945 CrossRef CAS PubMed.
- S. Geib, S. C. Martens, M. Marken, A. Rybina, H. Wadepohl and L. H. Gade, Chem. – Eur. J., 2013, 19, 13811–13822 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2237822–2237824. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3tc00462g |
| This journal is © The Royal Society of Chemistry 2023 |