Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A low-symmetry monothiatruxene-based hole transport material for planar n–i–p perovskite solar cells with 18.9% efficiency†
Ellie
Tanaka‡
 a,
Gyu Min
Kim§
a,
Gyu Min
Kim§
 b,
Michał R.
Maciejczyk¶
a,
Ayumi
Ishii||
b,
Michał R.
Maciejczyk¶
a,
Ayumi
Ishii||
 b,
Gary S.
Nichol
b,
Gary S.
Nichol
 a,
Tsutomu
Miyasaka
a,
Tsutomu
Miyasaka
 *b and
Neil
Robertson
*b and
Neil
Robertson
 *a
*a
aSchool of Chemistry, University of Edinburgh, Kings Buildings, Edinburgh, EH9 3FJ, UK. E-mail: neil.robertson@ed.ac.uk
bGraduate School of Engineering, Toin University of Yokohama, 1614 Kuroganecho, Aoba, Yokohama, Kanagawa 225-8503, Japan
First published on 20th March 2023
Abstract
Hole transport materials (HTMs) based on truxene cores have emerged as promising candidates in recent years. They are noted by properties such as higher hole mobility and higher glass transition temperature than the 2,2′,7,7′-tetrakis (N,N-di-p-methoxyphenamine)-9,9′-spirobiflourene (spiro-MeOTAD), as well as good hydrophobicity and energy alignment. Truxene derivatives have been studied for application in transistors, OLEDs, lasers, supercapacitors, etc., however, there are only a few studies on their use as HTMs in perovskite solar cells (PSCs). In this study, we synthesised a novel small organic molecule HTM with a monothiatruxene (TrxS) core, namely TrxS-2MeOTAD, and characterised its basic properties and ability as an HTM in n–i–p planar PSCs. The TrxS-2MeOTAD showed suitable electrochemical, optical, structural and thermal properties for an HTM, such as a relatively high glass transition temperature (145 °C) and stable amorphous nature when deposited as films. The PSCs using TrxS-2MeOTAD achieved 18.9% power conversion efficiency (PCE) compared to the reference spiro-MeOTAD at 19.3% PCE. The unencapsulated TrxS-2MeOTAD devices showed better operational stability than spiro-MeOTAD, with a 1.5 times longer lifetime under constant AM1.5G illumination. Our results suggest that small molecules based on the TrxS core can be a promising direction for the development of alternative HTMs.
10th Anniversary StatementI have been heavily involved with Journal of Materials Chemistry C as an Associate Editor for nearly nine years, hence for nearly all of its existence as a separate journal. Back in 2014, I was delighted to be offered the opportunity to join the editorial team and to contribute what I can to the journal's development. In addition to the overall quality of JMCC, the attraction for me has always been the breadth across so many types of organic and inorganic materials for applications including optical, electronic and magnetic topics. I don’t think any other journal would have given me the chance for such a complete overview of functional materials. I will complete my time as Associate Editor later this year, but am looking forward to continuing my involvement with JMCC as an author and reader, and am sure it will continue to go from strength to strength over its second decade. Neil Robertson, January 2023. |
Introduction
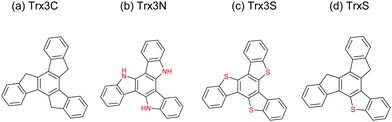
In recent years, perovskite solar cells (PSCs) have attracted tremendous interest as a readily-processed and efficient emerging solar technology.1–6 Organic small molecules such as 2,2′,7,7′-tetrakis[n,n-di(4-methoxyphenyl)amino]-9,9′-spirobifl-uorene (spiro-MeOTAD or spiro-OMeTAD) have been the classic hole transport materials (HTMs) in PSCs owing to the wide range of possible core/substituent designs, solubility and amorphous nature when deposited as thin films.7–11 Despite the relatively high performance ca. 20% that has been consistently achieved across many labs using spiro-MeOTAD as the HTM in PSCs,12–14 there remain some limitations: (1) the short lifetime of the final PSC due to the hygroscopic additives i.e. lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and tert-butyl pyridine (tBP) in the HTM, and (2) the costly synthesis and purification of the spiro-MeOTAD. When designing a new organic small molecule HTM, the selection of the core structure will greatly influence the properties and cost of the final HTM. Such examples include the spiro[fluorene-9,90-xanthene] (SFX) core and phenothiazine core, where the materials showed power conversion efficiencies (PCEs) comparable to spiro-MeOTAD with greatly reduced cost.15–18HTMs based on truxene cores (Fig. 1) have emerged as promising candidates in the last few years. They are noted by properties such as higher hole mobility and higher glass transition temperature than the spiro-MeOTAD as well as good hydrophobicity and energy alignment.19 Truxene derivatives have so far been studied for application in transistors, OLEDs, lasers, supercapacitors, etc., however, there are only a few studies of their use as HTMs in PSCs. Huang et al. demonstrated a truxene (Trx3C) based HTM and achieved 18.6% in an inverted p–i–n PSC.20 Rakstys et al. studied a number of triazatruxene (Trx3N) based HTMs to achieve 18–19% PCE in mesoporous n–i–p PSCs.21,22 Meanwhile, Maciejczyk et al. reported a new monothiatruxene core (TrxS),23 which displayed merged properties of the Trx3C and Trx3S, whereby the low symmetry may lead to a greater potential to form uniform, amorphous films. The oxidised form of TrxS (TrxSO2) has been demonstrated to display exceptional properties in blue organic light-emitting materials,24 however this remains the only application sought for this class of materials. In this study, we therefore explore the potential of TrxS-based materials as HTMs in PSCs by synthesising a simple methoxydiphenylamine (MeODPA) substituted derivative (TrxS-2MeOTAD). The design of the molecule was chosen based on computational DFT calculations. The newly synthesised HTM shows good solubility, high glass transition temperature, sufficient hole mobility and stability. Planar n–i–p PSCs using the new molecule achieved 18.9% PCE—comparable with the reference spiro-MeOTAD at 19.3% PCE. The unencapsulated solar cell using TrxS-2MeOTAD showed better operation stability than spiro-MeOTAD, with a 1.5 times longer lifetime under constant AM1.5 illumination.
Results and discussion
Materials and synthesis
| HOMOa (G)/eV | LUMOa (G)/eV | HOMOb (S)/eV | LUMOb (S)/eV | E g (G)/eV | E g (S)/eV | |
|---|---|---|---|---|---|---|
| a HOMO and LUMO levels calculated for the compound in gas state. b HOMO and LUMO levels calculated for the compound in the liquid state with DCM as solvent. c HOMO–LUMO gap derived from LUMO (G)–HOMO (G). d HOMO–LUMO gap derived from LUMO (S)–HOMO (S). | ||||||
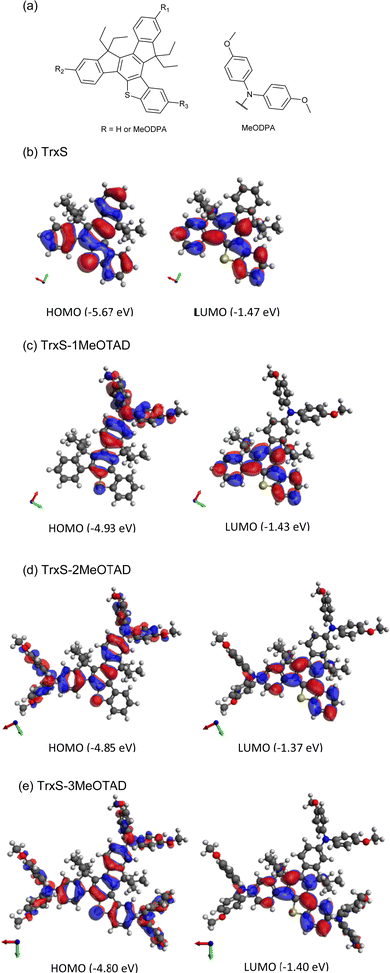
| TrxS | −5.52 | −1.35 | −5.67 | −1.47 | 4.17 | 4.20 |
| TrxS-1MeOTAD | −4.78 | −1.24 | −4.93 | −1.43 | 3.54 | 3.50 |
| TrxS-2MeOTAD | −4.65 | −1.14 | −4.85 | −1.37 | 3.51 | 3.48 |
| TrxS-3MeOTAD | −4.56 | −1.13 | −4.80 | −1.40 | 3.43 | 3.40 |
Table 1 shows the rise in HOMO level when the number of MeODPA moieties increases. The wide bandgaps derived from the liquid-state calculations suggest the compounds to be optically transparent in the visible, which is advantageous for an HTM since it will not absorb any of the light that should be harvested by the perovskite. The HOMO level of the HTM should be assessed based on the valence band edge energy of the lead-halide perovskite, typically in the range of ca. −5.9 eV to −5.3 eV (CsPbI3: −5.3 eV;25 MAPbI3: −5.4 eV;26 (FAPbI3)0.95(MAPbBr3)0.05: −5.5 eV;27 CsPbI2Br: −5.8 eV;28 CsPbBr3: −5.9 eV).25 The HOMO of the HTM should be higher than the valence band energy of the perovskite, therefore TrxS with predicted HOMO (L) = −5.67 eV would possibly work for some of the perovskite materials studied but probably not with the commonest perovskite formulas such as MAPbI3. The energy levels in general are seen to shift to lower values when the solvent field is applied. This can be explained by the enhanced stability of the more polarizable state by DCM solvent. The energy levels are expected to lower further in the solid state. The >0.7 eV upward shift in HOMO level from TrxS to xMeOTAD (x from 1 to 3) can be explained by the extended HOMO over the MeODPA moiety once substituted. HOMO/LUMO locations for TrxS, TrxS-2MeOTAD and TrxS-3MeOTAD are shown in Fig. 2. When MeODPA is substituted, the HOMO evenly distributes over the MeODPA, bridged by the TrxS core, while the LUMO is concentrated on the TrxS core.
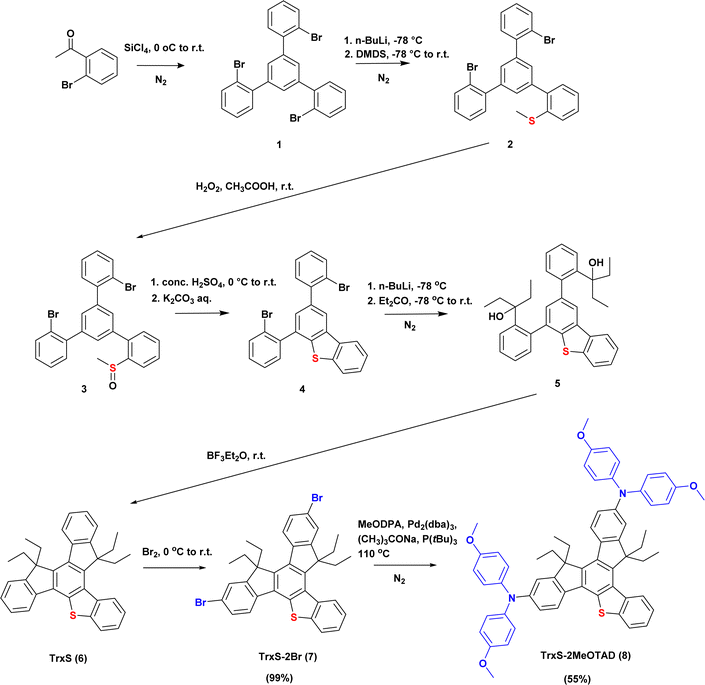
TrxS-2MeOTAD with two MeODPA substituents was selected as the target molecule in this study, based on the predicted energy levels and predicted simplicity of the synthesis. The overall synthesis scheme is described in Fig. 3.
Synthesis of TrxS-2MeOTAD
The synthesis of TrxS is based on the reports by Maciejczyk et al.23,24 TrxS-2Br and TrxS-2MeOTAD were synthesised for the first time in this study. The bromination of TrxS resulted in an excellent yield of 99% and further purification of TrxS-2Br was not required before the synthesis of TrxS-2MeOTAD. The final TrxS-2MeOTAD was synthesised by Buchwald–Hartwig amination of the TrxS-2Br with 4,4′-dimethoxydiphenylamine. Details of the syntheses are provided in the ESI.†Electrochemical analysis
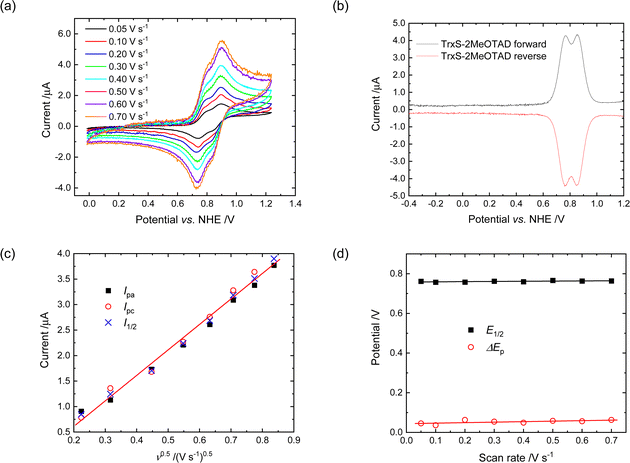
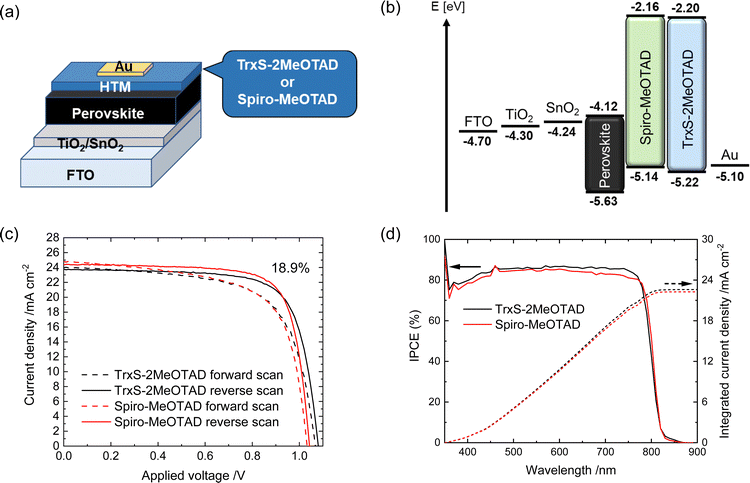
The electrochemical properties of TrxS-2MeOTAD were investigated by cyclic voltammetry (Fig. 4(a)) in DCM solution, with ferrocene used as internal standard.29 Two oxidation peaks with a difference of 90 mV were detected as presented in the square wave voltammogram in Fig. 4(b). For the first oxidation peak the proportionality of I1/2 (mean of Ipa and Ipc) against the square-root of the scan rate (ν0.5) (Fig. 4(c)); the negligible difference between Ipa and Ipc; the scan rate independence of E1/2; and ΔEp values around 60 mV (Fig. 4(d)) indicate good chemical and electrochemical reversibility, important for good stability of the HTM in the PV cell, and rapid electron transfer, important for high hole mobility.The HOMO level of TrxS-2MeOTAD is similar to the value reported for spiro-MeOTAD (−5.22 eV vs. −5.14 eV)15,30 and is in a suitable range for most common perovskites for PSCs. However, the experimental HOMO value turned out to be lower than the calculated value by 0.37 eV, which may be too low for some perovskites and in such cases the HOMO level of TrxS-3MeOTAD may be in a more suitable range.
Optical properties
The absorption and emission properties of the TrxS-2MeOTAD are shown in Fig. S1(a) (ESI†). The absorption peak (λmax) and emission peak (λem) do not shift from solution to film, which is an indication of the amorphous nature for the film. Compared to the solution, the TrxS-2MeOTAD film shows a tail at lower energies for both absorption and emission, which could be interpreted as some intermolecular interaction. The film is colourless, which is confirmed by the absence of any absorption in the visible. The experimental values for the electrochemical and optical data are summarised in Table 2.| HTM | λ max /nm (ε/105 M−1 cm−1) | λ em /nm | E ox /V | E HOMO /eV | E LUMO /eV | E g /eV |
|---|---|---|---|---|---|---|
| a Absorption peak from UV/Vis absorption (molar extinction coefficient in parenthesis). b Emission peak detected by excitation at λmax. c Half-wave potential (E1/2) from CV (peak potential from SWV in parenthesis), referenced to NHE. d Calculated from EHOMO [eV] = −EOX [V] − 4.456 (value from SWV in parenthesis).30 e Calculated from ELUMO = EHOMO + Eg. f Optical gap derived from UV/Vis absorption and PL measurements. g Data from the literature.15 | ||||||
| TrxS-2MeOTAD | 360a (7.37) | 440 | 0.761 (0.764) | −5.22 (−5.22) | −2.20 | 3.02 |
| Spiro-MeOTADg | 385 | 424 | — | −5.14 | −2.16 | 2.98 |
Thermal properties
The thermal properties were assessed by thermo gravimetric-differential thermal analysis (TG-DTA) (Fig. 5(a)) and differential scanning calorimetry (DSC) (Fig. 5(b). The properties are summarised in Table S1 (ESI†). TrxS-2MeOTAD displays an endothermic peak at 223 °C (or 221 °C from the DSC), accompanied by a small endothermic peak at 260 °C (or 261 °C from the DSC). With both points having no weight losses, the first prominent peak at 223 °C is apparently the melting point (Tm), while the small peak could be related to some polymorphic change of the material. The TG curve shows one step of weight loss from 333 °C (0.5% weight loss at ca. 400 °C) with an exothermic peak at 460 °C, assignable to decomposition. 45% of the initial weight was lost during this step until the end of the measurement at 590 °C. In Fig. S2 (ESI†), a shift of the baseline before the melting point suggests that TrxS-2MeOTAD has a glass transition temperature (Tg) at 145 °C. This is higher than that of spiro-MeOTAD at 122 °C, indicating that TrxS-2MeOTAD has relatively high stability in its rigid glassy state. No cold crystallisation was observed, in contrast to the case for some organic small molecules including spiro-MeOTAD.15 To further explore the thermal stability we compared SEM images of the spin-coated TrxS-2MeOTAD before heating, after heating for 10 minutes at 135 °C, and after heating for 10 minutes at 155 °C (i.e. 10 °C below and above Tg respectively) (Fig. S8, ESI†). We observe very limited change in the images after heating, with a similar amorphous appearance at higher temperature. Overall, the results indicate that TrxS-2MeOTAD has high thermal stability as an organic small molecule, making it compatible for an HTM in PSCs.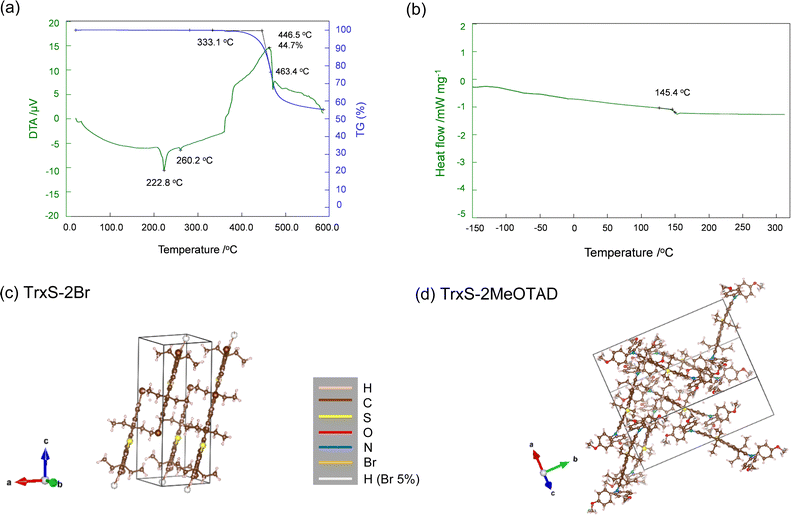 | ||
| Fig. 5 (a) TG-DTA curves of TrxS-2MeOTAD. The blue curve indicates the TG, with a decomposition onset at 333 °C. (b) Second heating profile of the DSC to determine the glass temperature (Tg). (c) The crystal structures of TrxS-2Br (CCDC 2235428†) and (d) that of TrxS-2MeOTAD (CCDC 2235429†) from the SXRD analysis. The images were created by the Vesta software.37 White spheres are marked in the TrxS-2Br image because the recrystallised crystal was found to contain ca. 5% of tribrominated TrxS-3Br. This, we believe, does not affect the interpretation of the SXRD analysis. | ||
Structural properties of TrxS-2MeOTAD
The crystal structures of TrxS-2Br and TrxS-2MeOTAD were solved from single crystals as shown in Fig. 5(c and d). Full crystal parameters are listed in Table S2 (ESI†). TrxS-2Br was found to crystallise in triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, whereas TrxS-2MeOTAD was found to crystallise in monoclinic P21/c space group. The TrxS-2Br molecules displayed π–π stacking, with interactions of 3.557 Å. The crystal packing of TrxS-2MeOTAD is more complicated, with the molecules stacking in a zigzag form. This apparently comes from the twisted MeODPA moiety, with a dihedral angle of 123.3°. π–π interactions of 3.68 Å were observed, which explains the high Tg and melting point. The X-ray powder diffraction (PXRD) patterns of TrxS-2MeOTAD in powder form and as a spin-coated film on microscope glass slide along with simulated PXRD pattern from the SXRD analysis are shown in Fig. S3 (ESI†). TrxS-2MeOTAD powder showed preferred orientation towards the (100) facet, with one prominent peak at 2θ = 5.4° followed by several other small peaks. The spin-coated film from chlorobenzene did not show any peaks, indicating that the TrxS-2MeOTAD film is amorphous.
space group, whereas TrxS-2MeOTAD was found to crystallise in monoclinic P21/c space group. The TrxS-2Br molecules displayed π–π stacking, with interactions of 3.557 Å. The crystal packing of TrxS-2MeOTAD is more complicated, with the molecules stacking in a zigzag form. This apparently comes from the twisted MeODPA moiety, with a dihedral angle of 123.3°. π–π interactions of 3.68 Å were observed, which explains the high Tg and melting point. The X-ray powder diffraction (PXRD) patterns of TrxS-2MeOTAD in powder form and as a spin-coated film on microscope glass slide along with simulated PXRD pattern from the SXRD analysis are shown in Fig. S3 (ESI†). TrxS-2MeOTAD powder showed preferred orientation towards the (100) facet, with one prominent peak at 2θ = 5.4° followed by several other small peaks. The spin-coated film from chlorobenzene did not show any peaks, indicating that the TrxS-2MeOTAD film is amorphous.
Hole mobility of TrxS-2MeOTAD
The space-charge limited current (SCLC) was measured for hole-only devices to assess the hole mobility of TrxS-2MeOTAD. A hole only device with the structure 〈ITO/PEDOT:PSS/HTM/Au〉 (ITO = indium-doped tin oxide, PEDOT:PSS = poly(3,4-ethylenedioxythiophene polystyrene sulfonate) was tested and compared with a similar device using spiro-MeOTAD as the HTM. Both HTMs were doped with the optimised ratio of dopants for each. The J–V curves that were used to derive the hole mobility are shown in Fig. 6(a). The slope of J0.5vs. V (denoted as C0.5) was used to derive the hole mobility μ from the following relation: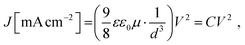 | (1) |
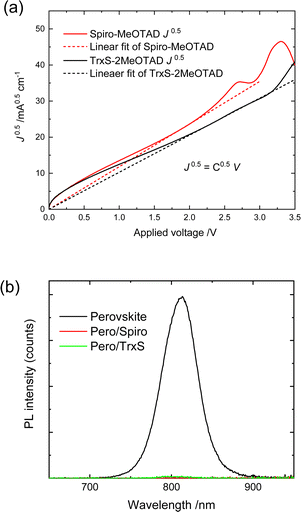 | ||
| Fig. 6 (a) J0.5–V curves used to derive the hole mobility from SCLC measurements. (b) Steady-state photoluminescence spectra of spiro-MeOTAD/perovskite and TrxS-2MeOTAD/perovskite films. | ||
Photoluminescence quenching
The steady-state and time-resolved PL were measured for a device structure of 〈Glass/Perovskite/HTM〉 for TrxS-2MeOTAD and spiro-MeOTAD. The steady-state PL is shown in Fig. 6(b) and the time-resolved PL decay curves are shown in Fig. S4 (ESI†). While the EQE of the 〈glass/perovskite〉 derived from the steady-state PL was 1.1%, the EQE of 〈Glass/Perovskite/TrxS-2MeOTAD〉 and 〈Glass/Perovskite/spiro-MeOTAD〉 were both approximately zero. Both steady-state and time-resolved measurements confirm that the TrxS-2MeOTAD sufficiently quenches the PL of the perovskite by efficient hole extraction.Photovoltaic performance
The PSCs were fabricated in dry atmosphere, i.e., with normal oxygen content and humidity below 2%. The devices had a general structure of fluorine-doped tin oxide (FTO)/TiO2/SnO2/Perovskite/HTM/Au, where the perovskite was a triple-cation Cs0.04(FA0.83MA0.17)0.96Pb(I0.95Br0.05)3 (FA = formamidinium, MA = methylammonium) as shown in Fig. 7(a and b).32 The HTM and dopant (LiTFSI and tBP) concentration in the spin-coating solution were independently optimised for TrxS-2MeOTAD (Fig. S5 and S6, ESI†). The performance of the optimised TrxS-2MeOTAD PSC (40 mM HTM, HTM/LiTFSI/tBP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.55
0.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.3 (mol/mol)) and spiro-MeOTAD PSC (60 mM HTM, HTM/LiTFSI/tBP = 1
3.3 (mol/mol)) and spiro-MeOTAD PSC (60 mM HTM, HTM/LiTFSI/tBP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.55
0.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.3 (mol/mol)) are summarised in Table 3. The corresponding J–V curves are shown in Fig. 7(c) along with the incident photon-to-current efficiency (IPCE) spectra (Fig. 7(d)). The best TrxS-2MeOTAD PSC exhibited 18.9% PCE, nearing spiro-MeOTAD with 19.3% PCE. Interestingly, the JSC, VOC, ff and hysteresis of the TrxS-2MeOTAD were all very similar to the spiro-MeOTAD PSC. It is seen from Fig. 7(c) that the JSC is slightly higher for the spiro-MeOTAD and the VOC is slightly higher for the TrxS-2MeOTAD.
3.3 (mol/mol)) are summarised in Table 3. The corresponding J–V curves are shown in Fig. 7(c) along with the incident photon-to-current efficiency (IPCE) spectra (Fig. 7(d)). The best TrxS-2MeOTAD PSC exhibited 18.9% PCE, nearing spiro-MeOTAD with 19.3% PCE. Interestingly, the JSC, VOC, ff and hysteresis of the TrxS-2MeOTAD were all very similar to the spiro-MeOTAD PSC. It is seen from Fig. 7(c) that the JSC is slightly higher for the spiro-MeOTAD and the VOC is slightly higher for the TrxS-2MeOTAD.
| HTM | Scan | J SC/mA cm−2 | V OC/V | ff | PCE/% |
|---|---|---|---|---|---|
| TrxS-2MeOTAD | Forward | 24.0 | 1.07 | 0.66 | 16.9 |
| Reverse | 23.8 | 1.08 | 0.73 | 18.9 | |
| Spiro-MeOTAD | Forward | 24.8 | 1.03 | 0.66 | 16.8 |
| Reverse | 24.3 | 1.04 | 0.76 | 19.3 |
The IPCE spectra in Fig. 7(d) indicate that the light-to-electricity conversion is also similar for the whole spectral absorption range. The integrated JSC derived from the IPCE spectra was 22.6 mA cm−2 for TrxS-2MeOTAD and 22.3 mA cm−2 for spiro-MeOTAD, which are sufficiently close to the values obtained by the J–V measurements. Overall, the results confirm that the TrxS-2MeOTAD HTM works efficiently in n–i–p PSCs.
Structural properties of the TrxS-2MeOTAD PSCs
Fig. S7(a) (ESI†) shows the PXRD patterns of the optimised PSCs incorporating TrxS-2MeOTAD or spiro-MeOTAD as the HTM. Both patterns only show peaks associated with the perovskite or FTO substrate, which indicates that the HTM layers are amorphous in the PSC. This is consistent with the PXRD pattern of the TrxS-2MeOTAD film coated on a microscope glass slide (Fig. S3, ESI†).SEM images of the cross-section of the PSCs employing TrxS-2MeOTAD and spiro-MeOTAD are shown in Fig. S7(b and c) (ESI†). A uniform layer and smooth interfaces with the perovskite and gold are observed for both HTMs. No grain boundaries are seen in either of the samples, indicating again that the TrxS-2MeOTAD forms amorphous layers similar to spiro-MeOTAD.
Stability of the PSCs
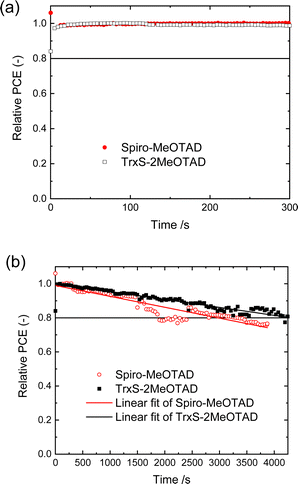
The operational stability of the PSC is equally important to the performance itself. Fig. 8 compares the performance of unencapsulated PSCs using TrxS-2MeOTAD and spiro-MeOTAD as HTM, tracked under constant illumination at 1 sun in ambient air for an hour (stability assessment protocol ISOS-L-1).33 The device was held at a constant voltage at the initial maximum power point and the photocurrent was constantly recorded. The PCE was derived by multiplying the recorded current density with the fixed voltage. Fig. 8(a) shows that both spiro-MeOTAD and TrxS-2MeOTAD PSCs maintained a constant power output for the first 300 s. The PCE at this point was taken as the “initial” PCE in Fig. 8(b). Fig. 8(b) indicates that the TrxS-2MeOTAD PSC has a longer lifetime than the spiro-MeOTAD PSC. The linear fitting was y = 1 − 6.79 × 10−5x for spiro-MeOTAD and y = 1 − 4.63 × 10−5x for TrxS-2MeOTAD, where y is the relative PCE (%) and x is the time [s]. The fitting for TrxS-MeOTAD results in an operational lifetime of T80 = 4320 s (time until 20% PCE drop), which is ca. 1.5 times longer compared to the spiro-MeOTAD PSC at T80 = 2946 s. The higher operational stability of TrxS-2MeOTAD could be attributed to the properties of the HTM itself, or to the lower amount of LiTFSI contained in the corresponding PSC (as demonstrated by the lower concentration of the HTM in the spin-coating solution and the thinner film observed by SEM). Further insights on the photo/thermo/moisture stability could be gained if the devices were measured in a designated environment for each specific condition. Overall, The TrxS-MeOTAD has been demonstrated to display relatively high stability in addition to the excellent performance in PSCs.Conclusions
In summary, a novel organic small molecule TrxS-2MeOTAD with a monothiatruxene core was successfully synthesised and its basic properties were investigated. The electrochemical, optical, structural and thermal properties were found to be suitable for an HTM in n–i–p PSCs. The TrxS-2MeOTAD showed higher glass transition temperature compared to the spiro-MeOTAD, with a stable amorphous nature when deposited as films. The TrxS-2MeOTAD displayed similar photovoltaic performance to spiro-MeOTAD in n–i–p planar PSCs when doped with common dopants LiTFSI and tBP. Hole mobility and PL decay analyses confirmed that the material works as an efficient hole extractor and transporter. Finally, the operational stability of the PSC using TrxS-2MeOTAD was demonstrated to be higher than that using spiro-MeOTAD, which we assume is due to the better thermal stability and lower level of doping required with the new HTM. Interestingly, using spiro-MeOTAD, we see a partial recovery of PCE at 2500 s before the degradation resumes. Such partial recovery has been observed before, albeit associated with a rest period for the cell.34–36 These results all together indicate that TrxS based HTMs can be a promising direction for the development of alternative HTMs. More widely, the study of HTMs based on a low-symmetry core may prove a convenient route to amorphous films of good thermal stability. In addition, the close crystal packing and high orientation observed in the crystalline form provokes interest in testing these materials as HTMs in inverted planar PSCs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
ET acknowledges JASSO for a PhD studentship. The authors acknowledge the Royal Society of Chemistry for a Researcher Mobility Grant towards collaborative research. TM would like to thank Mitsubishi Chemical Corporation for the TG-DTA and DSC measurements. Some crystal data were collected remotely at beam line I-19 of Diamond Light Source (award CY22240). We thank Jiayi Zhao and Xuerui Yi for carrying out the heated-sample SEM experiments.References
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef CAS PubMed.
- H.-S. Kim, C.-R. Lee, J.-H. Im, K.-B. Lee, T. Moehl, A. Marchioro, S.-J. Moon, R. Humphry-Baker, J.-H. Yum, J. E. Moser, M. Grätzel and N.-G. Park, Sci. Rep., 2012, 2, 591 CrossRef PubMed.
- M. A. Green and A. Ho-Baillie, ACS Energy Lett., 2017, 2, 822–830 CrossRef CAS.
- A. K. Jena, A. Kulkarni and T. Miyasaka, Chem. Rev., 2019, 119, 3036–3103 CrossRef CAS PubMed.
- T. Wu, Z. Qin, Y. Wang, Y. Wu, W. Chen, S. Zhang, M. Cai, S. Dai, J. Zhang, J. Liu, Z. Zhou, X. Liu, H. Segawa, H. Tan, Q. Tang, J. Fang, Y. Li, L. Ding, Z. Ning, Y. Qi, Y. Zhang and L. Han, Nano-Micro Lett., 2021, 13, 1–18 CrossRef PubMed.
- H. D. Pham, L. Xianqiang, W. Li, S. Manzhos, A. K. K. Kyaw and P. Sonar, Energy Environ. Sci., 2019, 12, 1177–1209 RSC.
- L. Nakka, Y. Cheng, A. G. Aberle and F. Lin, Adv. Energy Sustainability Res., 2022, 3, 2200045 CrossRef CAS.
- H. D. Pham, Z. Wu, L. K. Ono, S. Manzhos, K. Feron, N. Motta, Y. Qi and P. Sonar, Adv. Electron. Mater., 2017, 3, 1700139 CrossRef.
- H. D. Pham, S. M. Jain, M. Li, S. Manzhos, K. Feron, S. Pitchaimuthu, Z. Liu, N. Motta, H. Wang, J. R. Durrant and P. Sonar, J. Mater. Chem. A, 2019, 7, 5315–5323 RSC.
- H. D. Pham, S. M. Jain, M. Li, Z. K. Wang, S. Manzhos, K. Feron, S. Pitchaimuthu, Z. Liu, N. Motta, J. R. Durrant and P. Sonar, Adv. Electron. Mater., 2020, 6, 1900884 CrossRef CAS.
- F. M. Rombach, S. A. Haque and T. J. Macdonald, Energy Environ. Sci., 2021, 14, 5161–5190 RSC.
- Z. Hawash, L. K. Ono and Y. Qi, Adv. Mater. Interfaces, 2018, 5, 1700623 CrossRef.
- S. Gangala and R. Misra, J. Mater. Chem. A, 2018, 6, 18750–18765 RSC.
- M. Maciejczyk, A. Ivaturi and N. Robertson, J. Mater. Chem. A, 2016, 4, 4855–4863 RSC.
- B. Xu, D. Bi, Y. Hua, P. Liu, M. Cheng, M. Grätzel, L. Kloo, A. Hagfeldt and L. Sun, Energy Environ. Sci., 2016, 9, 873–877 RSC.
- J. Salunke, X. Guo, Z. Lin, J. R. Vale, N. R. Candeias, M. Nyman, S. Dahlström, R. Österbacka, A. Priimagi, J. Chang and P. Vivo, ACS Appl. Energy Mater., 2019, 2, 3021–3027 CrossRef CAS.
- M. R. Maciejczyk, R. Chen, A. Brown, N. Zheng and N. Robertson, J. Mater. Chem. C, 2019, 7, 8593–8598 RSC.
- K. H. Lin, A. Prlj and C. Corminboeuf, J. Phys. Chem. C, 2017, 121, 21729–21739 CrossRef CAS.
- C. Huang, W. Fu, C. Z. Li, Z. Zhang, W. Qiu, M. Shi, P. Heremans, A. K. Y. Jen and H. Chen, J. Am. Chem. Soc., 2016, 138, 2528–2531 CrossRef CAS PubMed.
- K. Rakstys, A. Abate, M. I. Dar, P. Gao, V. Jankauskas, G. Jacopin, E. Kamarauskas, S. Kazim, S. Ahmad, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2015, 137, 16172–16178 CrossRef CAS PubMed.
- K. Rakstys, S. Paek, P. Gao, P. Gratia, T. Marszalek, G. Grancini, K. T. Cho, K. Genevicius, V. Jankauskas, W. Pisula and M. K. Nazeeruddin, J. Mater. Chem. A, 2017, 5, 7811–7815 RSC.
- M. R. Maciejczyk, J. A. G. Williams, N. Robertson and M. Pietraszkiewicz, RSC Adv., 2017, 7, 49532–49535 RSC.
- M. R. Maciejczyk, S. Zhang, G. J. Hedley, N. Robertson, I. D. W. Samuel and M. Pietraszkiewicz, Adv. Funct. Mater., 2018, 1807572, 1–13 Search PubMed.
- C. Liu, M. Hu, X. Zhou, J. Wu, L. Zhang, W. Kong, X. Li, X. Zhao, S. Dai, B. Xu and C. Cheng, NPG Asia Mater., 2018, 10, 552–561 CrossRef CAS.
- J. Liu, Y. Wu, C. Qin, X. Yang, T. Yasuda, A. Islam, K. Zhang, W. Peng, W. Chen and L. Han, Energy Environ. Sci., 2014, 7, 2963–2967 RSC.
- N. J. Jeon, H. Na, E. H. Jung, T. Y. Yang, Y. G. Lee, G. Kim, H. W. Shin, S. Il Seok, J. Lee and J. Seo, Nat. Energy, 2018, 3, 682–689 CrossRef CAS.
- Z. Guo, A. K. Jena, I. Takei, G. M. Kim, M. A. Kamarudin, Y. Sanehira, A. Ishii, Y. Numata, S. Hayase and T. Miyasaka, J. Am. Chem. Soc., 2020, 142, 9725–9734 CAS.
- R. R. Gagne, C. A. Koval and G. C. Lisensky, Inorg. Chem., 1980, 19, 2854–2855 CrossRef CAS.
- C. M. Cardona, W. Li, A. E. Kaifer, D. Stockdale and G. C. Bazan, Adv. Mater., 2011, 23, 2367–2371 CrossRef CAS PubMed.
- Y. Li, R. G. Clevenger, L. Jin, K. V. Kilway and Z. Peng, J. Mater. Chem. C, 2014, 2, 7180 RSC.
- S. S. Mali, J. V. Patil, H. Arandiyan and C. K. Hong, J. Mater. Chem. A, 2019, 7, 17516–17528 RSC.
- M. V. Khenkin, E. A. Katz, A. Abate, G. Bardizza, J. J. Berry, C. Brabec, F. Brunetti, V. Bulović, Q. Burlingame, A. Di Carlo, R. Cheacharoen, Y.-B. Cheng, A. Colsmann, S. Cros, K. Domanski, M. Dusza, C. J. Fell, S. R. Forrest, Y. Galagan, D. Di Girolamo, M. Grätzel, A. Hagfeldt, E. von Hauff, H. Hoppe, J. Kettle, H. Köbler, M. S. Leite, S. (Frank) Liu, Y.-L. Loo, J. M. Luther, C. Q. Ma, M. Madsen, M. Manceau, M. Matheron, M. McGehee, R. Meitzner, M. K. Nazeeruddin, A. F. Nogueira, Ç. Odabaşı, A. Osherov, N.-G. Park, M. O. Reese, F. De Rossi, M. Saliba, U. S. Schubert, H. J. Snaith, S. D. Stranks, W. Tress, P. A. Troshin, V. Turkovic, S. Veenstra, I. Visoly-Fisher, A. Walsh, T. Watson, H. Xie, R. Yıldırım, S. M. Zakeeruddin, K. Zhu and M. Lira-Cantu, Nat. Energy, 2020, 5, 35–49 CrossRef.
- G. Wenson, H. Thakkar, H. Tsai, J. Stein, R. Singh and W. Nie, J. Mater. Chem. A, 2022, 10, 13519–13526 RSC.
- S. W. Lee, S. Kim, S. Bae, K. Cho, T. Chung, L. E. Mundt, S. Lee, S. Park, H. Park, M. C. Schubert, S. W. Glunz, Y. Ko, Y. Jun, Y. Kang, H. S. Lee and D. Kim, Sci. Rep., 2016, 6, 38150 CrossRef CAS PubMed.
- J. Carolus, T. Merckx, Z. Purohit, B. Tripathi, H. G. Boyen, T. Aernouts, W. De Ceuninck, B. Conings and M. Daenen, Sol. RRL, 2019, 3, 1900226 CrossRef CAS.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Solar cell fabrication and characterization; synthesis of compounds; single crystal X-ray diffraction; fabrication and characterisation of SCLC devices; UV/Vis absorption and photoluminescence; computational calculations; cyclic voltammetry; powder X-ray diffraction; scanning electron microscopy. CCDC 2235428 and 2235429. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3tc00119a |
| ‡ Current address: Science & Innovation Center, Mitsubishi Chemical Corporation, 1000 Kamoshidacho, Aoba, Yokohama, Kanagawa, 227-8502, Japan. |
| § Current address: Faculty of Food Biotechnology and Chemical Engineering, Hankyong National University, Anseong, Gyeonggi-Do, 17579, Republic of Korea. |
| ¶ Current address: Cambridge Display Technology Limited, Unit 12 Cardinal Park, Cardinal Way, Godmanchester, Cambridgeshare, PE29 2XG, UK. |
| || Current address: Faculty of Life and Environmental Sciences, Teikyo University of Science, 2525 Yatsusawa, Uenohara, Yamanashi, 409-0193, Japan. |
| This journal is © The Royal Society of Chemistry 2023 |